A Colorimetric Probe Based on Functionalized Gold Nanorods for Sensitive and Selective Detection of As(III) Ions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Characterization
2.3. Preparation and Modification of AuNRs
2.4. Samples
2.5. Detection of As(III) Ions by Colorimetric Probe
3. Results
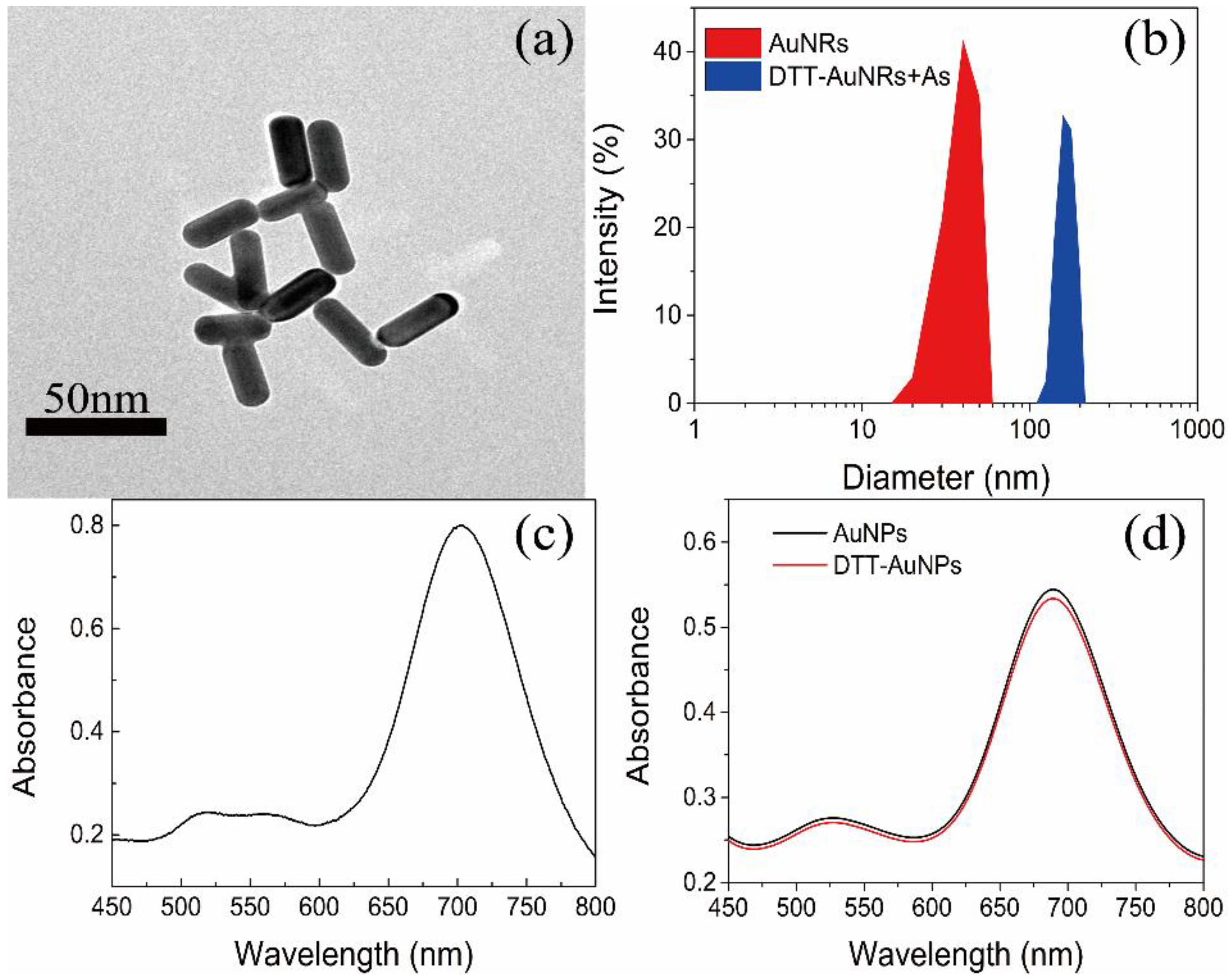
3.1. Characterization of Functionalized AuNRs
3.2. Stability Evaluation of AuNRs
3.3. Optimization of Experimental Conditions
3.3.1. Effect of DTT Concentration
3.3.2. Effect of pH
3.3.3. Effect of Reaction Time
3.3.4. Effect of NaCl Concentration
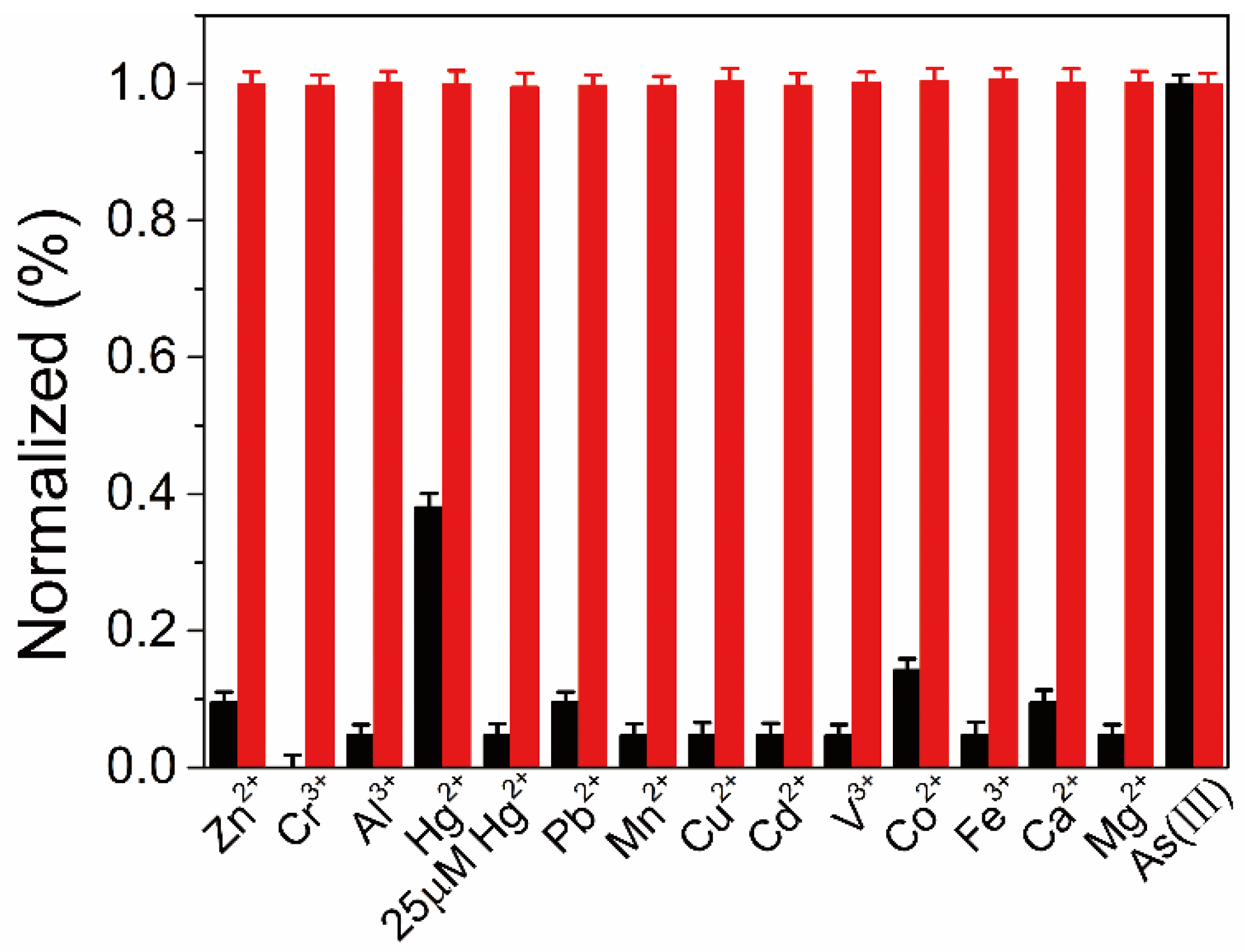
3.4. Selectivity
3.5. Determination of As(III)
3.6. Analysis of Samples
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sun, H.J.; Rathinasabapathi, B.; Wu, B.; Luo, J.; Pu, L.P.; Ma, L.Q. Arsenic and selenium toxicity and their interactive effects in humans. Environ. Int. 2014, 69, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Vermehren, J.; Polta, A.; Zimmermann, O.; Herrmann, E.; Poynard, T.; Hofmann, W.P.; Bojunga, J.; Sarrazin, C.; Zeuzem, S.; Friedrich-Rust, M. Comparison of acoustic radiation force impulse imaging with transient elastography for the detection of complications in patients with cirrhosis. Liver Int. 2012, 32, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Sengupta, M.K.; Yuan, D.; Dasgupta, P.K. Speciation and detection of arsenic in aqueous samples: A review of recent progress in non-atomic spectrometric methods. Anal. Chim. Acta 2014, 831, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Rager, J.E.; Bailey, K.A.; Smeester, L.; Miller, S.K.; Parker, J.S.; Laine, J.E.; Drobná, Z.; Currier, J.; Douillet, C.; Olshan, A.F. Prenatal arsenic exposure and the epigenome: Altered microRNAs associated with innate and adaptive immune signaling in newborn cord blood. Environ. Mol. Mutagen. 2014, 55, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, S.; Naqshbandi, A.; Farooqui, Z.; Khan, A.A.; Khan, F. Protective effect of dietary flaxseed oil on arsenic-induced nephrotoxicity and oxidative damage in rat kidney. Food Chem. Toxicol. 2014, 68, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Mulvihill, M.; Tao, A.; Benjauthrit, K.; Arnold, J.; Yang, P. Surface-enhanced Raman spectroscopy for trace arsenic detection in contaminated water. Angew. Chem. Int. Ed. Engl. 2008, 47, 6456–6460. