Characteristics of Surface Acoustic Wave Sensors with Nanoparticles Embedded in Polymer Sensitive Layers for VOC Detection
Abstract
:1. Introduction
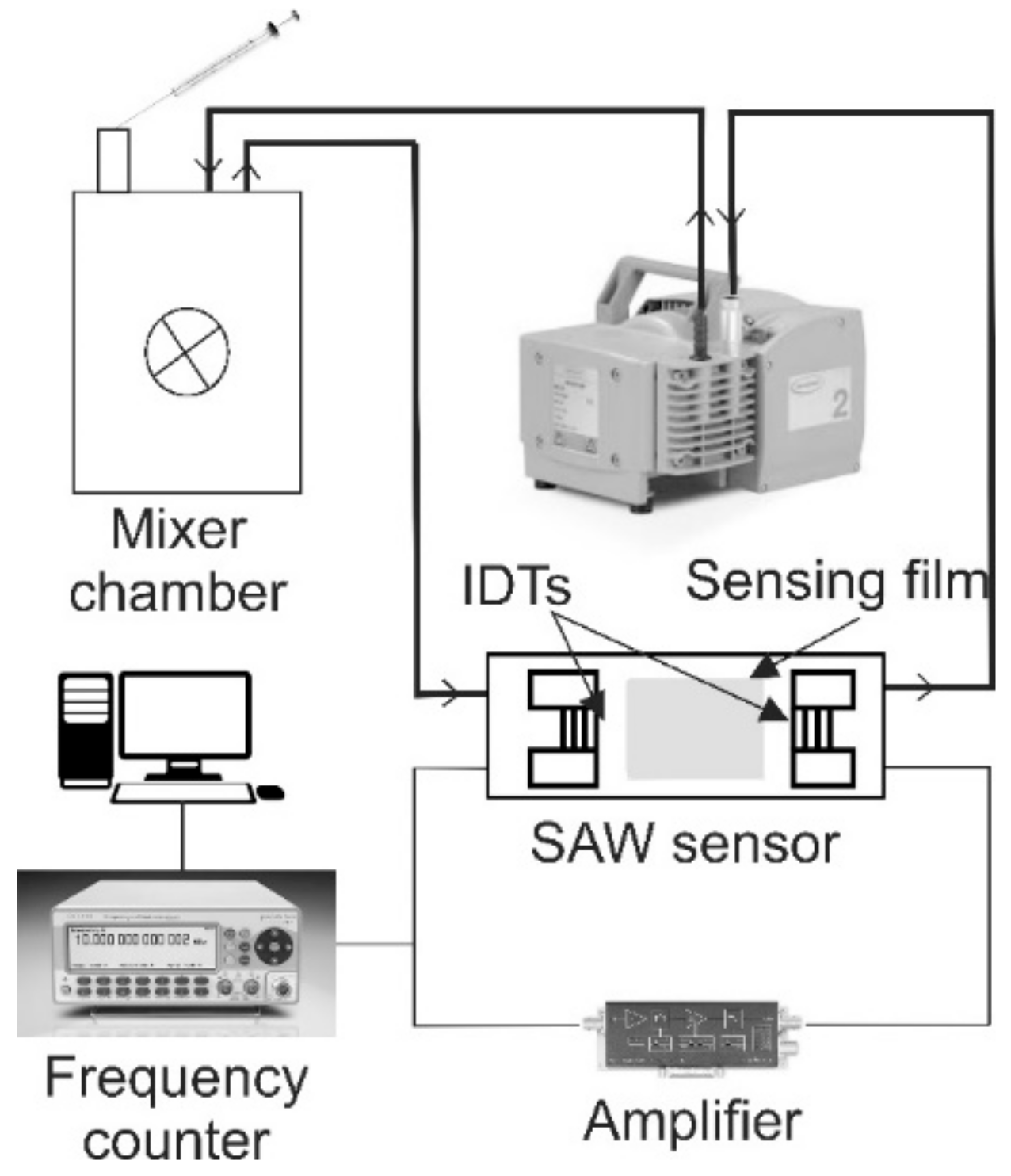
2. Materials and Methods
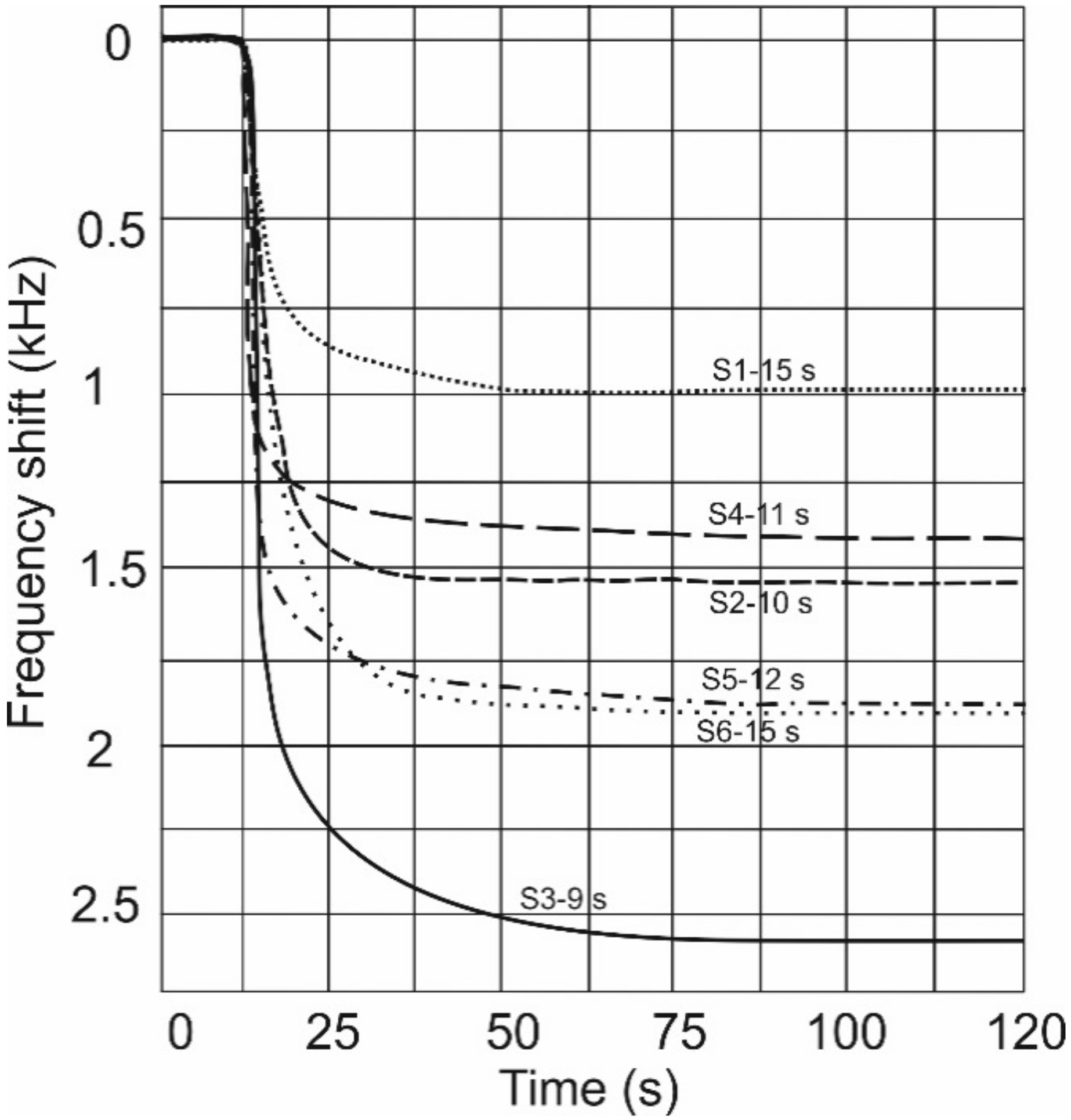
3. Results
4. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Devkota, J.; Ohodnicki, P.R.; Greve, D.W. SAW Sensors for Chemical Vapors and Gases. Sensors 2017, 17, 801. [Google Scholar] [CrossRef] [PubMed]
- Hubert, T.; Boon-Brett, L.; Black, G.; Banach, U. Hydrogen sensors—A review. Sens. Actuators B Chem. 2011, 157, 392–352. [Google Scholar] [CrossRef]
- Buryakov, I.A.; Buryakov, T.I.; Matsaev, V.T. Mass-Sensitive Micro- and Nanosensors for Detecting the Vapors of Explosives and Associated Substances. J. Anal. Chem. 2014, 69, 299–310. [Google Scholar] [CrossRef]
- Viespe, C. Surface Acoustic Wave Sensors based on Nanoporous Films for Hydrogen Detection. Materials and applications for sensors and transducers III. Key Eng. Mater. 2014, 605, 331–334. [Google Scholar] [CrossRef]
- Yang, L.; Yin, C.; Zhang, Z.; Zhou, J.; Xu, H. The investigation of hydrogen gas sensing properties of SAW gas sensor based on Pd surface modified SnO2 thin film. Mater. Sci. Semicond. Process. 2017, 60, 16–28. [Google Scholar] [CrossRef]
- Ippolito, S.J.; Ponzoni, A.; Kalantar-Zadeh, K.; Wlodarski, W.; Comini, E.; Faglia, G. Layered WO3/ZnO/36° LiTaO3 SAW gas sensor sensitive towards ethanol vapour and humidity. Sens. Actuators B Chem. 2006, 117, 442–450. [Google Scholar] [CrossRef]
- Jakubik, W.P. Surface acoustic wave-based gas sensors. Thin Solid Films 2011, 520, 986–993. [Google Scholar] [CrossRef]
- Viespe, C.; Miu, D. Surface Acoustic Wave Sensor with Pd/ZnO bilayer structure for room temperature hydrogen detection. Sensors 2017, 17, 1529. [Google Scholar] [CrossRef] [PubMed]
