Bimetallic Layers Increase Sensitivity of Affinity Sensors Based on Surface Plasmon Resonance
Abstract
:Introduction
Experimental
Computer simulation
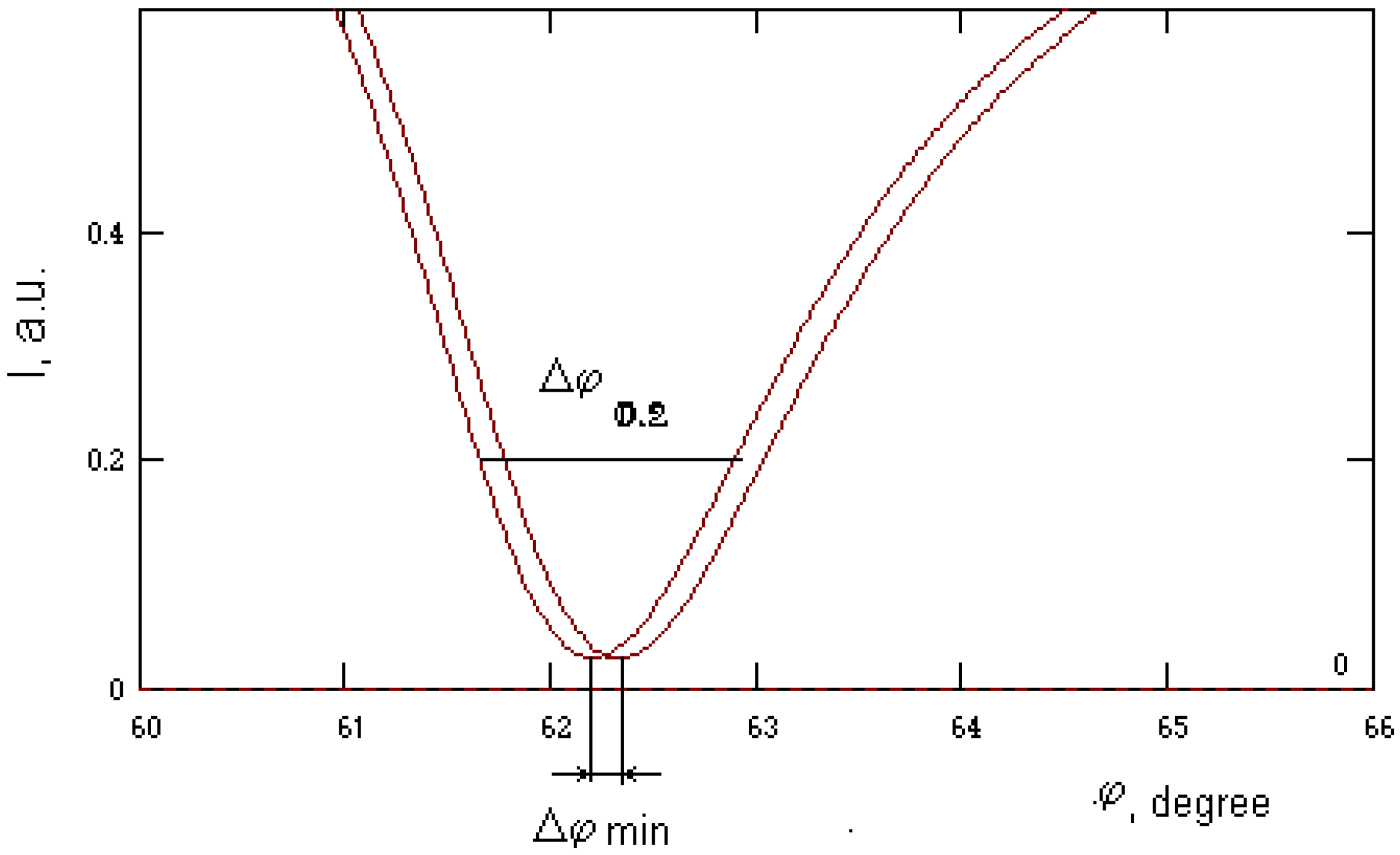
| Nglass / dAg (nm) / dAu (nm) | Δϕmin, degree | Δϕ 0.2, degree | Δϕmin /Δϕ 0.2 | |
|---|---|---|---|---|
| 1 | 1.61 / 0 / 50 | 0.14 | 1.91 | 0.073 |