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Verma, N.C.; Khan, S.; Nandi, C.K. Carbon dots for naked eye colorimetric ultrasensitive arsenic and glutathione detection. Biosens. Bioelectron. 2016, 81, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, W.; Hu, Y.; Cheng, H. Extraction and detection of organoarsenic feed additives and common arsenic species in environmental matrices by HPLC–ICP-MS. Microchem. J. 2013, 108, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Corns, W.T.; Stockwell, P.B.; Huang, J.-H. Accurate fast screening for total and inorganic arsenic in rice grains using hydride generation atomic fluorescence spectrometry (HG-AFS). Anal. Methods 2014, 6, 7554–7558. [Google Scholar] [CrossRef]
- Fang, Y.; Sun, X.; Yang, W.; Ma, N.; Xin, Z.; Fu, J.; Liu, X.; Liu, M.; Mariga, A.M.; Zhu, X.; et al. Concentrations and health risks of lead, cadmium, arsenic, and mercury in rice and edible mushrooms in China. Food Chem. 2014, 147, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Jackson, B.; Liba, A.; Nelson, J. Advantages of reaction cell ICP-MS on doubly charged interferences for arsenic and selenium analysis in foods. J. Anal. At. Spectrom. 2015, 30, 1179–1183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jena, B.K.; Raj, C.R. Gold nanoelectrode ensembles for the simultaneous electrochemical detection of ultratrace arsenic, mercury, and copper. Anal. Chem. 2008, 80, 4836–4844. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.; Vandergiessen, J.; Savarimuthu, X. Implementation of electrochemical sensors in arsenic-contaminated areas of West Bengal in India toward rapid and point-of-use detection of arsenic in drinking water. In Proceedings of the Global Humanitarian Technology Conference, San Jose, CA, USA, 10–13 October 2014; pp. 474–478. [Google Scholar]
- Ramesha, G.K.; Sampath, S. In-situ formation of graphene–lead oxide composite and its use in trace arsenic detection. Sens. Actuators B Chem. 2011, 160, 306–311. [Google Scholar] [CrossRef]
- Linhart, O.; Smolejová, J.; Červený, V.; Hraníček, J.; Nováková, E.; Resslerová, T.; Rychlovský, P. Determination of As by UV-photochemical generation of its volatile species with AAS detection. Monatsh. Chem. Chem. Mon. 2016, 147, 1447–1454. [Google Scholar] [CrossRef]
