Planar Amperometric Glucose Sensor Based on Glucose Oxidase Immobilized by Chitosan Film on Prussian Blue Layer
Abstract
:Introduction
Experimental
Chemicals
Microelectrode preparation
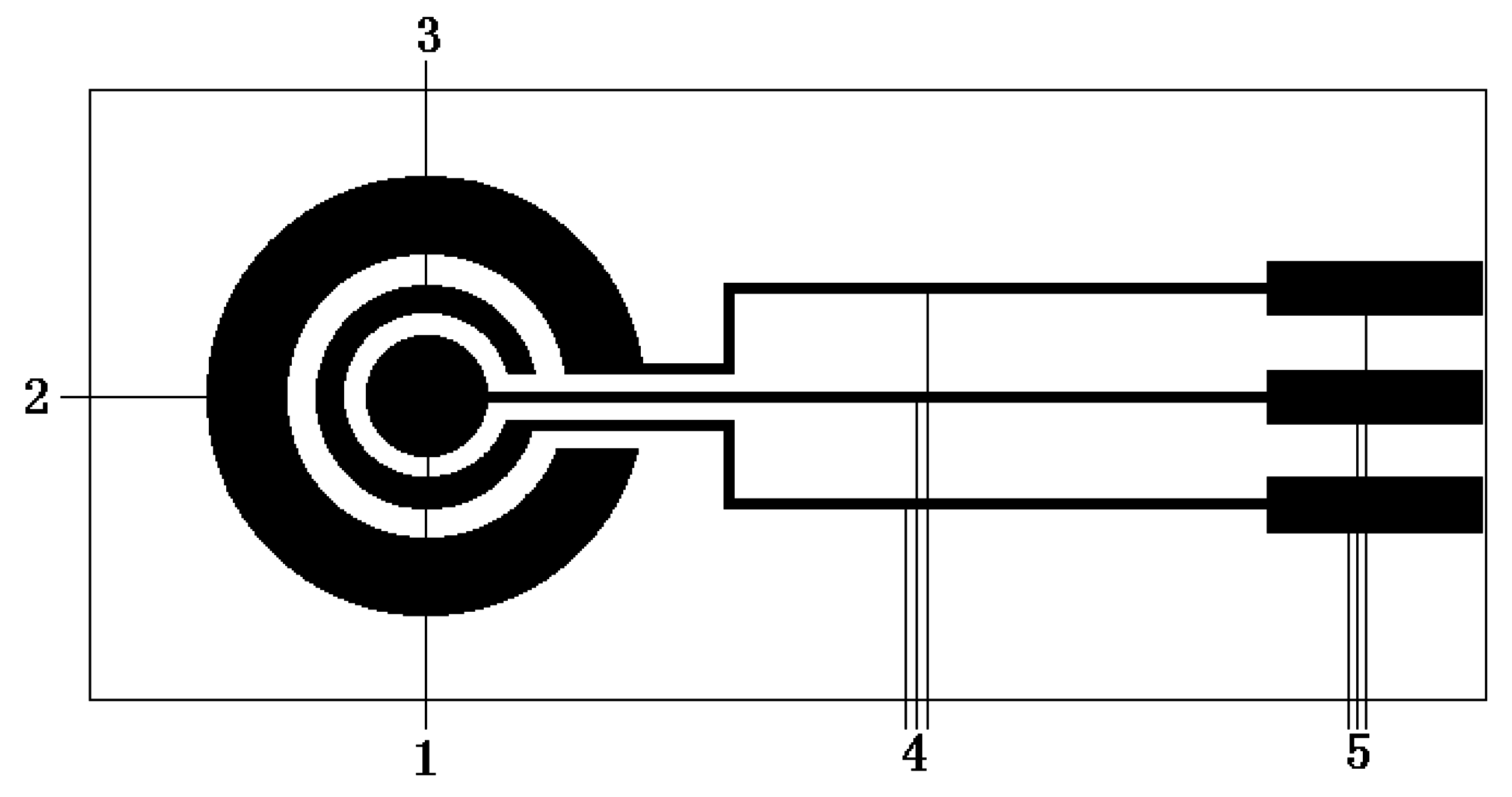
PB layer electropolymerization
GOD immobilization
Measurements
Results and Discussion
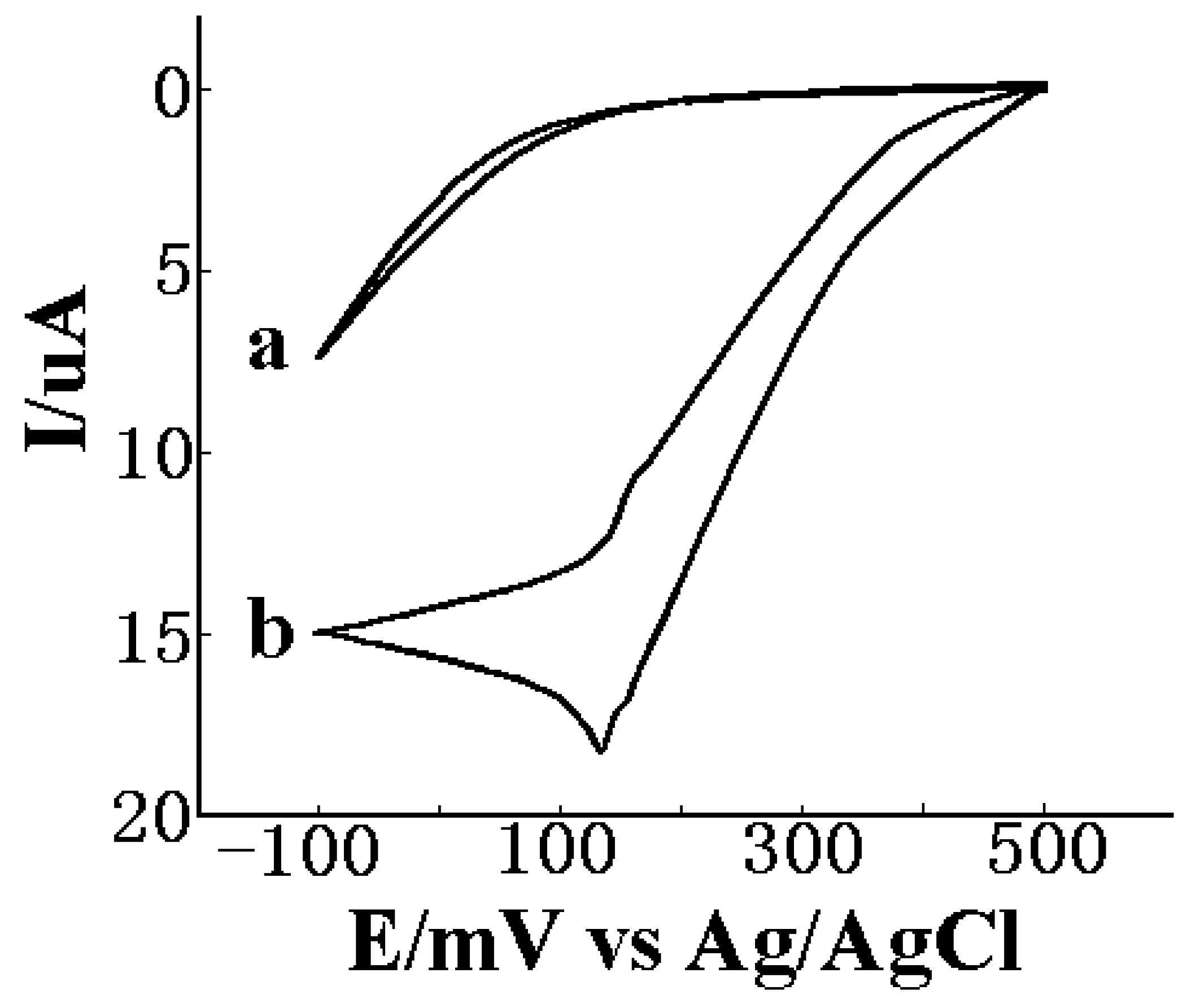