| 2 | 1.61 / 25 / 25 | 0.12 | 1.43 | 0.083 |
| 3 | 1.61 / 32 / 18 | 0.12 | 1.22 | 0.097 |
| 4 | 1.61 / 40 / 10 | 0.12 | 1.06 | 0.113 |
| 5 | 1.61 / 50 / 0 | 0.12 | 0.75 | 0.159 |
| 6 | 1.51 / 0 / 50 | 0.2 | 2.55 | 0.078 |
| 7 | 1.51 / 40 / 10 | 0.18 | 1.75 | 0.102 |
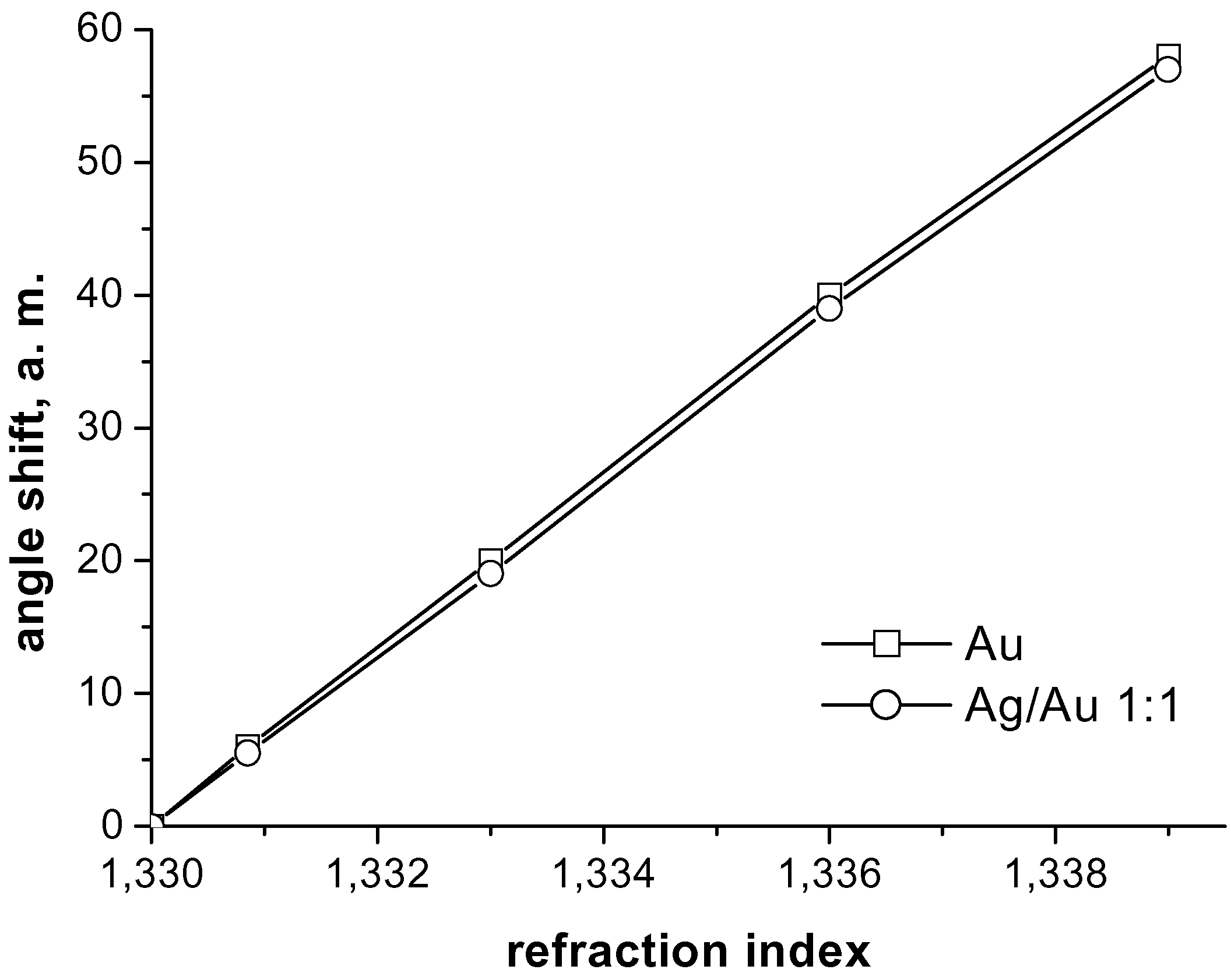
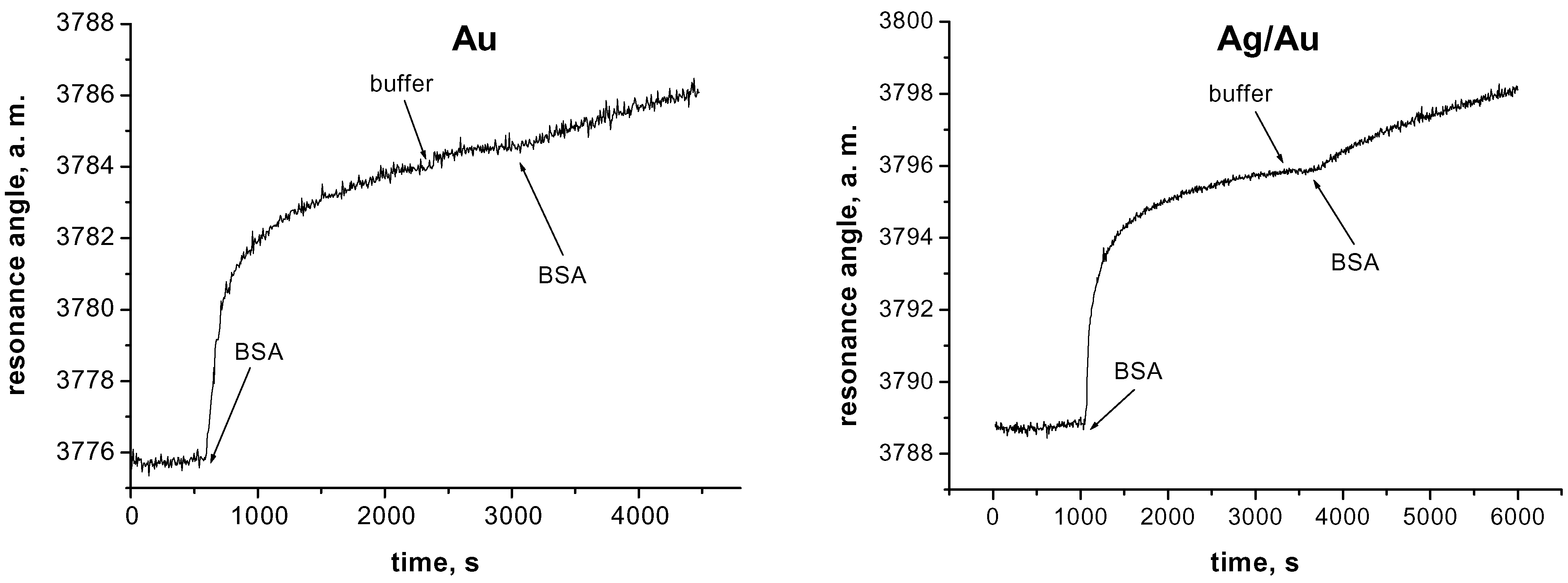
Experimental Results