- Butwong, N.; Noipa, T.; Burakham, R.; Srijaranai, S.; Ngeontae, W. Determination of arsenic based on quenching of CdS quantum dots fluorescence using the gas-diffusion flow injection method. Talanta 2011, 85, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Shrivas, K.; Shankar, R.; Dewangan, K. Gold nanoparticles as a localized surface plasmon resonance based chemical sensor for on-site colorimetric detection of arsenic in water samples. Sens. Actuators B Chem. 2015, 220, 1376–1383. [Google Scholar] [CrossRef]
- Kalluri, J.R.; Arbneshi, T.; Afrin Khan, S.; Neely, A.; Candice, P.; Varisli, B.; Washington, M.; McAfee, S.; Robinson, B.; Banerjee, S.; et al. Use of Gold Nanoparticles in a Simple Colorimetric and Ultrasensitive Dynamic Light Scattering Assay: Selective Detection of Arsenic in Groundwater. Angew. Chem. 2009, 121, 9848–9851. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, Y.; Xu, B.; Zhang, H.; Gao, Y.; Zhang, H.; Song, D. A novel surface plasmon resonance biosensor based on graphene oxide decorated with gold nanorod-antibody conjugates for determination of transferrin. Biosens. Bioelectron. 2013, 45, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; Wang, H.; Fu, Q.; Peng, J.; Wang, Y.; Du, J.; Zhou, Y.; Zhan, L. Gold nanorod-based localized surface plasmon resonance biosensor for sensitive detection of hepatitis B virus in buffer, blood serum and plasma. Biosens. Bioelectron. 2010, 26, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Wei, Q.; Wei, A.; Cheng, J.X. Gold nanorods as contrast agents for biological imaging: Optical properties, surface conjugation and photothermal effects. Photochem. Photobiol. 2009, 85, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Alkilany, A.M.; Thompson, L.B.; Boulos, S.P.; Sisco, P.N.; Murphy, C.J. Gold nanorods: Their potential for photothermal therapeutics and drug delivery, tempered by the complexity of their biological interactions. Adv. Drug Deliv. Rev. 2012, 64, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, Y.; Wang, T.; Ma, Z.; Su, Z. Biorecognition-Driven Self-Assembly of Gold Nanorods: A Rapid and Sensitive Approach toward Antibody Sensing. Chem. Mater. 2007, 19, 5809–5811. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, I.H.; Qian, W.; El-Sayed, M.A. Cancer cells assemble and align gold nanorods conjugated to antibodies to produce highly enhanced, sharp, and polarized surface Raman spectra: A potential cancer diagnostic marker. Nano Lett. 2007, 7, 1591–1597. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Jain, P.K.; Elsayed, I.H.; Elsayed, M.A. Gold nanoparticles: Interesting optical properties and recent applications in cancer diagnostics and therapy. Nanomedicine 2007, 2, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Qu, C.; Liu, X.; Huang, S.; Xu, Z.; Zhu, Y.; Chu, P.K. Amplification of localized surface plasmon resonance signals by a gold nanorod assembly and ultra-sensitive detection of mercury. Chem. Commun. 2011, 47, 6897–6899. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.X.; Wang, L.; Zhang, H.; Han, Z.; Wu, X. A colorimetric probe for the rapid and selective determination of mercury(II) based on the disassembly of gold nanorods. Microchim. Acta 2012, 179, 345–350. [Google Scholar] [CrossRef]
- Rex, M.; Hernandez, F.E.; Campiglia, A.D. Pushing the limits of mercury sensors with gold nanorods. Anal. Chem. 2006, 78, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Pissuwan, D.; Valenzuela, S.M.; Cortie, M.B. Prospects for Gold Nanorod Particles in Diagnostic and Therapeutic Applications. Biotechnol. Genet. Eng. Rev. 2008, 25, 93–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- And, C.Y.; Irudayaraj, J. Multiplex Biosensor Using Gold Nanorods. Anal. Chem. 2007, 79, 572–579. [Google Scholar]
- Mayer, K.M.; Lee, S.; Liao, H.; Rostro, B.C.; Fuentes, A.; Scully, P.T.; Nehl, C.L.; Hafner, J.H. A Label-Free Immunoassay Based Upon Localized Surface Plasmon Resonance of Gold Nanorods. ACS Nano 2008, 2, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Li, P.C.; Shieh, D.B.; Wang, C.R.; Wei, C.W.; Liao, C.K.; Ding, A.A.; Wu, Y.N.; Poe, C.; Jhan, S. In vivo photoacoustic molecular imaging with simultaneous multiple selective targeting using antibody-conjugated gold nanorods. Opt. Express 2008, 16, 18605–18615. