Membranes of 5,10,15,20-Tetrakis(4-Methoxyphenyl) Porphyrinatocobalt (TMOPP-Co) (I) as MoO42- - Selective Sensors
Abstract
:Introduction
Experimental
Reagents
Apparatus
Synthesis of 5,10,15,20-tetrakis(4-methoxyphenyl)porphyrinatocobalt (TMOPP-Co) (I)

Membrane preparation
Potential Measurements
| Internal reference electrode (SCE) | Internal solution 0.1 mol 1-1 | Membrane | Test Solution | External reference electode (SCE) |
Results and Discussion
Membrane Characteristics
| Sensor / Membrane No. | Composition of the membrane (w/w) | Working Concentration range (M) | Slope (±1.0mV/decade of activity) | Response time (s) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (I) | PVC | DBP | DOP | TBP | CN | DBBP | ||||
| 1 | 12 | 100 | - | - | - | - | - | 8.9×10-4-1.0×10-2 | 38.0 | 60 |
| 2 | 12 | 100 | 60 | - | - | - | - | 5.0×10-5-1.0×10-1 | 32.0 | 18 |
| 3 | 12 | 100 | - | 55 | - | - | - | 7.0×10-4-1.0×10-1 | 36.5 | 30 |
| 4 | 12 | 100 | - | - | 54 | - | - | 5.0×10-4-1.0×10-1 | 35.0 | 25 |
| 5 | 12 | 100 | - | - | - | 48 | - | 3.2×10-4-1.0×10-1 | 34.0 | 25 |
| 6 | 12 | 100 | - | - | - | - | 70 | 8.9×10-5-1.0×10-1 | 33.5 | 20 |
Working Concentration Range and Slope
Responses and Lifetime

| Sensor / Membrane No. | Composition of the membrane (w/w) | Working Concentration range (M) | Slope (±1.0mV/decade of activity) | Response time (s) | ||
|---|---|---|---|---|---|---|
| (I) | DBP | PVC | ||||
| A | 10.0 | 60 | 100 | 1.0×10-4-1.0×10-1 | 31.5 | 25 |