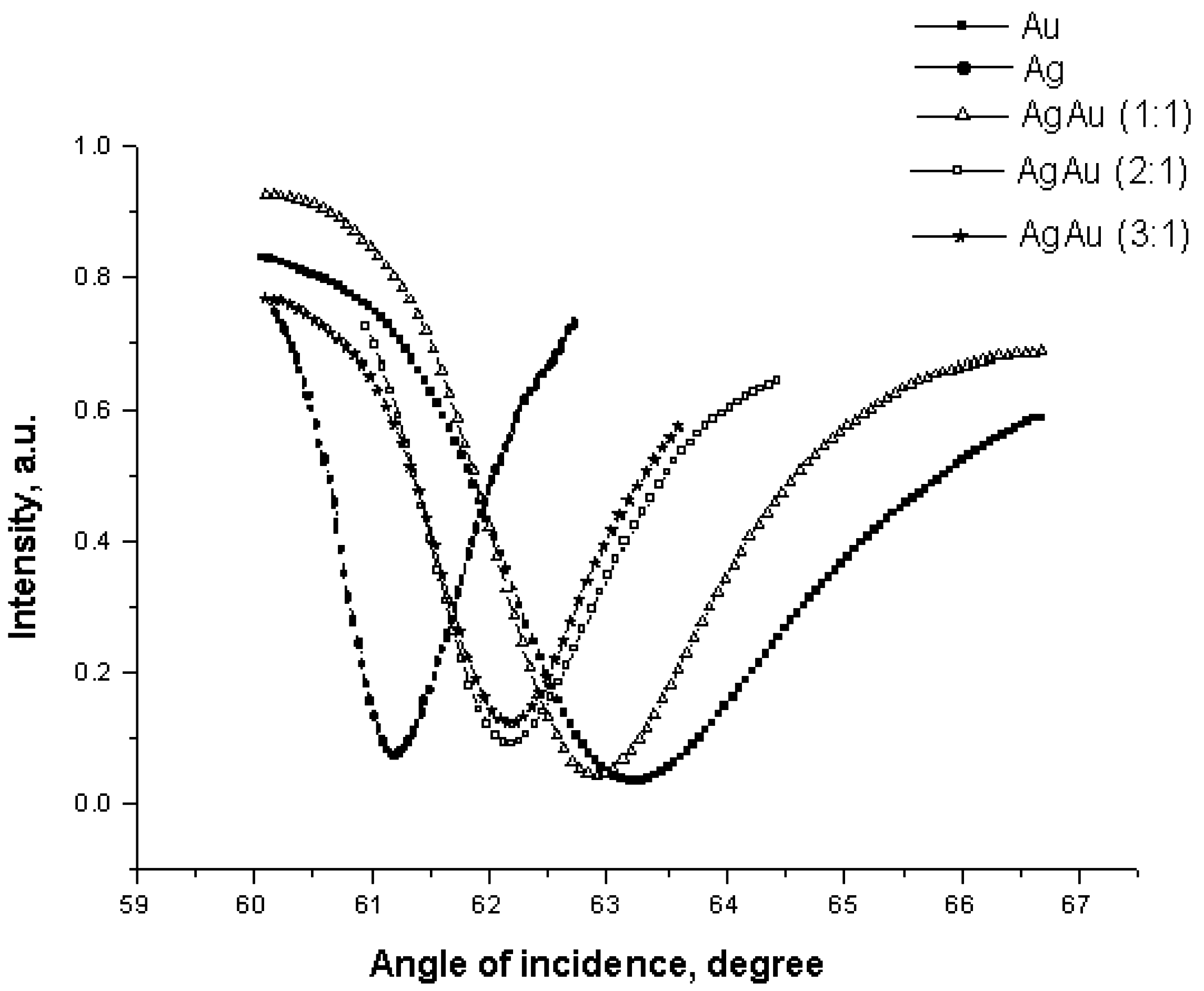
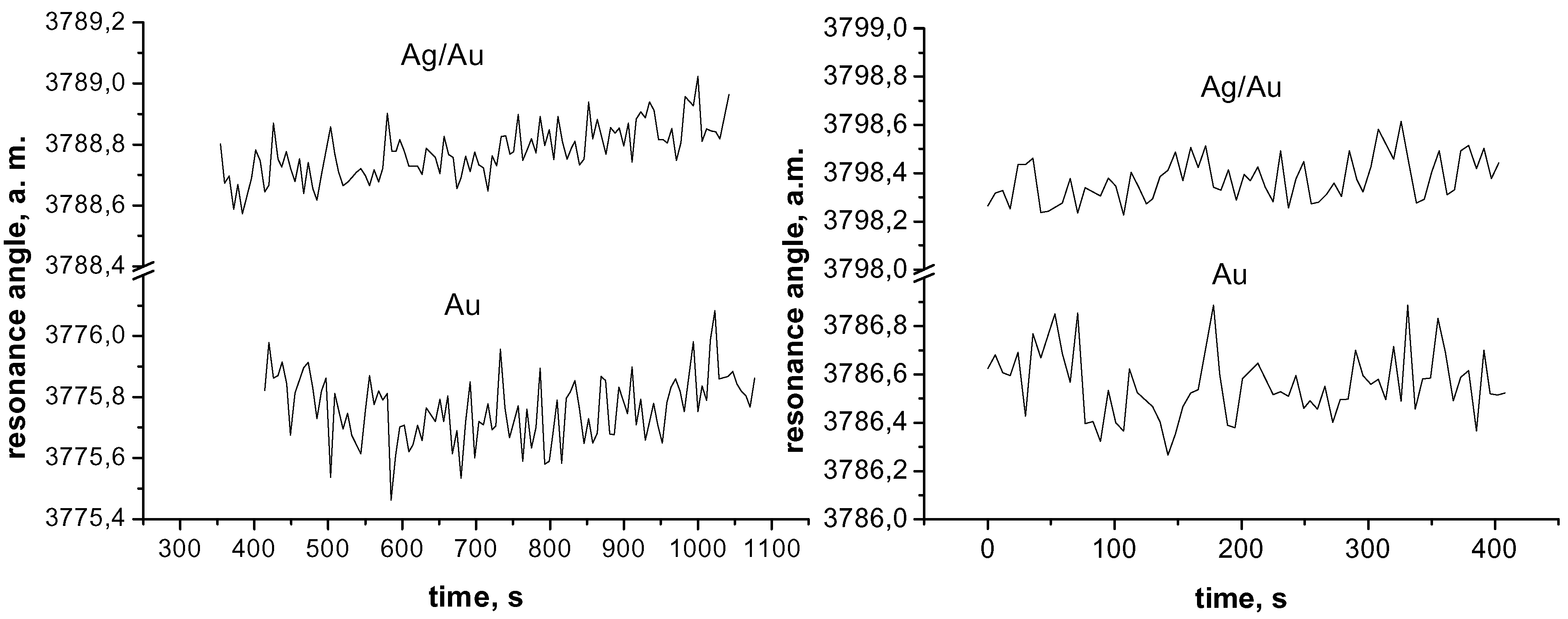
| Active layer | Stand. deviation, a. m. H2O/NaCl | Stand. deviation, a. m. H2O/ethanol |
|---|---|---|
| Au | 0,124457 | 0,104036 |
| Ag/Au 1:1 | 0,114879 | 0,099494 |
| Ag/Au 2:1 | 0,073425 | 0,063679 |
| Ag | 0,054529 | 0,052653 |
| Active layer | Stand. deviation, a. m. 150 mM NaCl, pH 7.0 | Stand. deviation, a. m. 150 mM NaCl, pH 7.0 + BSA, 20 µg/ml |
|---|---|---|
| Au | 0,11 | 0,10 - 0,14 |
| Ag/Au 1:1 | 0,09 | 0,06 - 0,09 |
References
- Jonsson, U.; Fagerstam, L.; Ivarsson, B. Real-time biospecific interaction analysis using surface plasmon resonance and a sensor chip technology. BioTechniques 1991, 11, 620–627. [Google Scholar] [PubMed]
- Woodbury, R.G.; Wendin, C.; Clendenning, J.; Mendelez, J.; Elkind, J.; Bartholomew, D.; Brown, S.; Furlong, C. Construction of biosensors using a gold-binding polypeptide and a miniature integrated surface plasmon resonance sensor. Biosens. Bioelectron. 1998, 13, 1117–1126. [Google Scholar] [CrossRef]
- www.micro-systems.de; www.biocore.com.