Electrocatalytic properties to H2O2 for PB/Pt electrode
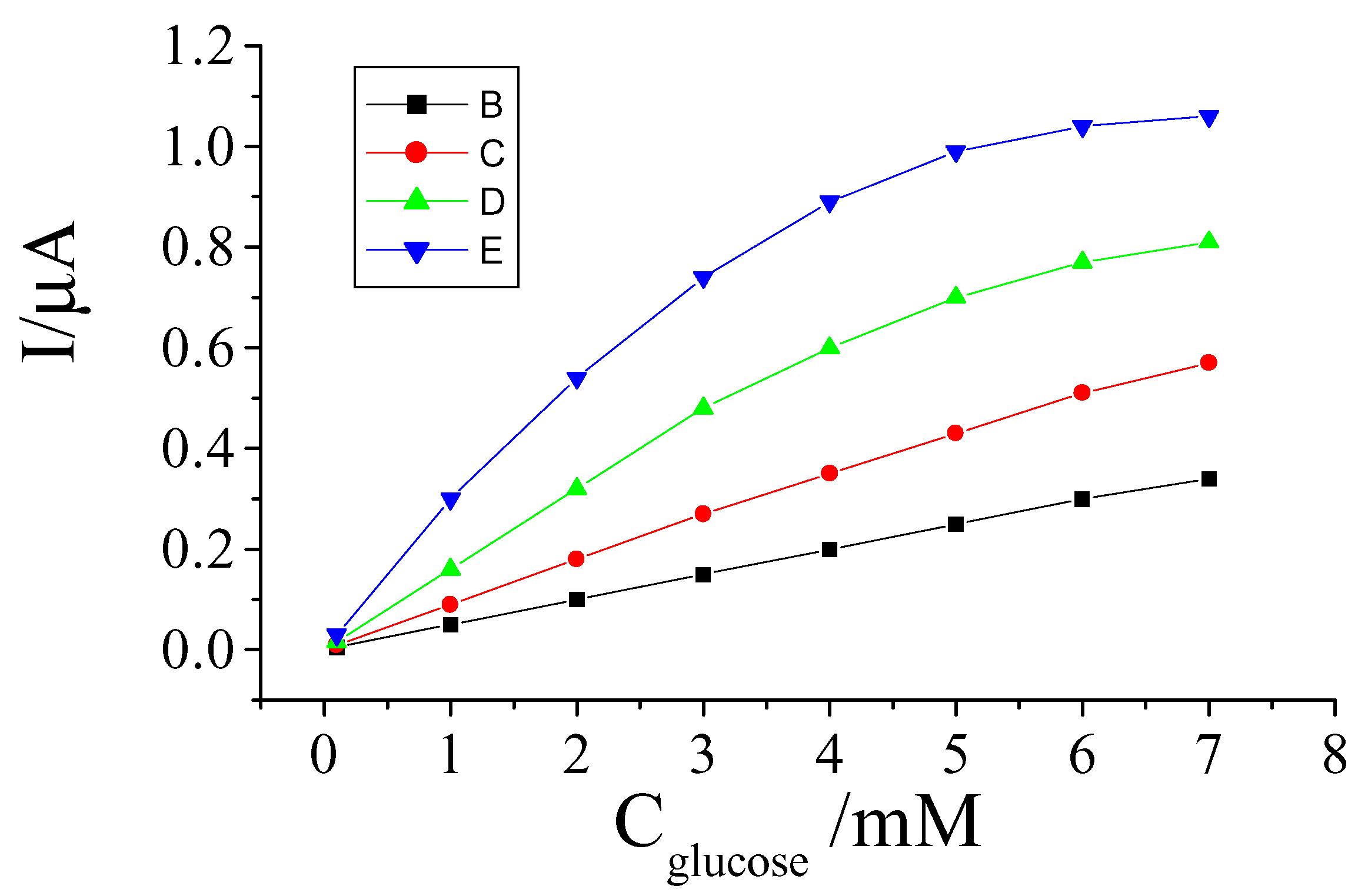
Effects of experimental parameter on performances of the sensor
Effect of PB film thickness
Effect of enzyme quantity