| B | 11.0 | 60 | 100 | 6.5×10-5-1.0×10-1 | 32.0 | 20 |
| C | 12.0 | 60 | 100 | 5.0×10-5-1.0×10-1 | 32.0 | 18 |
| D | 13.0 | 60 | 100 | 5.0×10-5-1.0×10-1 | 32.0 | 18 |
| E | 14.0 | 60 | 100 | 5.1×10-5-1.0×10-1 | 32.0 | 18 |
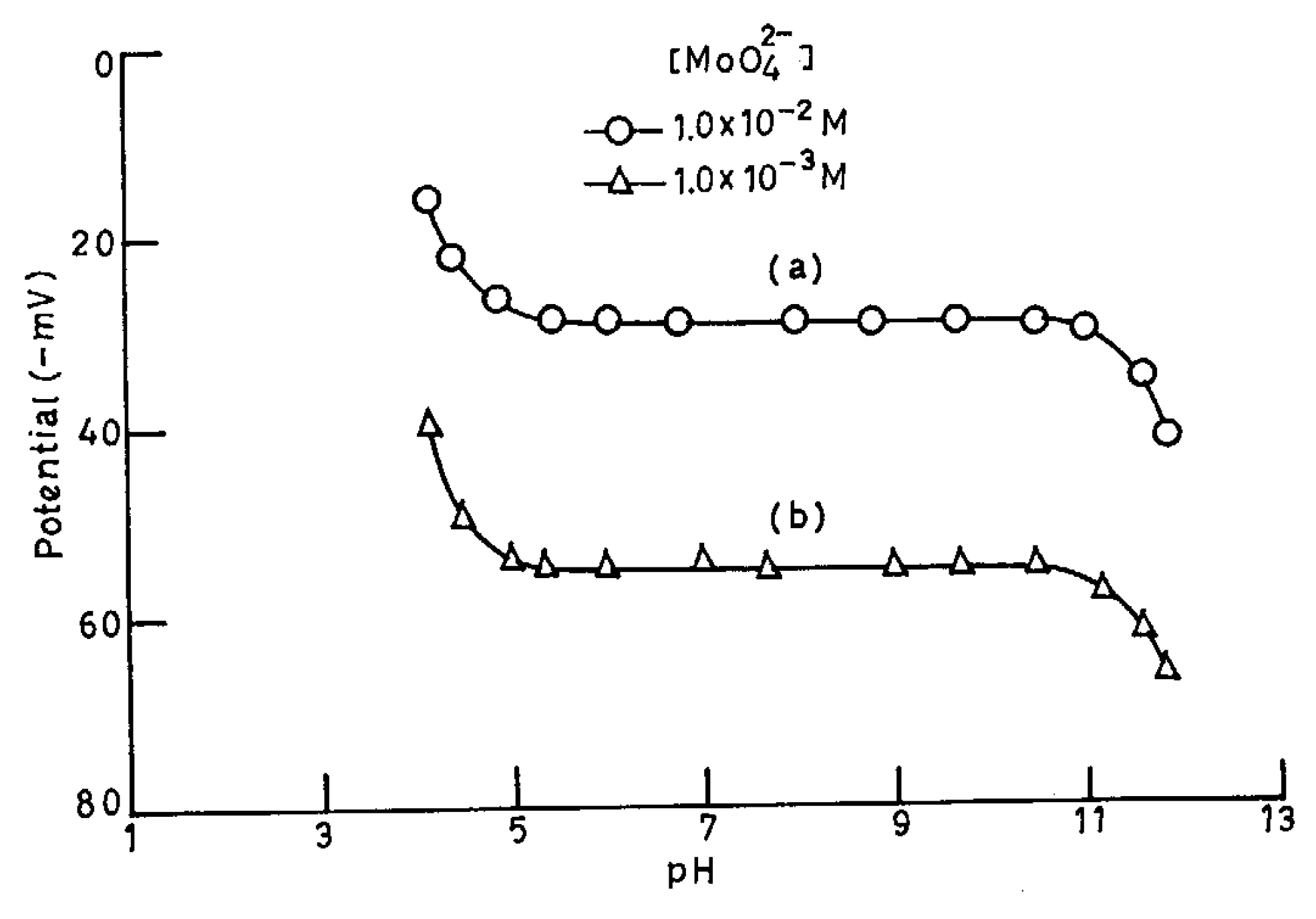
pH and Solvent Effect
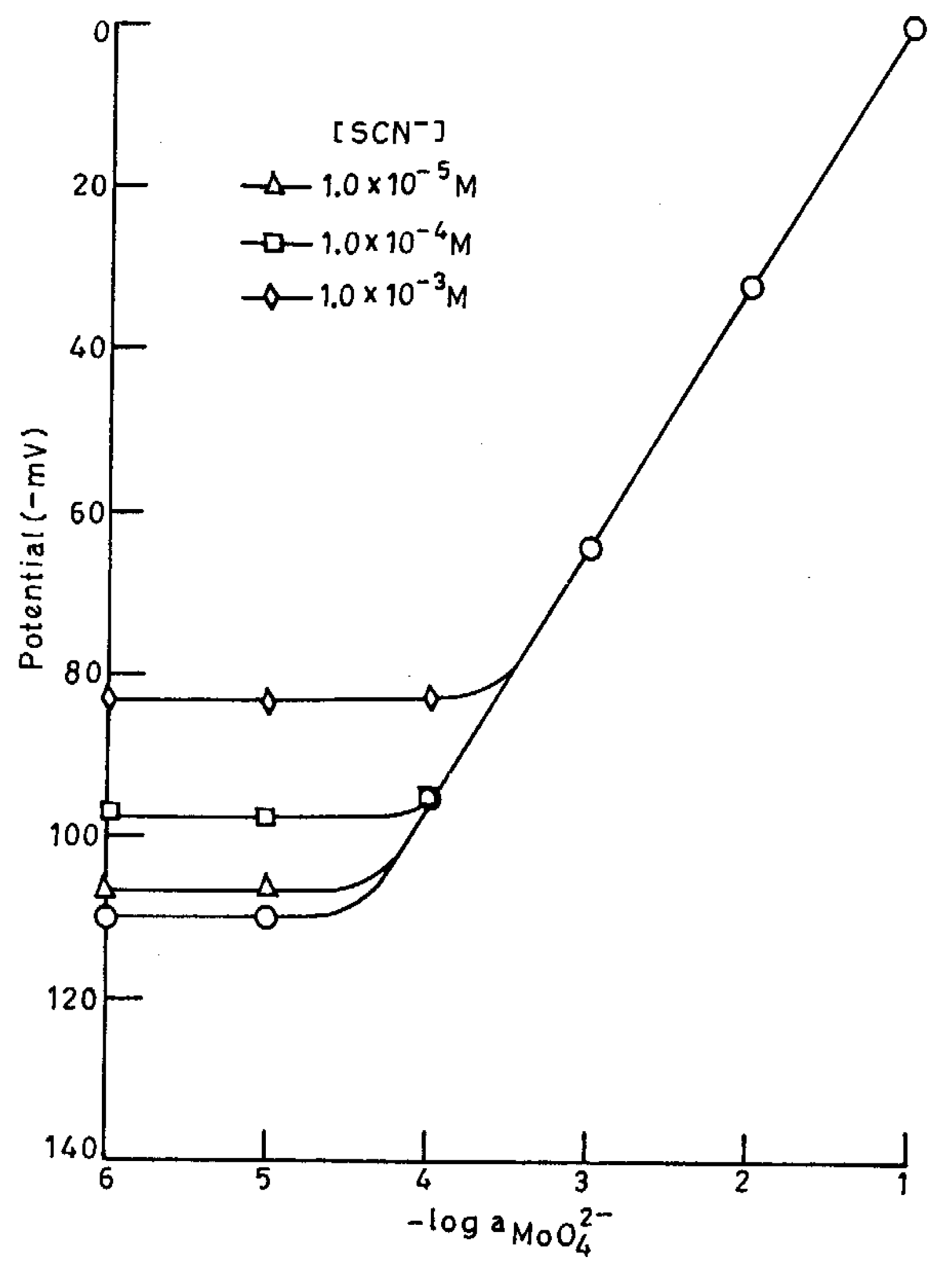
Potentiometric Selectivity
| Non-aqueous content (% v/v) | Working concentration range (M) | Slope (± 1.0 mV/decade of activity) |
|---|---|---|
| 0 | 5.0×10-5-1.0×10-1 | 32.0 |
| Methanol | ||
| 15 | 5.0×10-5-1.0×10-1 | 32.0 |
| 20 | 4.8×10-5-1.0×10-1 | 31.8 |
| 25 | 4.5×10-5-1.0×10-1 | 31.5 |
| 30 | 1.2×10-4-1.0×10-1 | 28.0 |
| Ethanol | ||
| 15 | 5.0×10-5-1.0×10-1 | 32.0 |
| 20 | 4.9×10-5-1.0×10-1 | 32.0 |
| 25 | 4.5×10-5-1.0×10-1 | 31.7 |
| 30 | 2.5×10-4-1.0×10-1 | 28.3 |

| Interfering ion (B) | Selectivity coefficient |
|---|---|
| Cl- | 1.8×10-1 |
| I- | 3.9×10-2 |
| Br- | 5.6×10-2 |
| NO3- | 1.0×10-2 |
| NO2- | 1.7×10-2 |
| ClO4- | 1.9×10-2 |
| IO43- | 2.8×10-2 |
| SCN- | 2.1×10-1 |
| SO42- | 1.5×10-2 |
| C2O42- | 1.2×10-2 |
| PO43- | 1.3×10-2 |
Conclusion
Acknowledgements
References
- Buegmayer, S. J. N.; Stiefel, E. I. J. Chem. Educ. 1991, 62, 1627.
- Marczenko, Z.; Lobinski, R. Pure Appl. Chem. 1991, 63, 1627.
- Malik, W.U.; Srivastava, S. K.; Bansal, A. Anal. Chem. 1982, 54, 1399.
- Srivastava, S.K.; Sharma, A. K.; Jain, C. K. Talanta 1983, 30, 285. [PubMed]