- Marcu, A.; Nicolae, I.; Viespe, C. Active surface geometrical control of noise in nanowire-SAW sensors. Sens. Actuators B Chem. 2016, 231, 469–473. [Google Scholar] [CrossRef]
- Penza, M.; Tagliente, M.A.; Aversa, P.; Cassano, G. Organic-vapor detection using carbon-nanotubes nanocomposite microacoustic sensors. Chem. Phys. Lett. 2005, 409, 349–354. [Google Scholar] [CrossRef]
- Al-Mashat, L.; Tran, H.D.; Wlodarski, W.; Kaner, R.B.; Kalanter-zadeh, K. Polypyrrole nanofiber surface acoustic wave gas sensors. Sens. Actuators B Chem. 2008, 134, 826–831. [Google Scholar] [CrossRef]
- Joo, B.-S.; Huh, J.-S.; Lee, D.-D. Fabrication of polymer SAW sensor array to classify chemical warfare agents. Sens. Actuators B Chem. 2007, 121, 47–53. [Google Scholar] [CrossRef]
- Horrillo, M.C.; Fernandez, M.J.; Fontecha, J.L.; Sagayo, I.; Garcia, M.; Aleixandre, M.; Santos, J.P.; Ares, L.; Gutierrez, J.; Garcia, I.; et al. Detection of volatile organic compounds using surface acoustic wave sensors with different polymer coatings. Thin Solid Films 2004, 467, 234–238. [Google Scholar] [CrossRef]
- Matatagui, D.; Marti, J.; Fernandez, M.J.; Fontecha, J.L.; Gutierrez, J.; Gracia, I.; Cane, C.; Horillo, M.C. Optimized design of a SAW sensor array for chemical warfare agents simulants detection. Procedia Chem. 2009, 1, 232–235. [Google Scholar] [CrossRef]
- Ballantine, D.S.; White, R.M.; Martin, S.I.; Ricco, A.J.; Zellers, E.T.; Frye, G.C.; Wohltjen, H. Acoustic Wave Sensors, Theory, Design and Physico-Chemical Applications; Academic Press: San Diego, CA, USA, 1997; pp. 300–306. [Google Scholar]
- Dinca, V.; Fardel, R.; Shaw-Stewart, F.; Di Pietrantonio, D.; Cannata, M.; Benetti, E.; Verona, A.; Palla-Papavlu, A.; Dinescu, M.; Lippert, T. Laser-induced forward transfer: An approach to single-step polymer microsensor fabrication. Sens. Lett. 2010, 8, 436–440. [Google Scholar] [CrossRef]
