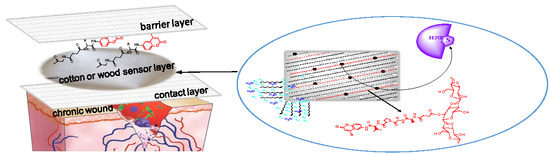
Peptide-Cellulose Conjugates on Cotton-Based Materials Have Protease Sensor/Sequestrant Activity
Abstract
:1. Introduction
Chronic Wounds and Protease-Modulating Dressings
2. Materials and Methods
2.1. General
2.2. Summary of Synthetic Approach of Peptide-Cellulose Conjugates
2.3. Response and Sensitivity Assay
2.4. Surface Charge
2.5. Sequestration of Elastase from Wound-Like Fluid by Cellulosic Transducers
2.6. Water Contact Angle (WCA) Measurements
2.7. Moisture Absorption
2.8. Degree of Substitution
2.9. Specific Surface Area and Average Fibril Diameter
2.10. Microscopy Studies of NAs
3. Results and Discussion
3.1. Structure/Function and Physical Property Considerations
3.1.1. Sensor Protease Detection Levels Relevant to Chronic Wounds
3.1.2. Effect of Transducer Porosity and Pore Size on Elastase Detection
3.1.3. Surface Wetting, Absorption, and Permeability of the Nanocellulosic Aerogel
3.1.4. Protease Sequestration Activity
3.1.5. Biosensor Properties as a Function of an Intelligent Semiocclusive Dressing Motif
3.2. Future Prospects
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hasan, A.; Murata, H.; Falabella, A.; Ochoa, S.; Zhou, L.; Badiavas, E.; Falanga, V. Dermal fibroblasts from venous ulcers are unresponsive to the action of transforming growth factor-β 11. J. Dermatol. Sci. 1997, 16, 59–66. [Google Scholar] [CrossRef]
- Ågren, M.S.; Steenfos, H.H.; Dabelsteen, S.; Hansen, J.B.; Dabelsteen, E. Proliferation and Mitogenic Response to PDGF-BB of Fibroblasts Isolated from Chronic Venous Leg Ulcers is Ulcer-Age Dependent1. J. Investig. Dermatol. 1999, 112, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Cook, H.; Stephens, P.; Davies, K.J.; Thomas, D.W.; Harding, K.G. Defective Extracellular Matrix Reorganization by Chronic Wound Fibroblasts is Associated with Alterations in TIMP-1, TIMP-2, and MMP-2 Activity. J. Investig. Dermatol. 2000, 115, 225–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yager, D.R.; Nwomeh, B.C. The proteolytic environment of chronic wounds. Wound Repair Regen. 1999, 7, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Yager, D.R.; Chen, S.M.; Ward, S.I.; Olutoye, O.O.; Diegelmann, R.F.; Kelman Cohen, I. Ability of chronic wound fluids to degrade peptide growth factors is associated with increased levels of elastase activity and diminished levels of proteinase inhibitors. Wound Repair Regen. 1997, 5, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Wound Management Forecast. Wound Management: An $18.5 Billion+ Worldwide Market in 2021. Available online: http://blog.mediligence.com/2013/06/10/wound-management-an-18-5-billion-worldwide-market-in-2021/ (accessed on 15 September 2017).