Selection of WE potential
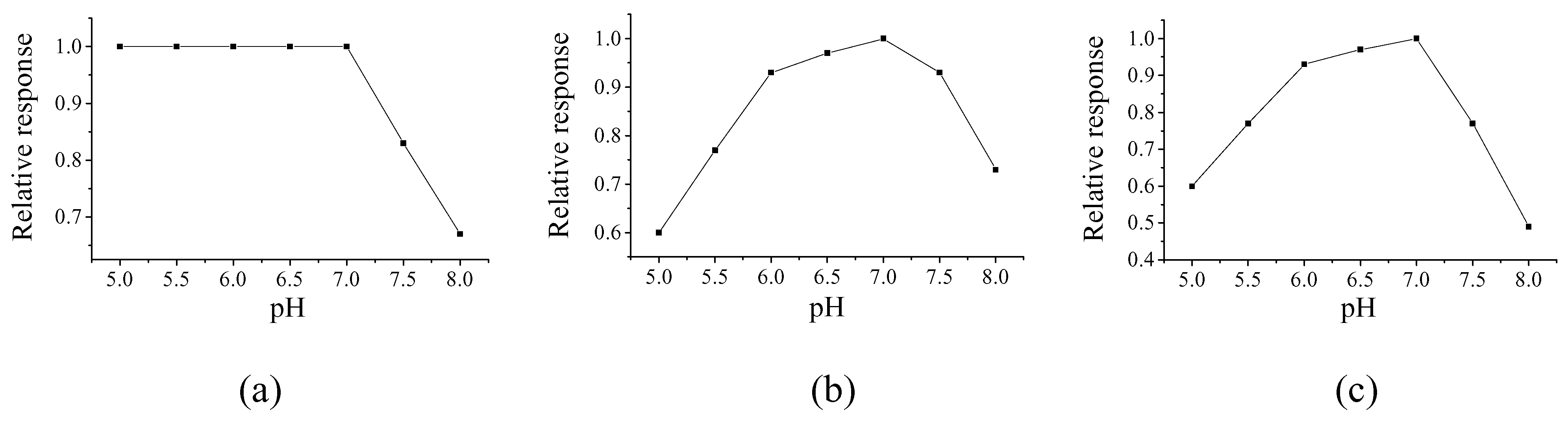
Selection of pH value
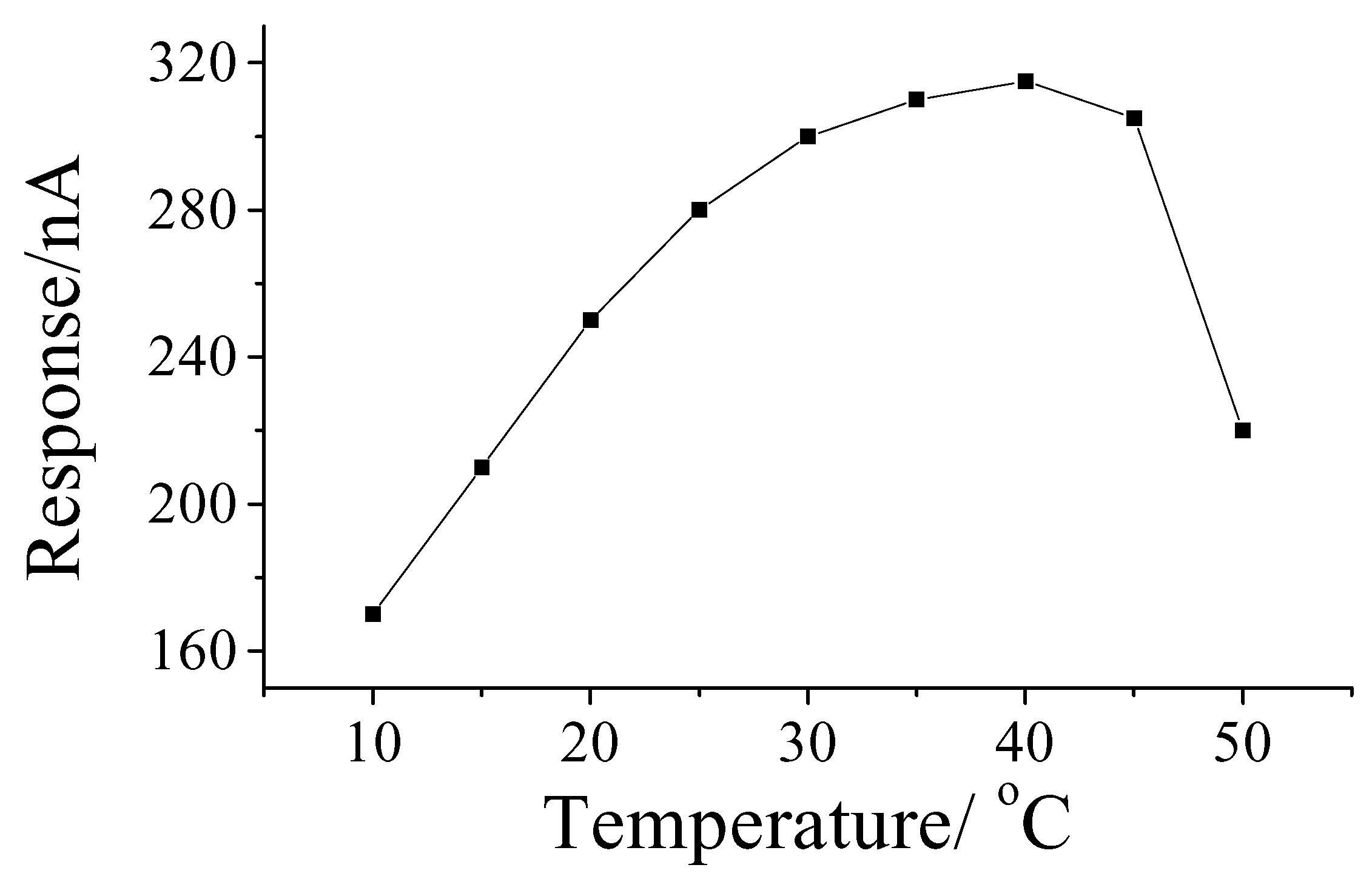
Effect of temperature on response