- Dinca, V.; Palla-Papavlu, A.; Dinescu, M.; Shaw-Stewart, F.; Lippert, T.; Di Pietrantonio, D.; Cannata, M.; Benetti, E.; Verona, A. Polymer pixel enhancement by laser-induced forward transfer for sensor applications. Appl. Phys. A 2010, 101, 559–565. [Google Scholar] [CrossRef]
- Wei, D.W.; Wang, L.S.; Ma, J.Y.; Jiang, H.M. Synthesis and evaluation of hexafluoroisopropanol-functionalized polysiloxane as a new coating material for sensors. J. Appl. Polym. Sci. 2012, 5, 4136–4140. [Google Scholar] [CrossRef]
- Viespe, C.; Grigoriu, C. Surface acoustic wave sensors with carbon nanotubes and SiO2/Si nanoparticles based nanocomposites for VOC detection. Sens. Actuators B Chem. 2010, 147, 43–47. [Google Scholar] [CrossRef]
- Kim, H.; Choi, Y.-J.; Kang, K.-M.; Park, H.-H. Directly patternable SnO2 thin films incorporating Pt nanoparticles. Mater. Res. Bull. 2014, 52, 6–10. [Google Scholar] [CrossRef]
- Kaushik, A.; Kumar, R.; Arya, S.K.; Nair, M.; Malhotra, B.D.; Bhansali, S. Organic-Inorganic Hybrid Nanocomposite-Based Gas Sensors for Environmental Monitoring. Chem. Rev. 2015, 115, 4571–4606. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Ahmad, S. Recent developments in hybrid conducting polymers: Synthesis, applications and future prospects. J. Ind. Eng. Chem. 2018, 60, 53–84. [Google Scholar] [CrossRef]
- Su, P.G.; Peng, Y.-T. Fabrication of a room-temperature H2S gas sensor based on PPy/WO3 nanocomposite films by in-situ photopolimerization. Sens. Actuators B Chem. 2014, 193, 637–643. [Google Scholar] [CrossRef]
- Fu, C.; Lee, K.J.; Yang, S.S. Low intensity ultraviolet detection using a surface-acoustic-wave sensor with a Ag-doped ZnO nanoparticle films. Smart Mater. Struct. 2014, 24, 015010. [Google Scholar] [CrossRef]
- Nicolae, I.; Viespe, C.; Grigoriu, C. Nanocomposite sensitive polymeric films for SAW sensors deposited by the MAPLE direct write technique. Sens. Actuators B Chem. 2011, 158, 418–422. [Google Scholar] [CrossRef]
