Immuno Nanosensor for the Ultrasensitive Naked Eye Detection of Tuberculosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Conjugation of Streptavidin with Catalase
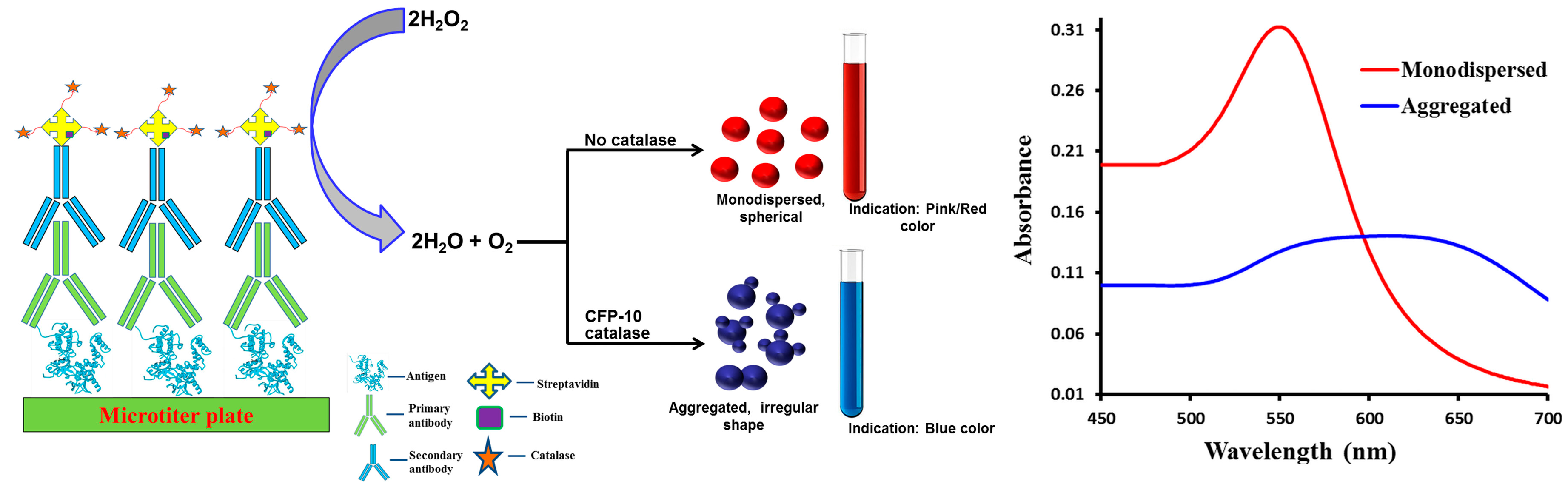
2.3. ELISA
2.4. Instrumental Analysis
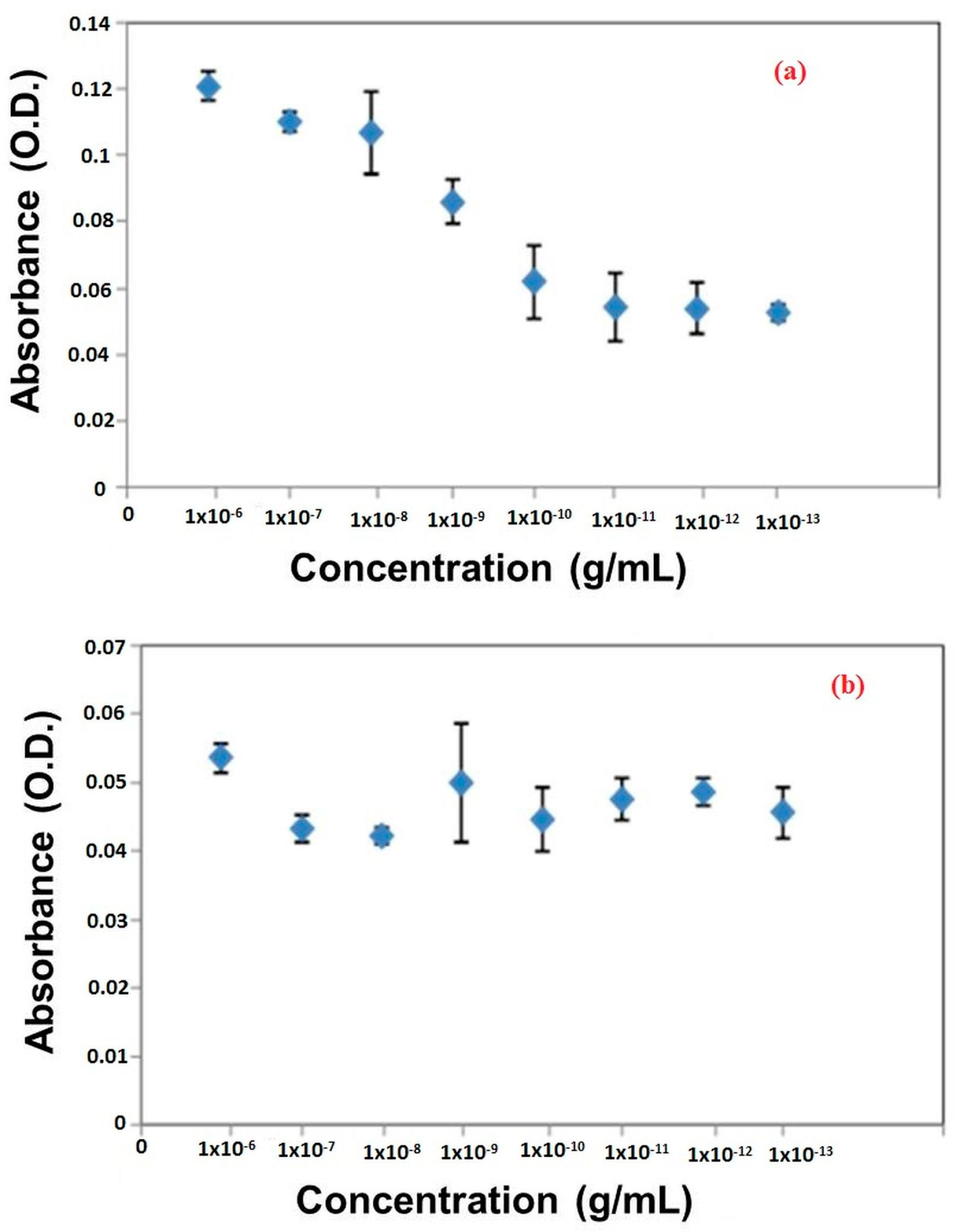
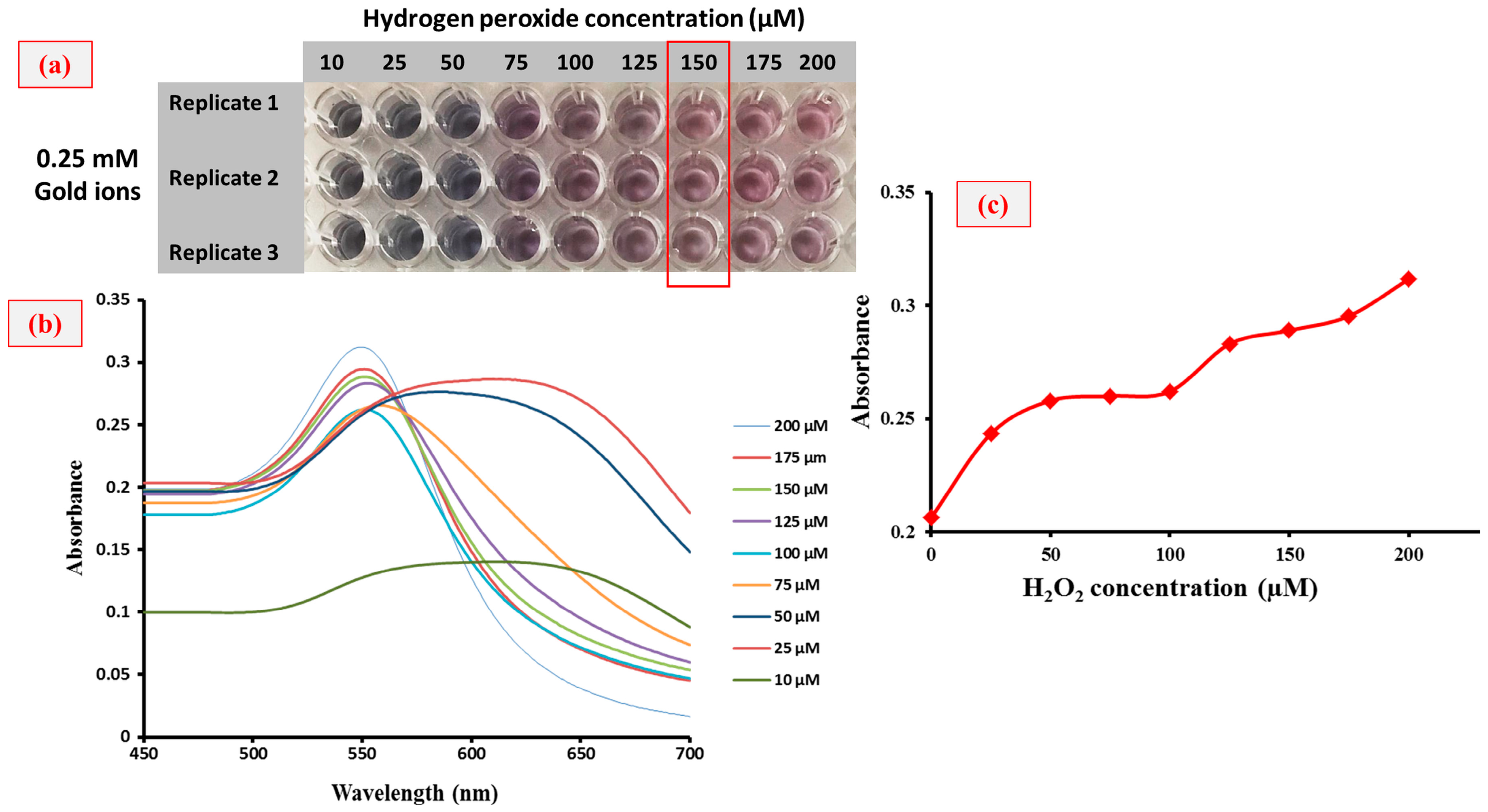
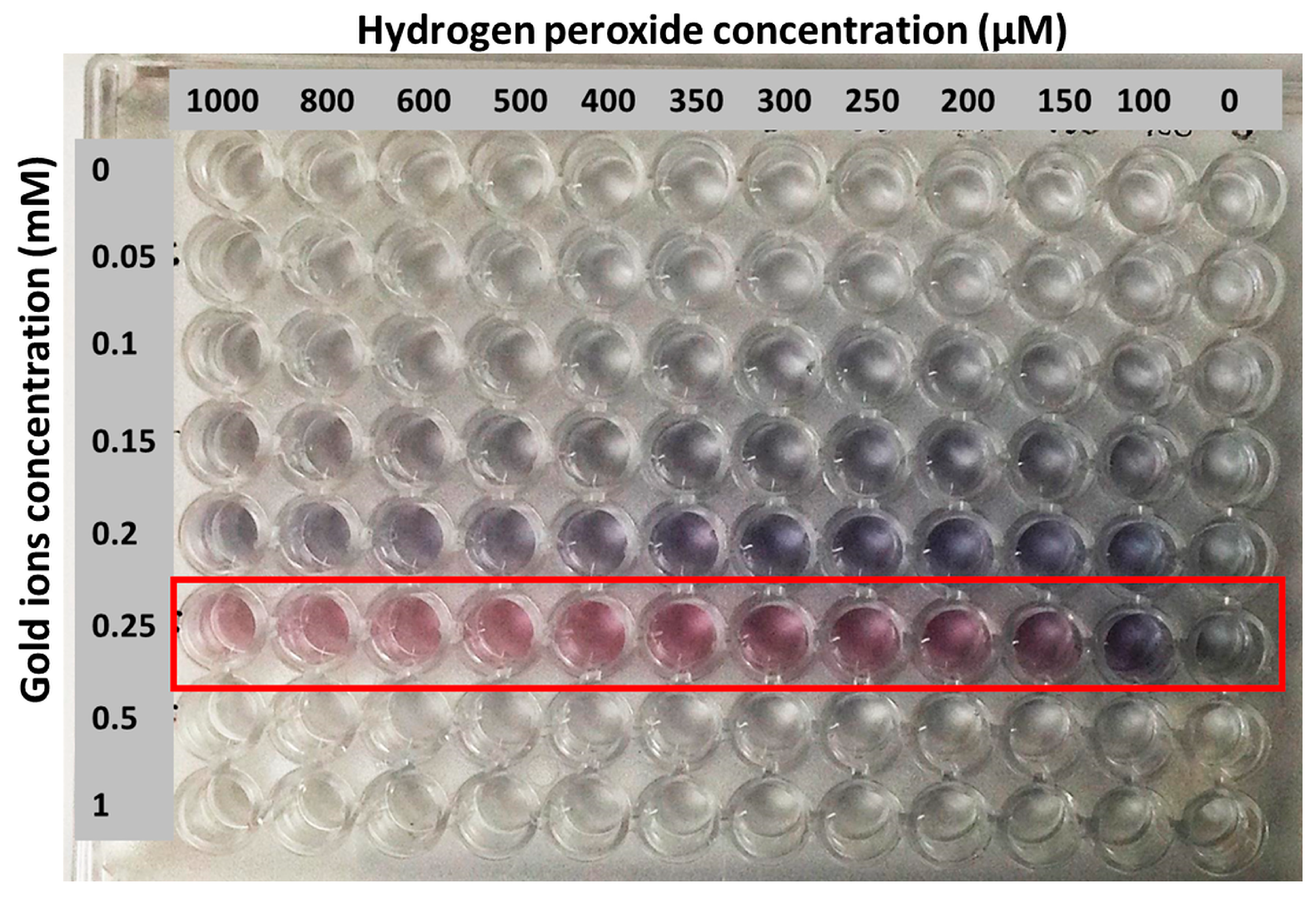
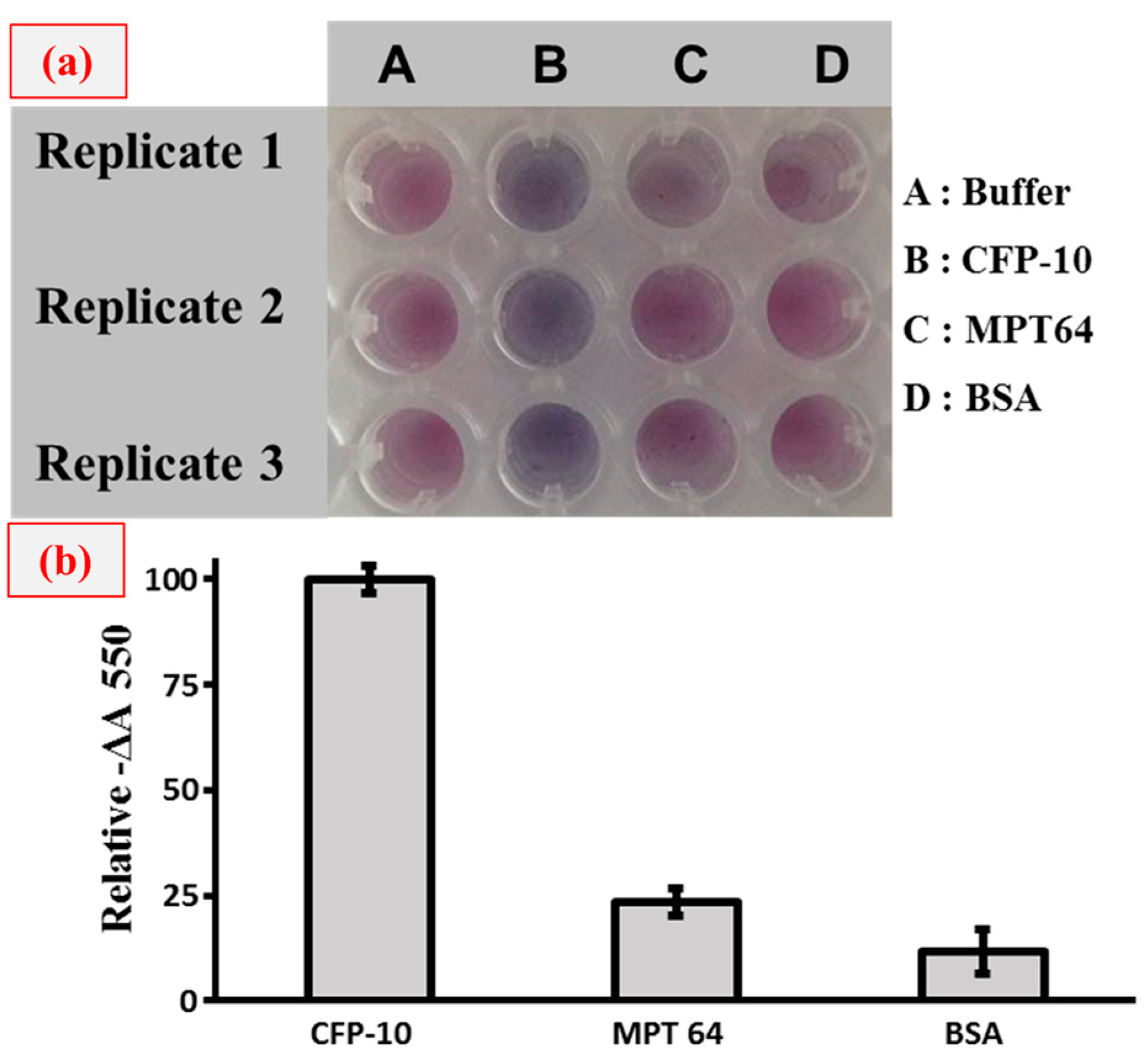
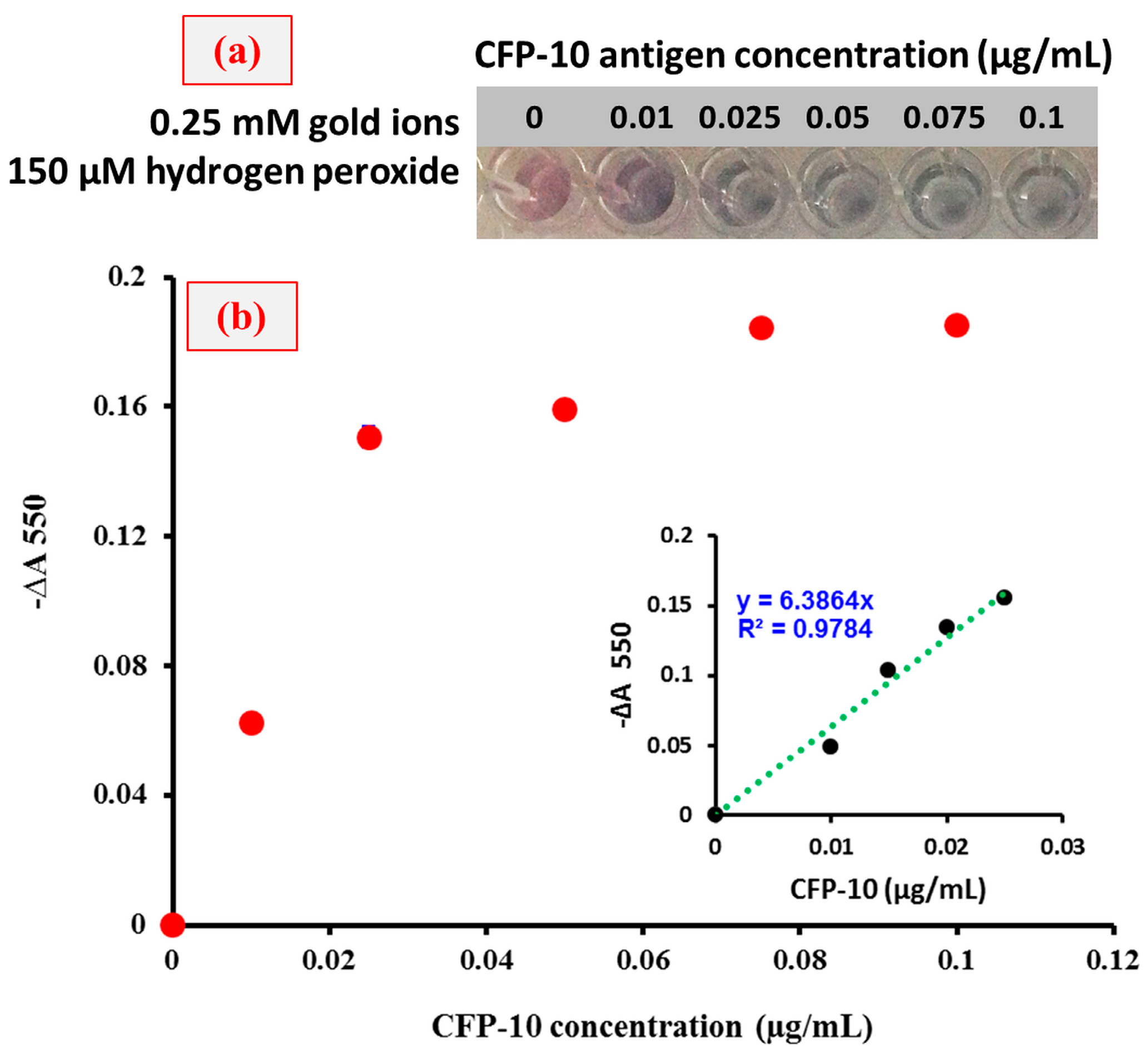
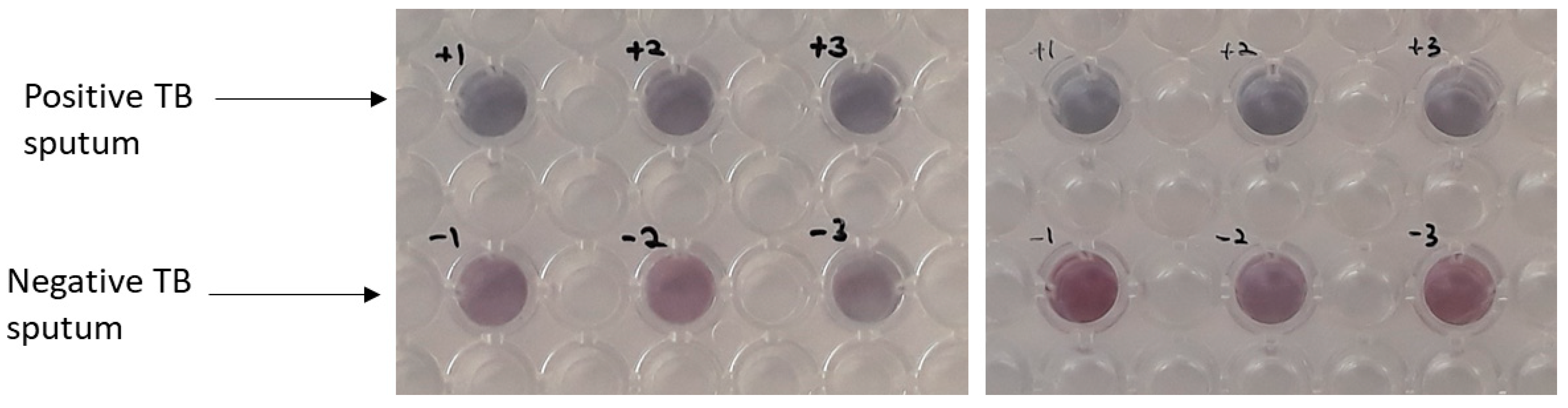
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hamada, Y.; Sidibe, A.; Matteelli, A.; Dadu, A.; Aziz, M.A.; del Granado, M.; Nishikiori, N.; Floyd, K.; Getahun, H. Policies and practices on the programmatic management of latent tuberculous infection: Global survey. Int. J. Tuberc. Lung Dis. 2016, 20, 1566–1571. [Google Scholar] [CrossRef] [PubMed]