- Homola, J. On the sensitivity of surface plasmon resonance sensors with spectral interrogation. Sens. Actuators 1997, B 41, 207–211. [Google Scholar] [CrossRef]
- Ho, H.P.; Wu, S.Y.; Yang, M.; Cheung, A.C. Application of white light-emitting diode to surface plasmon resonance sensors. Sensors Actuators 2001, B 80, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Nikitin, P.I.; Beloglazov, A.A.; Valeiko, M.V.; Creighton, J.A.; Wright, J.D. Silicon-based surface plasmon resonance combined with surface-enhancement Raman scattering for chemical sensing. Rev. Sci. Instrum. 1997, 68, 2254–2257. [Google Scholar] [CrossRef]
- Nikitin, P.I.; Grigorenko, A.N.; Beloglazov, A.A.; Valeiko, M.V.; Savchuk, A.I.; Savchuk, O.A. Surface plasmon resonance interferometry for micro-array biosensing. Proc. EUROSENSORS XIII 1999, 235–238. [Google Scholar] [CrossRef]
- Slavik, R.; Homola, J.; Ctyroky, J.; Brynda, E. Novel spectral fiber optic sensor based on surface plasmon resonance. Sens. Actuators 2001, B 74, 106–111. [Google Scholar] [CrossRef]
- Stamm, Ch.; Dangel, R.; Lukosz, W. Biosensing with integrated-optical difference interferometer: dual-wavelenth operation. Opt. Comm. 1988, 153, 347–359. [Google Scholar] [CrossRef]
- Janunts, N.A.; Markarian, Sh.A.; Nekarayan, Kh.V. A chemo-optical sensor based on coupling of surface plasmon modes. Sensors Actuators 2001, A 89, 205–209. [Google Scholar] [CrossRef]
- Johnston, K.S.; Jorgenson, R.C.; Yee, S.S.; Russel, A. characterization of porous sol-gel films on fiber optic surface plasmon resonance sensors. Proc.SPIE-Int.Soc.Opt.Eng. 1994, 2068, 87–93. [Google Scholar]
- Cai, W.; Hofmeister, H.; Rainer, T. Surface effect on the size evolution of surface plasmon resonances of Ag and Au nanoparticles dispersed within mesoporous silica. Physica E: Low-Dimensional Systems & Nanostructures 2001, 11, 339–344. [Google Scholar]
- Velge-Roussel, F.; Breton, P.; Guillon, X.; Lescure, F.; Bru, N.; Bout, D.; Hoebeke, J. Immunochemical characterization of antibody-coated nanoparticles. Experientia 1996, 52, 803–806. [Google Scholar] [CrossRef] [PubMed]
- Hutter, E.; Fendler, J.H.; Roy, D. Surface plasmon resonance studies of gold and silver nanoparticles linked to gold and silver substrates by 2-aminoethanethiol and 1,6-hexanedithiol. J. Phys. Chem. B 2001, 105, 11159–11168. [Google Scholar] [CrossRef]
- Astilean, S.; Lalanne, P.; Palamaru, M. Resonance-enhanced light transmission through metal nanochannels. Proc. SPIE-Int. Soc. Opt. Eng. 2000, 4068, 513–519. [Google Scholar]
- Leung, P.T.; Pollard-Knight, D.; Malan, G.P.; Finlan, M.F. Modeling of particle-enhanced sensitivity of the surface-plasmon-resonance biosensor. Sens. Actuators B 1994, 22, 175–180. [Google Scholar] [CrossRef]