- Norman, G.; Westby, M.J.; Stubbs, N.; Dumville, J.C.; Cullum, N. A ‘test and treat’ strategy for elevated wound protease activity for healing in venous leg ulcers. Cochrane Database Syst. Rev. 2016. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.V.; Fontenot, K.R.; Prevost, N.T.; Haldane, D.; Nicole, P.; Liebner, F.; French, A.D.; Condon, B.D. Protease biosensors based on peptide-nanocellulose conjugates: From molecular design to dressing interface. Int. J. Med. Nano Res. 2016, 3, 018. [Google Scholar] [CrossRef]
- Dabiri, G.; Damstetter, E.; Phillips, T. Choosing a Wound Dressing Based on Common Wound Characteristics. Adv. Wound Care 2016, 5, 32–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarlton, J.F.; Munro, H.S. Use of Modified Superabsorbent Polymer Dressings for Protease Modulation in Improved Chronic Wound Care. Wounds 2013, 25, 51–57. [Google Scholar] [PubMed]
- Schultz, G.S.; Sibbald, R.G.; Falanga, V.; Ayello, E.A.; Dowsett, C.; Harding, K.; Romanelli, M.; Stacey, M.C.; Teot, L.; Vanscheidt, W. Wound bed preparation: A systematic approach to wound management. Wound Repair Regen. 2003, 11, S1–S28. [Google Scholar] [CrossRef] [PubMed]
- Krejner, A.; Grzela, T. Modulation of matrix metalloproteinases MMP-2 and MMP-9 activity by hydrofiber-foam hybrid dressing—relevant support in the treatment of chronic wounds. Cent.-Eur. J. Immunol. 2015, 40, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.V.; Yager, D.R.; Cohen, I.K.; Diegelmann, R.F.; Montante, S.; Bertoniere, N.; Bopp, A.F. Modified cotton gauze dressings that selectively absorb neutrophil elastase activity in solution. Wound Repair Regen. 2001, 9, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.A.F. Overview and General Considerations of Wound Repair. In The Molecular and Cellular Biology of Wound Repair; Clark, R.A.F., Henson, P.M., Eds.; Springer: Boston, MA, USA, 1988; pp. 3–33. [Google Scholar]
- Cullen, B.; Smith, R.; McCulloch, E.; Silcock, D.; Morrison, L. Mechanism of action of PROMOGRAN, a protease modulating matrix, for the treatment of diabetic foot ulcers. Wound Repair Regen. 2002, 10, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Serena, T.E.; Cullen, B.M.; Bayliff, S.W.; Gibson, M.C.; Carter, M.J.; Chen, L.; Yaakov, R.A.; Samies, J.; Sabo, M.; DeMarco, D.; et al. Defining a new diagnostic assessment parameter for wound care: Elevated protease activity, an indicator of nonhealing, for targeted protease-modulating treatment. Wound Repair Regen. 2016, 24, 589–595. [Google Scholar] [CrossRef] [PubMed]
- International Consensus. The role of proteases in wound diagnostics. An expert working group review. Wounds Int. 2011, 1, 1–13. [Google Scholar]
- Edwards, J.; Fontenot, K.; Liebner, F.; Pircher, N.; French, A.; Condon, B. Structure/Function Analysis of Cotton-Based Peptide-Cellulose Conjugates: Spatiotemporal/Kinetic Assessment of Protease Aerogels Compared to Nanocrystalline and Paper Cellulose. Int. J. Mol. Sci. 2018, 19, 840. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.V.; Fontenot, K.R.; Prevost, N.T.; Pircher, N.; Liebner, F.; Condon, B.D. Preparation, Characterization, and Activity of a Peptide-Cellulosic Aerogel Protease Sensor from Cotton. Sensors 2016, 16, 1789. [Google Scholar] [CrossRef] [PubMed]
- Fontenot, K.R.; Edwards, J.V.; Haldane, D.; Graves, E.; Citron, M.S.; Prevost, N.T.; French, A.D.; Condon, B.D. Human neutrophil elastase detection with fluorescent peptide sensors conjugated to cellulosic and nanocellulosic materials: Part II, structure/function analysis. Cellulose 2016, 16, 1789. [Google Scholar] [CrossRef]
- Yuan, Y.; Lee, T.R. Contact Angle and Wetting Properties. In Surface Science Techniques; Bracco, G., Holst, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 51, pp. 3–34. [Google Scholar]
- Aulin, C.; Netrval, J.; Wagberg, L.; Lindstrom, T. Aerogels from nanofibrillated cellulose with tunable oleophobicity. Soft Matter 2010, 6, 3298–3305. [Google Scholar] [CrossRef]