- Komurcuoglu, B.; Senol, G.; Balci, G.; Yalnız, E.; Ozden, E. Drug resistance in pulmonary tuberculosis in new and previously treated cases: Experience from Turkey. J. Infect. Public Health 2013, 6, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Brouqui, P.; Puro, V.; Fusco, F.M.; Bannister, B.; Schilling, S.; Follin, P.; Gottschalk, R.; Hemmer, R.; Maltezou, H.C.; Ott, K.; et al. Infection control in the management of highly pathogenic infectious diseases: Consensus of the european network of infectious disease. Lancet Infect. Dis. 2009, 9, 301–311. [Google Scholar] [CrossRef]
- Leem, A.L.; Song, J.H.; Lee, E.H.; Lee, H.; Sim, B.; Kim, S.Y.; Chung, K.S.; Kim, E.Y.; Jung, J.Y.; Park, M.S.; et al. Changes in cytokine responses to TB antigens ESAT-6, CFP-10 and TB 7.7 and infammatory markers in peripheral blood during therapy. Sci. Rep. 2018, 8, 1159. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, K.A.; Counoupas, C.; Leotta, L.; Eto, C.; Bitter, W.; Winter, N.; Triccas, J.A. The Ag85B protein of the BCG vaccine facilitates macrophage uptake but is dispensable for protection against aerosol Mycobacterium tuberculosis infection. Vaccine 2016, 34, 2608–2615. [Google Scholar] [CrossRef] [PubMed]
- Torati, S.R.; Reddy, V.; Yoon, S.S.; Kim, C. Electrochemical biosensor for Mycobacterium tuberculosis DNA detection based on gold nanotubes array electrode platform. Biosens. Bioelectron. 