- Lyon, L.A.; Musick, M.D.; Natan, M.J. Colloidal Au-Enhanced Surface Plasmon Resonance Immunosensing. Anal. Chem. 1998, 70, 5177–5183. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Musick, M.D.; Nicewarner, S.R.; Salinas, F.G.; Benkovic, S.J.; Natan, M.J.; Keating, C.D. Colloidal Au-enhanced surface plasmon resonance for ultrasensitive detection of DNA hybridization. J. Am. Chem. Soc. 2000, 122, 9071–9077. [Google Scholar] [CrossRef]
- Yeatman, E.M.; Ash, E.A. Surface plasmon scanning microscopy. Proc. SPIE - Int. Soc. Opt. Eng. 1988, 897, 100–107. [Google Scholar]
- De Bruijn, H.E.; Kooyman, R.P.H.; Greve, J. Choice of metal and wavelength for surface-plasmon resonance sensors: some considerations. Appl. Opt. 1992, 31, 440–442. [Google Scholar] [CrossRef] [PubMed]
- Abdelghani, A.; Veillas, C.; Chovelon, J.M.; Jaffrezic-Renault, N.; Gagnaire, H. Stabilization of a surface plasmon resonance (SPR) optical fiber sensor with an ultra-thin organic film: application to the detection of chloro-fluoro-carbon (CFC). Synth.Met. 1997, 90, 193–198. [Google Scholar] [CrossRef]
- Roy, D. Surface plasmon resonance spectroscopy of dielectric coated gold and silver films on supporting metal layers: reflectivity formulas in the Kretschmann formalism. Appl. Spectrosc. 2001, 55, 1046–1052. [Google Scholar] [CrossRef]
- Lin, W.B.; Lacroix, M.; Chovelon, J.M.; Jaffrezic-Renault, N.; Gagnaire, H. Development of a fiber-optic sensor based on surface plasmon resonance on silver film for monitoring aqueous media. Sens. Actuators B 2001, B 75, 203–209. [Google Scholar] [CrossRef]
- Sunderland, R.F. Production of carriers for surface plasmon resonance. US Patent 5955153, 1996. [Google Scholar]
- Rengevich, O.V.; Shirshov, Yu. M.; Ushenin, Yu.V.; Beketov, A.G. Separate determination of thickness and optical parameters by surface plasmon resonance: accuracy consideration. Semicond. Phys. Quant. Electron. Optoelectron 1999, 2, 28–35. [Google Scholar]
- Sample Availability: Available from the author.
© 2002 by MDPI (http://www.mdpi.net). Reproduction is permitted for noncommercial purposes.
Share and Cite
Zynio, S.A.; Samoylov, A.V.; Surovtseva, E.R.; Mirsky, V.M.; Shirshov, Y.M. Bimetallic Layers Increase Sensitivity of Affinity Sensors Based on Surface Plasmon Resonance. Sensors 2002, 2, 62-70. https://doi.org/10.3390/s20200062
Zynio SA, Samoylov AV, Surovtseva ER, Mirsky VM, Shirshov YM. Bimetallic Layers Increase Sensitivity of Affinity Sensors Based on Surface Plasmon Resonance. Sensors. 2002; 2(2):62-70. https://doi.org/10.3390/s20200062
Chicago/Turabian StyleZynio, Stepan A., Anton V. Samoylov, Elena R. Surovtseva, Vladimir M. Mirsky, and Yuri M. Shirshov. 2002. "Bimetallic Layers Increase Sensitivity of Affinity Sensors Based on Surface Plasmon Resonance" Sensors 2, no. 2: 62-70. https://doi.org/10.3390/s20200062