Performances of the sensor
Response time

Precision
| No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| I/nA | 291 | 294 | 298 | 292 | 297 | 295 | 290 | 289 | 299 | 295 | 294 |
| AV | 294 | ||||||||||
| RSD | 3.0% | ||||||||||
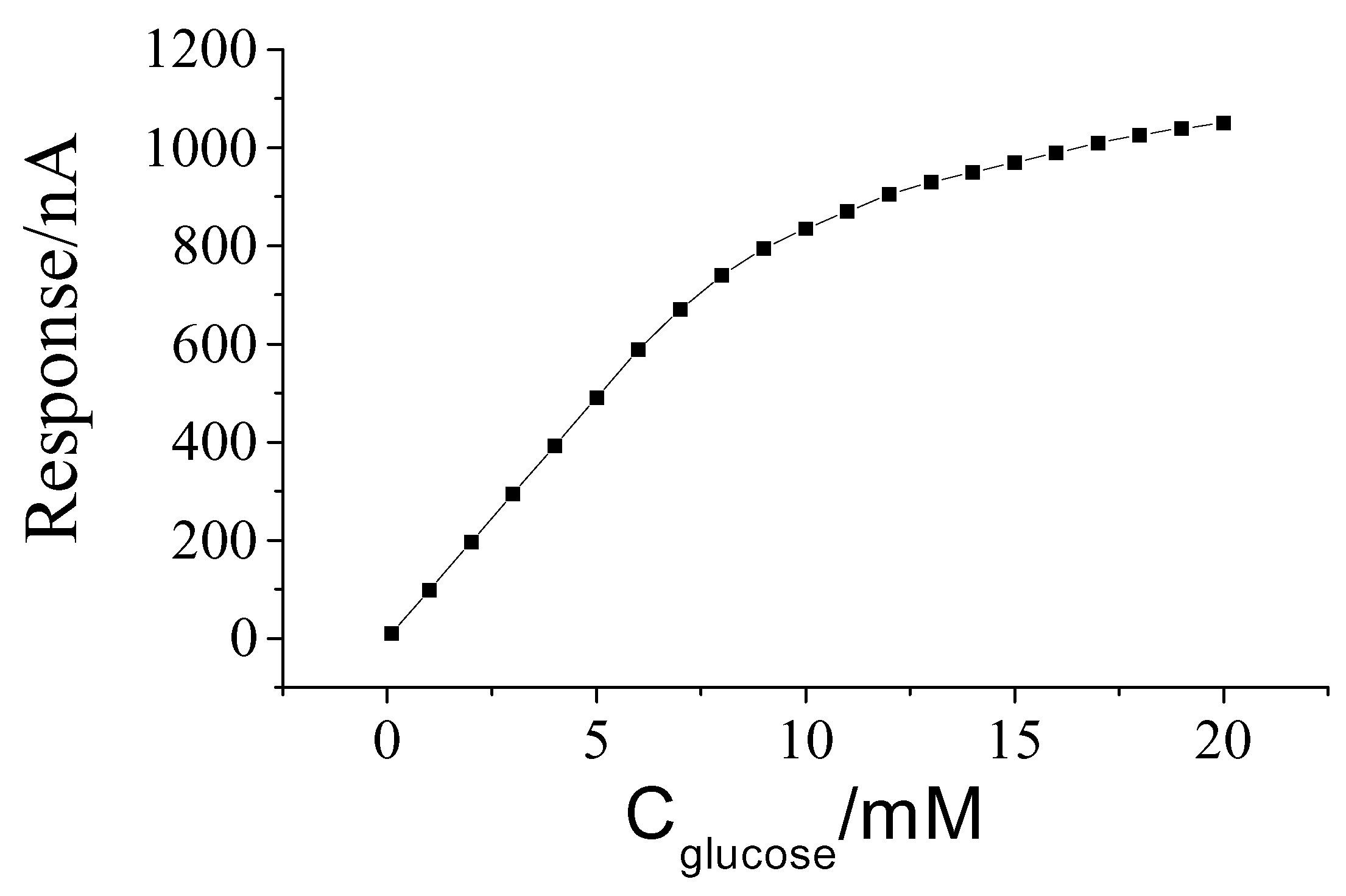
Calibration curve
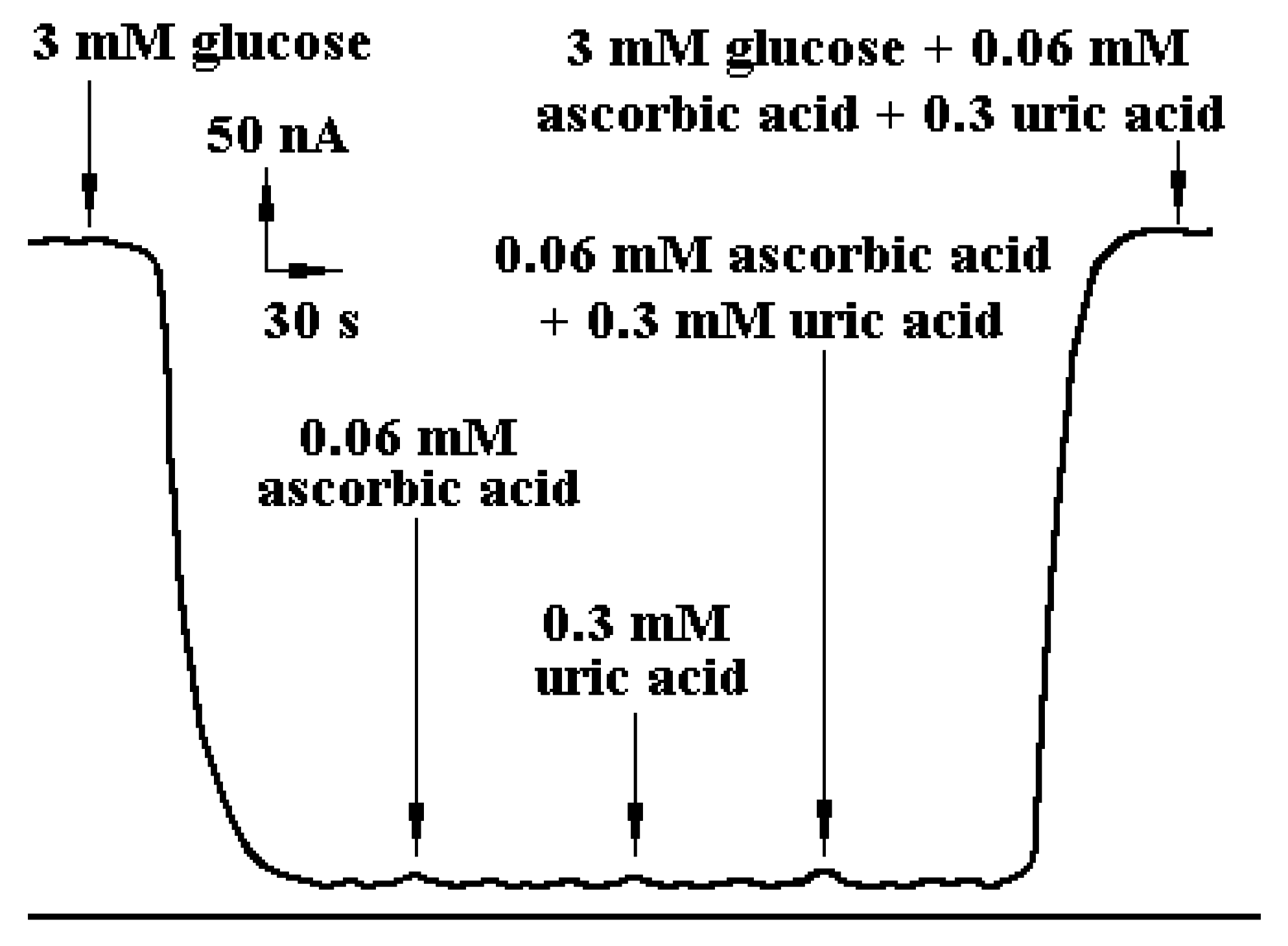
Testing of anti-interference