- Sergeev, G. M.; Korenman, I. M.; Stepanova, L. I. Zh. Anal. Khim. 1983, 38, 1816.
- Shpigun, L. K.; Abanina, E. N.; Gallai, Z. A.; Sheina, N. M. Talanta 1985, 32, 659. [PubMed]
- Lee, D. J.; Chen, K. L. Talanta 1990, 37, 901. [PubMed]
- Dorough, G. D.; Miller, J.R.; Huennekens, F. M. J. Am Chem. Soc. 1951, 73, 4315. [CrossRef]
- Craggs, A.; Moody, G. J.; Thomas, J. D. R. Chem. Educ. 1974, 51, 541.
- Srivastava, S. K.; Gupta, V. K.; Dwivedi, M. K.; Jain, S. Anal. Proc. 1995, 32, 21.
- Sa’ez de Viteri, F. J.; Diamond, D. Analyst 1994, 119, 749.
- Gadzekpo, V.P.Y.; Christian, G. D. Anal. Chim. Acta 1984, 164, 279. [CrossRef]
- Sample Availability: Available from the authors.
© 2002 by MDPI (http://www.mdpi.net). Reproduction is permitted for noncommercial purposes.
Share and Cite
Gupta, V.K.; Chandra, S.; Chauhan, D.K.; Mangla, R. Membranes of 5,10,15,20-Tetrakis(4-Methoxyphenyl) Porphyrinatocobalt (TMOPP-Co) (I) as MoO42- - Selective Sensors. Sensors 2002, 2, 164-173. https://doi.org/10.3390/s20500164
Gupta VK, Chandra S, Chauhan DK, Mangla R. Membranes of 5,10,15,20-Tetrakis(4-Methoxyphenyl) Porphyrinatocobalt (TMOPP-Co) (I) as MoO42- - Selective Sensors. Sensors. 2002; 2(5):164-173. https://doi.org/10.3390/s20500164
Chicago/Turabian StyleGupta, Vinod K., S. Chandra, D. K. Chauhan, and R. Mangla. 2002. "Membranes of 5,10,15,20-Tetrakis(4-Methoxyphenyl) Porphyrinatocobalt (TMOPP-Co) (I) as MoO42- - Selective Sensors" Sensors 2, no. 5: 164-173. https://doi.org/10.3390/s20500164