- Holmes, M.A.; Mackay, M.E.; Giunta, R.K. Nanoparticles for dewetting suppression of thin polymer films used in chemical sensors. J. Nanopart. Res. 2007, 9, 753–763. [Google Scholar] [CrossRef]
- Viespe, C.; Grigoriu, C. SAW sensor based on highly sensitive nanoporous palladium thin film for hydrogen detection. Microelectron. Eng. 2013, 108, 218–222. [Google Scholar] [CrossRef]
- Huotari, J.; Kekkonen, V.; Haapalainen, T.; Leidinger, M.; Sauerwald, T.; Puustinen, J.; Liimatainen, J.; Lappalainen, J. Pulsed laser deposition of metal nanostructures for highly sensitive gas sensor applications. Sens. Actuators B Chem. 2016, 236, 978–987. [Google Scholar] [CrossRef]
- Makarov, G.N. Laser Applications in nanotechnology: Nanofabrication using laser ablation and laser nanolithography. Physics-Uspekhi 2013, 56, 643–682. [Google Scholar] [CrossRef]
- Radu, M.; Dinu, D.; Sima, C.; Burlacu, R.; Hermenean, A.; Ardelean, A.; Dinischiotu, A. Magnetite nanoparticles induced adaptive mechanisms countered cell death in human pulmonary fibroblasts. Toxicol. In Vitro 2015, 29, 1492–1502. [Google Scholar] [CrossRef] [PubMed]
- Nicolae, I.; Viespe, C.; Serban, N.; Negrila, C.C.; Teodorescu, V.S.; Trupina, L. Increased Diffusion Coefficient of Polymeric Nanocomposite Layer for Gas Sensing Applications. Sens. Lett. 2013, 11, 2327–2332. [Google Scholar] [CrossRef]
- Dharmelingam, G.; Joy, N.A.; Grisafe, B.; Carpenter, M.A. Plasmonics-based detection of H2 and CO: Discrimination between reducing gases facilitated by material control. Beilstein J. Nanotechnol. 2012, 3, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Van, S.N.; Hadji, R.; Vincent, B.; Rouxel, D.; Sarry, F.; Bauer, F. P(VDF-TrFE)/Al2O3 piezoelectric thin films. In Proceedings of the 2010 IEEE International Symposium on the Applications of Ferroelectrics (ISAF), Edinburgh, UK, 9–12 August 2010; pp. 1–4. [Google Scholar]