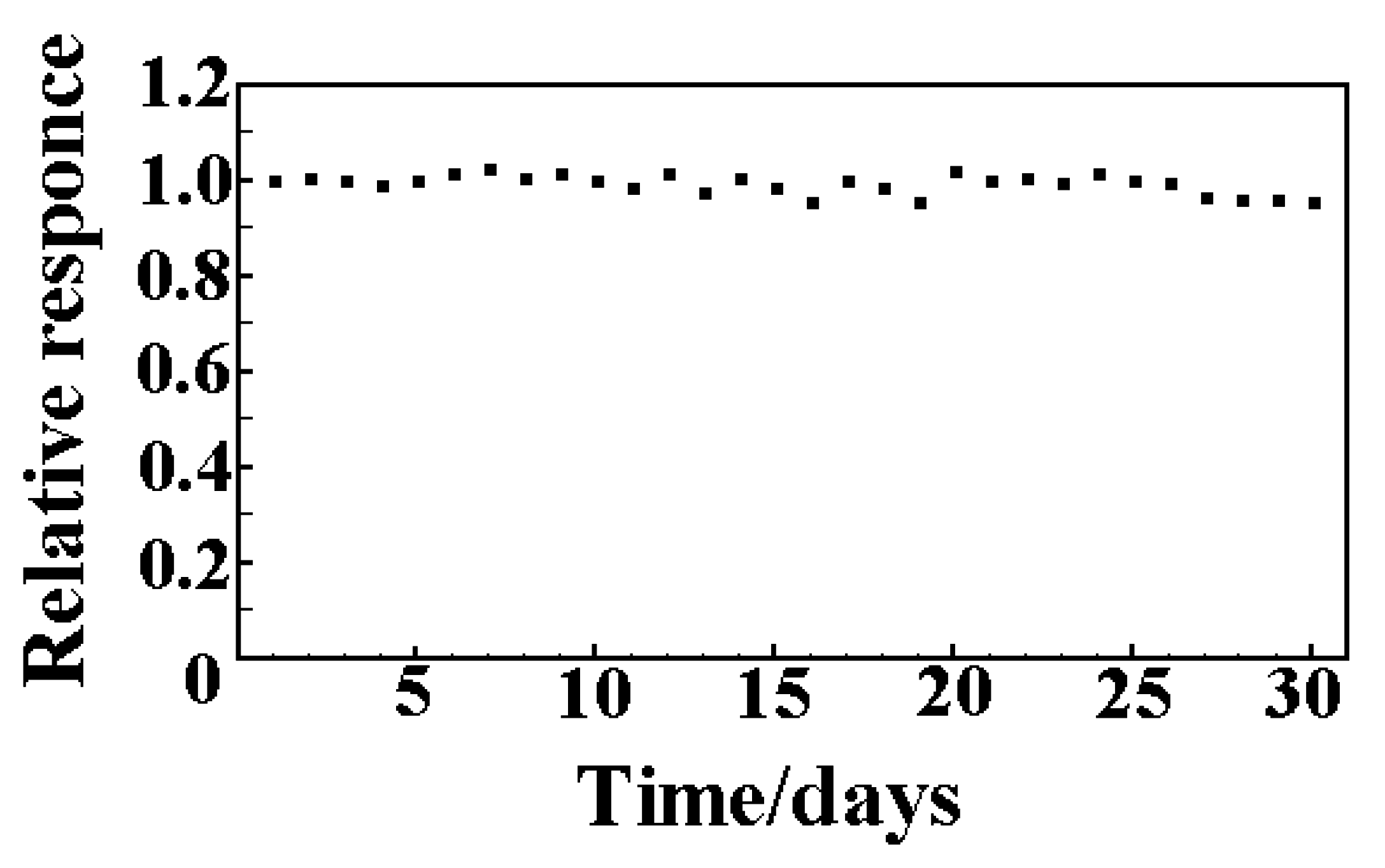
Long-term stability
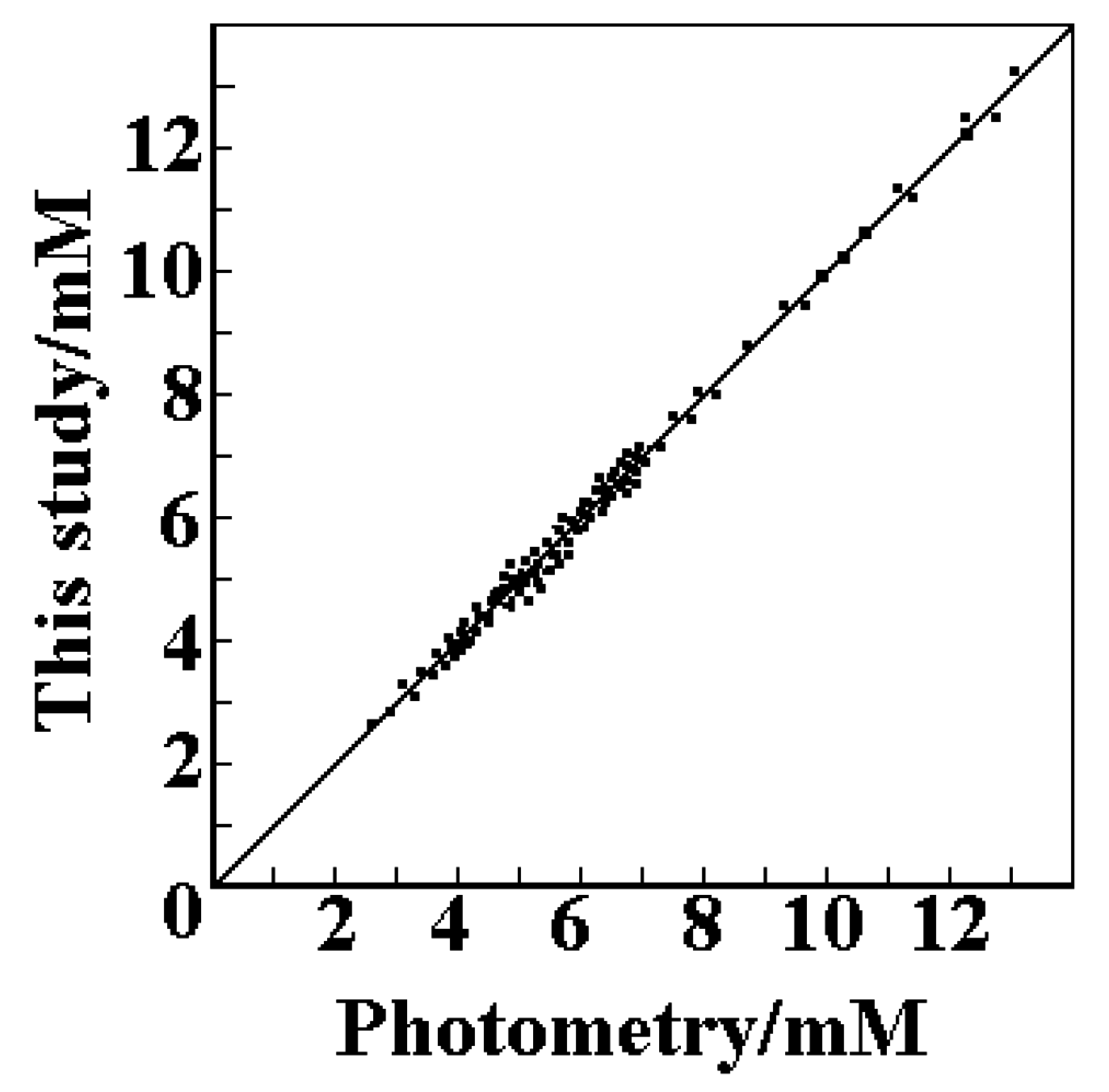
Accuracy
Acknowledgements
References
- Bacon, N. C.; Hall, E. A. H. Electroanalysis 1999, 11, 749.