2016, 78, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Abe, C.; Hirano, K.; Wada, M.; Kazumi, Y.; Takahashi, M.; Fukasawa, Y.; Yoshimura, T.; Miyagi, C.; Goto, S. Detection of mycobacterium tuberculosis in clinical specimens by polymerase chain reaction and gen-probe amplified mycobacterium tuberculosis direct test. J. Clin. Microbiol. 1993, 31, 3270–3274. [Google Scholar] [CrossRef]
- Mukherjee, A.; Lodha, R.; Kabra, S.K. Recent advances in diagnosis of tuberculosis. Pediatr. Infect. Dis. 2012, 4, 45–50. [Google Scholar]
- Steingart, K.R.; Henry, M.; Ng, V.; Hopewell, P.C.; Ramsay, A.; Cunningham, J.; Urbanczik, R.; Perkins, M.; Aziz, M.A.; Pai, M. Fluorescence versus conventional sputum smear microscopy for tuberculosis: A systematic review. Lancet Infect. Dis. 2006, 6, 570–581. [Google Scholar] [CrossRef]
- Camargos, P.; Fonseca, A.C.; Amantéa, S.; Oliveira, E.; Benfica, M.; Chamone, G.C. The role of latex agglutination test for the etiological diagnosis of pleural effusion in children and adolescents. Clin. Respir. J. 2017, 11, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Tsao, Z.; Chang, S.T.; Juang, R.H.; Wang, L.C.; Chang, C.M.; Wang, C.H. Development of an antigen-capture enzyme-linked immunosorbent assay using monoclonal antibodies for detecting H6 avian influenza viruses. J. Microbiol. Immunol. Infect. 2012, 45, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Shekar, S.; Yeo, Z.X.; Wong, J.C.L.; Chan, M.K.L.; Ong, D.C.T.; Tongyoo, P.; Wong, S.Y.; Lee, A.S.G. Detecting novel genetic variants associated with isoniazid-resistant mycobacterium tuberculosis. PLoS ONE 2014, 9, e102383. [Google Scholar] [CrossRef] [PubMed]