- Essabir, H.; Raji, M.; Essassi, E.M.; Rodrigue, D.; Bouhfid, R.; Qaiss, A. Morphological, thermal, mechanical, electrical and magnetic properties of ABS/PA6/SBR blends with Fe3O4 nano-particles. J. Mater. Sci. Mater. Electron. 2017, 28, 17120–17130. [Google Scholar] [CrossRef]
- Weidenfelder, B.; Hoefer, M.; Schilling, F. Thermal and electrical properties of magnetite filled polymers. Compos. Part A Appl. Sci. Manuf. 2002, 33, 1041–1053. [Google Scholar] [CrossRef]





| Sensor | Mean NP Diameter (nm) | NP Concentration (mg/mL) |
|---|---|---|
| S1 | Polymer (PEI) only | 0 |
| S2 | 13 | 0.4 |
| S3 | 7 | 0.4 |
| S4 | 50 | 0.2 |
| S5 | 50 | 0.4 |
| S6 | 50 | 0.8 |
| Sensor | Sensitivity (Hz/ppm) | LOD (ppm) | ||||
|---|---|---|---|---|---|---|
| Ethanol | Methanol | Toluene | Ethanol | Methanol | Toluene | |
| S1 | 0.56 | 0.50 | 0.69 | 320 | 360 | 262 |
| S2 | 0.97 | 0.81 | 1.43 | 139 | 166 | 95 |
| S3 | 1.63 | 1.00 | 1.94 | 65 | 105 | 54 |
| S4 | 0.81 | 0.53 | 1.00 | 203 | 311 | 165 |
| S5 | 1.06 | 0.66 | 1.13 | 212 | 343 | 200 |
| S6 | 1.13 | 0.88 | 1.31 | 240 | 309 | 206 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viespe, C.; Miu, D. Characteristics of Surface Acoustic Wave Sensors with Nanoparticles Embedded in Polymer Sensitive Layers for VOC Detection. Sensors 2018, 18, 2401. https://doi.org/10.3390/s18072401
Viespe C, Miu D. Characteristics of Surface Acoustic Wave Sensors with Nanoparticles Embedded in Polymer Sensitive Layers for VOC Detection. Sensors. 2018; 18(7):2401. https://doi.org/10.3390/s18072401
Chicago/Turabian StyleViespe, Cristian, and Dana Miu. 2018. "Characteristics of Surface Acoustic Wave Sensors with Nanoparticles Embedded in Polymer Sensitive Layers for VOC Detection" Sensors 18, no. 7: 2401. https://doi.org/10.3390/s18072401