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Senapati, D.; Wang, S.; Griffin, J.; Neely, A.; Candice, P.; Naylor, K.M.; Varisli, B.; Kalluri, J.R.; Ray, P.C. Gold Nanorod Based Selective Identification of Escherichia coli Bacteria Using Two-Photon Rayleigh Scattering Spectroscopy. ACS Nano 2009, 3, 1906–1912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Dai, Q.; Austin, L.; Coutts, J.; Knowles, G.; Zou, J.; Chen, H.; Huo, Q. A one-step homogeneous immunoassay for cancer biomarker detection using gold nanoparticle probes coupled with dynamic light scattering. J. Am. Chem. Soc. 2008, 130, 2780–2782. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, L.; Wang, J.; Jiang, X.; Li, X.; Hu, Z.; Ji, Y.; Wu, X.; Chen, C. Mesoporous silica-coated gold nanorods as a light-mediated multifunctional theranostic platform for cancer treatment. Adv. Mater. 2012, 24, 1418–1423. [Google Scholar] [CrossRef] [PubMed]
- Von Maltzahn, G.; Park, J.H.; Agrawal, A.; Bandaru, N.K.; Das, S.K.; Sailor, M.J.; Bhatia, S.N. Computationally guided photothermal tumor therapy using long-circulating gold nanorod antennas. Cancer Res. 2009, 69, 3892–3900. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.-H.; Lin, D.; Wang, J.; Yang, P.-H.; Cai, J. Controlled side-by-side assembly of gold nanorods: A strategy for lead detection. Sens. Actuators B Chem. 2014, 196, 252–259. [Google Scholar] [CrossRef]
- Bi, N.; Chen, Y.; Qi, H.; Zheng, X.; Chen, Y.; Liao, X.; Zhang, H.; Tian, Y. Spectrophotometric determination of mercury(II) ion using gold nanorod as probe. Sens. Actuators B Chem. 2012, 166–167, 766–771. [Google Scholar] [CrossRef]
- Liu, J.M.; Wang, H.F.; Yan, X.P. A gold nanorod based colorimetric probe for the rapid and selective detection of Cu2+ ions. Analyst 2011, 136, 3904–3910. [Google Scholar] [CrossRef] [PubMed]
- Placido, T.; Aragay, G.; Pons, J.; Comparelli, R.; Curri, M.L.; Merkoci, A. Ion-directed assembly of gold nanorods: A strategy for mercury detection. ACS Appl. Mater. Interfaces 2013, 5, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Li, F.-M.; Liu, J.-M.; Wang, X.-X.; Lin, L.-P.; Cai, W.-L.; Lin, X.; Zeng, Y.-N.; Li, Z.-M.; Lin, S.-Q. Non-aggregation based label free colorimetric sensor for the detection of Cr (VI) based on selective etching of gold nanorods. Sens. Actuators B Chem. 2011, 155, 817–822. [Google Scholar] [CrossRef]