- Li, Q. S. Anal. Lett. 1998, 31, 937.
- Mizutani, F.; Sato, Y.; Hirata, Y.; Sawaguchi, T.; Yabuki, S. Anal. Chim. Acta 1998, 364, 173. [CrossRef]
- Sasso, S. V.; Pierce, R. J.; Walla, R.; Yacynych, A. M. Anal Chem. 1990, 62, 1111. [CrossRef]
- Vaidya, R.; Wilkins, E. Electroanalysis 1994, 6, 677. [CrossRef]
- Vaidya, R.; Atanasov, P.; Wilkins, E. Med Eng Phys 1995, 17, 416. [CrossRef]
- McAteer, K.; O’Neill, R. D. Analyst 1996, 121, 773. [CrossRef]
- Zhu, J. Z.; Zhu, Z. Q.; Lai, Z. S. Sensors and Materials. in press.
- Garjonyte, R.; Malinauskas, A. Sensors and Actuators B 1999, 56, 85-92. 1-6.
- Karyakin, A.; Gitelmacher, O. V.; Karyakina, E. E. Anal. Chem. 1995, 67, 2419.
- Itaya, K.; Shoji, N.; Uchida, I. J. Am. Chem. Soc. 1984, 106, 3423. [CrossRef]
- Karyakin, A.; Karyakina, E. E.; Gorton, L. Electrochem. Commun. 1999, 1, 78.
- Malev, V. V.; Tikhomirova, A. V.; Kondratiev, V. V.; Rubashkin, A. A. Russ. J. Electrochem. 1999, 35, 1038.
- Kondratiev, V. V.; Tikhomirova, A. V.; Malev, V. V. Electrochim Acta 1999, 45, 751.
- Karyakin, A.; Karyakina, E. E.; Gorton, L. J. Electroanal. Chem. 1998, 456, 97. [CrossRef]
- De Mattos, L.; Gorton, L.; Laurell, T.; Malinauskas, A.; Karyakin, A. A. Talanta 2000, 52, 791–799. [PubMed]
- De Mattos, L.; Lukachova, L. V.; Gorton, L.; Laurell, T.; Karyakin, A. A. Talanta 2001, 54, 963–974.
- O’Halloran, M. P.; Pravda, M.; Guilbault, G. G. Talanta 2001, 55, 605–611.
- Sample Availability: Available from the authors.
© 2002 by MDPI (http://www.mdpi.net). Reproduction is permitted for noncommercial purposes.
Share and Cite
Zhu, J.; Zhu, Z.; Lai, Z.; Wang, R.; Guo, X.; Wu, X.; Zhang, G.; Zhang, Z.; Wang, Y.; Chen, Z. Planar Amperometric Glucose Sensor Based on Glucose Oxidase Immobilized by Chitosan Film on Prussian Blue Layer. Sensors 2002, 2, 127-136. https://doi.org/10.3390/s20400127
Zhu J, Zhu Z, Lai Z, Wang R, Guo X, Wu X, Zhang G, Zhang Z, Wang Y, Chen Z. Planar Amperometric Glucose Sensor Based on Glucose Oxidase Immobilized by Chitosan Film on Prussian Blue Layer. Sensors. 2002; 2(4):127-136. https://doi.org/10.3390/s20400127
Chicago/Turabian StyleZhu, Jianzhong, Ziqiang Zhu, Zongsheng Lai, Rong Wang, Xiaoming Guo, Xiaqin Wu, Guoxiong Zhang, Zongrang Zhang, Yiting Wang, and Zongyou Chen. 2002. "Planar Amperometric Glucose Sensor Based on Glucose Oxidase Immobilized by Chitosan Film on Prussian Blue Layer" Sensors 2, no. 4: 127-136. https://doi.org/10.3390/s20400127