- Vir, P.; Arrigucci, R.; Lakehal, K.; Pine, R.; Bushkin, Y.; Tyagi, S.; Davidow, A.; Lardizabal, A.; Gennaro, M. RNA fluorescence in situ hybridization and flow cytometry (FISH-Flow) detects antigen-specific T cell responses associated with latent tuberculosis infection (TECH3P.940). J. Immunol. 2015, 194, 207. [Google Scholar]
- Raston, N.H.A.; Nguyen, V.T.; Gu, M.B. A new lateral flow strip assay (LFSA) using a pair of aptamers for the detection of Vaspin. Biosens. Bioelectron. 2017, 93, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.P.; Gajewski, K. Rapid detection of bovine adipose tissue using lateral flow strip assay. Food Sci. Nutr. 2016, 4, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Jitendra, S.; Nirmal, P.; Debasish, M.; Soumyo, M. Plasmonic-ELISA: Expanding horizons. RSC Adv. 2016, 6, 85440–85456. [Google Scholar]
- El-Samadony, H.; Althani, A.; Tageldin, M.A.; Azzazy, H.M.E. Nanodiagnostics for tuberculosis detection. J. Expert Rev. Mol. Diag. 2017, 17, 427–443. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.T.; Huang, C.Y.; Chen, C.A.; Shen, S.W.; Wang, M.C.; Cheng, C.M.; Chen, C.F. Diagnosis of tuberculosis using colorimetric gold nanoparticles on a paper-based analytical device. ACS Sens. 2017, 2, 1345–1354. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.M.; Huang, R.; Dong, C.X.; Tang, L.J.; Gui, R.; Jiang, J.H. Plasmonic ELISA for the ultrasensitive detection of Treponema pallidum. Biosens. Bioelectron. 2014, 58, 314–319. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohd Bakhori, N.; Yusof, N.A.; Abdullah, J.; Wasoh, H.; Md Noor, S.S.; Ahmad Raston, N.H.; Mohammad, F. Immuno Nanosensor for the Ultrasensitive Naked Eye Detection of Tuberculosis. Sensors 2018, 18, 1932. https://doi.org/10.3390/s18061932
Mohd Bakhori N, Yusof NA, Abdullah J, Wasoh H, Md Noor SS, Ahmad Raston NH, Mohammad F. Immuno Nanosensor for the Ultrasensitive Naked Eye Detection of Tuberculosis. Sensors. 2018; 18(6):1932. https://doi.org/10.3390/s18061932
Chicago/Turabian StyleMohd Bakhori, Noremylia, Nor Azah Yusof, Jaafar Abdullah, Helmi Wasoh, Siti Suraiya Md Noor, Nurul Hanun Ahmad Raston, and Faruq Mohammad. 2018. "Immuno Nanosensor for the Ultrasensitive Naked Eye Detection of Tuberculosis" Sensors 18, no. 6: 1932. https://doi.org/10.3390/s18061932