- Durgadas, C.V.; Lakshmi, V.N.; Sharma, C.P.; Sreenivasan, K. Sensing of lead ions using glutathione mediated end to end assembled gold nanorod chains. Sens. Actuators B Chem. 2011, 156, 791–797. [Google Scholar] [CrossRef]
- Priyadarshni, N.; Nath, P.; Nagahanumaiah; Chanda, N. DMSA-Functionalized Gold Nanorod on Paper for Colorimetric Detection and Estimation of Arsenic (III and V) Contamination in Groundwater. ACS Sustain. Chem. Eng. 2018, 6, 6264–6272. [Google Scholar] [CrossRef]
- Joshi, P.P.; Yoon, S.J.; Hardin, W.G.; Emelianov, S.; Sokolov, K.V. Conjugation of Antibodies to Gold Nanorods through Fc Portion: Synthesis and Molecular Specific Imaging. Bioconjug. Chem. 2013, 24, 878–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orendorff, C.J.; Murphy, C.J. Quantitation of metal content in the silver-assisted growth of gold nanorods. J. Phys. Chem. B 2006, 110, 3990–3994. [Google Scholar] [CrossRef] [PubMed]






| Detection limit (3s)/nM | 38 |
| Linear range/μM | 0.13–10.01 |
| Calibration function (As, conc./μM) | ΔA = 0.00487 [As] + 0.02089 |
| Correlation coefficient (γ2) | 0.99878 |
| Precision (RSD, n = 11) (%) | 2.1 (2 μM) |
| Sample | Added Amount (μM) | Concentration (Mean ± s, n = 3/μM) | Recovery (Mean ± s, n = 3) (%) |
|---|---|---|---|
| Tap water | 0 | Not detectable | / |
| 3 | 2.86 ± 0.12 | 95.2 ± 4.0 | |
| 5 | 4.95 ± 0.17 | 99.0 ± 3.4 | |
| Lake water | 0 | Not detectable | / |
| 3 | 2.95 ± 0.04 | 98.4 ± 1.4 | |
| 5 | 5.02 ± 0.10 | 100.4 ± 1.9 | |
| River water | 0 | Not detectable | / |
| 3 | 2.93 ± 0.13 | 95.4 ± 4.3 | |
| 5 | 4.88 ± 0.19 | 97.7 ± 3.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, K.; Liu, J.; Fang, G.; Wang, P.; Zhang, D.; Wang, S. A Colorimetric Probe Based on Functionalized Gold Nanorods for Sensitive and Selective Detection of As(III) Ions. Sensors 2018, 18, 2372. https://doi.org/10.3390/s18072372
Ge K, Liu J, Fang G, Wang P, Zhang D, Wang S. A Colorimetric Probe Based on Functionalized Gold Nanorods for Sensitive and Selective Detection of As(III) Ions. Sensors. 2018; 18(7):2372. https://doi.org/10.3390/s18072372
Chicago/Turabian StyleGe, Kun, Jingmin Liu, Guozhen Fang, Peihua Wang, Dongdong Zhang, and Shuo Wang. 2018. "A Colorimetric Probe Based on Functionalized Gold Nanorods for Sensitive and Selective Detection of As(III) Ions" Sensors 18, no. 7: 2372. https://doi.org/10.3390/s18072372