- Edwards, J.V.; Prevost, N.; Sethumadhavan, K.; Ullah, A.; Condon, B. Peptide conjugated cellulose nanocrystals with sensitive human neutrophil elastase sensor activity. Cellulose 2013, 20, 1223–1235. [Google Scholar] [CrossRef]
- Yue, Y.; Zhou, C.; French, A.; Xia, G.; Han, G.; Wang, Q.; Wu, Q. Comparative properties of cellulose nano-crystals from native and mercerized cotton fibers. Cellulose 2012, 19, 1173–1187. [Google Scholar] [CrossRef]
- Edwards, J.V.; Fontenot, K.R.; Haldane, D.; Prevost, N.T.; Condon, B.D. Human Neutrophil Elastase peptide sensors conjugated to cellulosic and nanocellulosic materials: Part I, synthesis and characterization of fluorescent analogs. Cellulose 2016, 23, 1283–1295. [Google Scholar] [CrossRef]
- O’Donoghue, A.J.; Jin, Y.; Knudsen, G.M.; Perera, N.C.; Jenne, D.E.; Murphy, J.E.; Craik, C.S.; Hermiston, T.W. Global Substrate Profiling of Proteases in Human Neutrophil Extracellular Traps Reveals Consensus Motif Predominantly Contributed by Elastase. PLoS ONE 2013, 8, e75141. [Google Scholar] [CrossRef] [PubMed]
- Trengove, N.J.; Stacey, M.C.; Macauley, S.; Bennett, N.; Gibson, J.; Burslem, F.; Murphy, G.; Schultz, G. Analysis of the acute and chronic wound environments: The role of proteases and their inhibitors. Wound Repair Regen. 1999, 7, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.V.; Fontenot, K.R.; Prevost, N.; Nam, S.; Concha, M.; Condon, B. Synthesis and Assessment of Peptide Nanocellulosic Biosensors. In Nanocellulose, Cellulose Nanofibers, and Cellulose Nanocomposites; Mondal, I.H., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2016; pp. 475–494. [Google Scholar]
- Serena, T.E. Development of a Novel Technique to Collect Proteases from Chronic Wounds. Adv. Wound Care 2014, 3, 729–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patrick, A.G.; Ulijn, R.V. Hydrogels for the detection and Mangement of Protease Levels. Macromol. Biosci. 2010, 10, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Schyrr, B.; Boder-Pasche, S.; Ischer, R.; Smajda, R.; Voirin, G. Fiber-optic protease sensor based on the degradation of thin gelatin films. Sens. Bio-Sens. Res. 2015, 3, 65–73. [Google Scholar] [CrossRef]
- Edwards, J.V.; Prevost, N.T.; French, A.D.; Concha, M.; Condon, B.D. Kinetic and structural analysis of fluorescent peptides on cotton cellulose nanocrystals as elastase sensors. Carbohydr. Polym. 2015, 116, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Derikvand, F.; Yin, D.T.; Barrett, R.; Brumer, H. Cellulose-Based Biosensors for Esterase Detection. Anal. Chem. 2016, 88, 2989–2993. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, X.; Yang, Y.; Yuan, Q. Aptamer-functionalized carbon nanomaterials electrochemical sensors for detecting cancer relevant biomolecules. Carbon 2018, 129, 380–395. [Google Scholar] [CrossRef]
- Madhu, R.M.; Veeramani, V.; Chen, S.-M.; Veerakumar, P.; Liu, S.-B.; Miyamoto, N. Functional porous carbon-ZnO nanocomposites for high-performance biosensors and energy storage applications. Phys. Chem. Chem. Phys. 2016, 18, 16466–16475. [Google Scholar] [CrossRef] [PubMed]
- Kinser, E.R.; Padmanabhan, J.; Yu, R.; Corona, S.L.; Li, J.; Vaddiraju, S.; Legassey, A.; Loye, A.; Balestrini, J.; Solly, D.A.; et al. Nanopatterned Bulk Metallic Glass Biosensors. ACS Sens. 2017, 2, 1779–1787. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Kimura, S.; Togawa, E.; Wada, M. Crystal transition from cellulose II hydrate to cellulose II. Carbohydr. Polym. 2011, 86, 975–981. [Google Scholar] [CrossRef]
- Winter, G.D. Formation of the Scab and the Rate of Epithelization of Superficial Wounds in the Skin of the Young Domestic Pig. Nature 1962, 193, 293–294. [Google Scholar] [CrossRef] [PubMed]
- Falanga, V. Occlusive wound dressings: Why, when, which? Arch. Dermatol. 1988, 124, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, D.M.; Rovee, D.T.; Alvarez, O.M. Wound Dressings: Design and use. In Wound Healing: Biochemical and Clinical Aspects; Cohen, I.K., Diegelmann, R.F., Lindblad, W.J., Eds.; W.B. Saunders Company: Phildelphia, PA, USA, 1992; pp. 562–580. [Google Scholar]
- Morgan, D. Wounds-what should a dressing formulary include. Pharm. J. 2002, 9, 261–266. [Google Scholar]
- Abrigo, M.; McArthur, S.L.; Kingshott, P. Electrospun Nanofibers as Dressings for Chronic Wound Care: Advances, Challenges, and Future Prospects. Macromol. Biosci. 2014, 14, 772–792. [Google Scholar] [CrossRef] [PubMed]
- Baranoski, S.; Ayello, E.A. Wound Dressings: An Evolving Art and Science. Adv. Skin Wound Care 2012, 25, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Fisher, A.C.; Foo, P.P.; Queen, D.; Gaylor, J.D.S. In vitro assessment of water vapour transmission of synthetic wound dressings. Biomaterials 1995, 16, 171–175. [Google Scholar] [CrossRef]
- Queen, D.; Gaylor, J.D.S.; Evans, J.H.; Courtney, J.M.; Reid, W.H. The preclinical evaluation of the water vapour transmission rate through burn wound dressings. Biomaterials 1987, 8, 367–371. [Google Scholar] [CrossRef]
- Edwards, J.V.; Batiste, S.L.; Gibbins, E.M.; Goheen, S.C. Synthesis and activity of NH2- and COOH-terminal elastase recognition sequences on cotton. J. Pept. Res. 1999, 54, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.V. Future Structure and Properties of Mechanism-Based Wound Dressing. In Modified Fibers with Medical and Specialty Applications; Edwards, J.V., Buschle-Diller, G., Goheen, S.C., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 11–33. [Google Scholar]
- Edwards, J.V.; Howley, P.; Yachmenev, V.; Lambert, A.; Condon, B. Development of a Continuous Finishing Chemistry Process for Manufacture of a Phosphorylated Cotton Chronic Wound Dressing. J. Ind. Text. 2009, 39. [Google Scholar] [CrossRef]
- Edwards, J.V.; Prevost, N. Thrombin Production and Human Neutrophil Elastase Sequestration by Modified Cellulosic Dressings and Their Electrokinetic Analysis. J. Funct. Biomater. 2011, 2, 391. [Google Scholar] [CrossRef] [PubMed]
- Dargaville, T.R.; Farrugia, B.L.; Broadbent, J.A.; Pace, S.; Upton, Z.; Voelcker, N.H. Sensors and imaging for wound healing: A review. Biosens. Bioelectron. 2013, 41, 30–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, L.A.; Korber, A.; Grabbe, S.; Dissemond, J. Influence of pH on wound-healing: A new perspective for wound-therapy? Arch. Dermatol. Res. 2007, 298, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Fontenot, K.R.; Edwards, J.V.; Haldane, D.; Pircher, N.; Liebner, F.; Nam, S.; Condon, B.D. Structure/function relations of chronic wound dressings and emerging concepts on the interface of nanocellulosic sensors. In Lignocellulosics: Renewable Feedstock for (Tailored) Functional Materials and Nanotechnology; Nypelö, T.E., Filpponen, I., Peresin, M.S., Eds.; Elsevier Science: New York, NY, USA, 2016. [Google Scholar]
- Lindley, L.E.; Stojadinovic, O.; Pastar, I.; Tomic-Canic, M. Biology and Biomarkers for Wound Healing. Plast. Reconstr. Surg. 2016, 138, S18–S28. [Google Scholar] [CrossRef] [PubMed]






| Name | Porosity a | Average Pore Diameter | SSA c |
|---|---|---|---|
| (%) | (nm) | (m2g−1) | |
| CFP | 65.6 | 20–25 b | 0.020 |
| NA | 98.8 | 11 | 162.943 |
| NC | - | - | 186.200 |
| Name a | D.S. b |
|---|---|
| pCFP | 0.014 |
| pNA | 0.015 |
| pNC | 0.075 |
| Name | Surface Charge | Sequestration of HNE |
|---|---|---|
| (mV) | (%) | |
| pCFP | −10.2 ± 0.6 | 20 ± 1.0 |
| pNA | −19.0 ± 0.6 | 53 ± 1.0 |
| pNC | −26.1 ± 0.1 | 68 ± 4.0 |
| OSD | 2.5 ± 0.1 | 36 ± 3.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edwards, J.V.; Fontenot, K.R.; Liebner, F.; Condon, B.D. Peptide-Cellulose Conjugates on Cotton-Based Materials Have Protease Sensor/Sequestrant Activity. Sensors 2018, 18, 2334. https://doi.org/10.3390/s18072334
Edwards JV, Fontenot KR, Liebner F, Condon BD. Peptide-Cellulose Conjugates on Cotton-Based Materials Have Protease Sensor/Sequestrant Activity. Sensors. 2018; 18(7):2334. https://doi.org/10.3390/s18072334
Chicago/Turabian StyleEdwards, J. Vincent, Krystal R. Fontenot, Falk Liebner, and Brian D. Condon. 2018. "Peptide-Cellulose Conjugates on Cotton-Based Materials Have Protease Sensor/Sequestrant Activity" Sensors 18, no. 7: 2334. https://doi.org/10.3390/s18072334