Study on the Characteristics of Ag Doped CuO-BaTiO3 CO2 Sensors
Abstract
:Introduction
Experimental
Results and Discussion
Influence of Doping Elements on CO2 Sensitivity and Operating Temperature
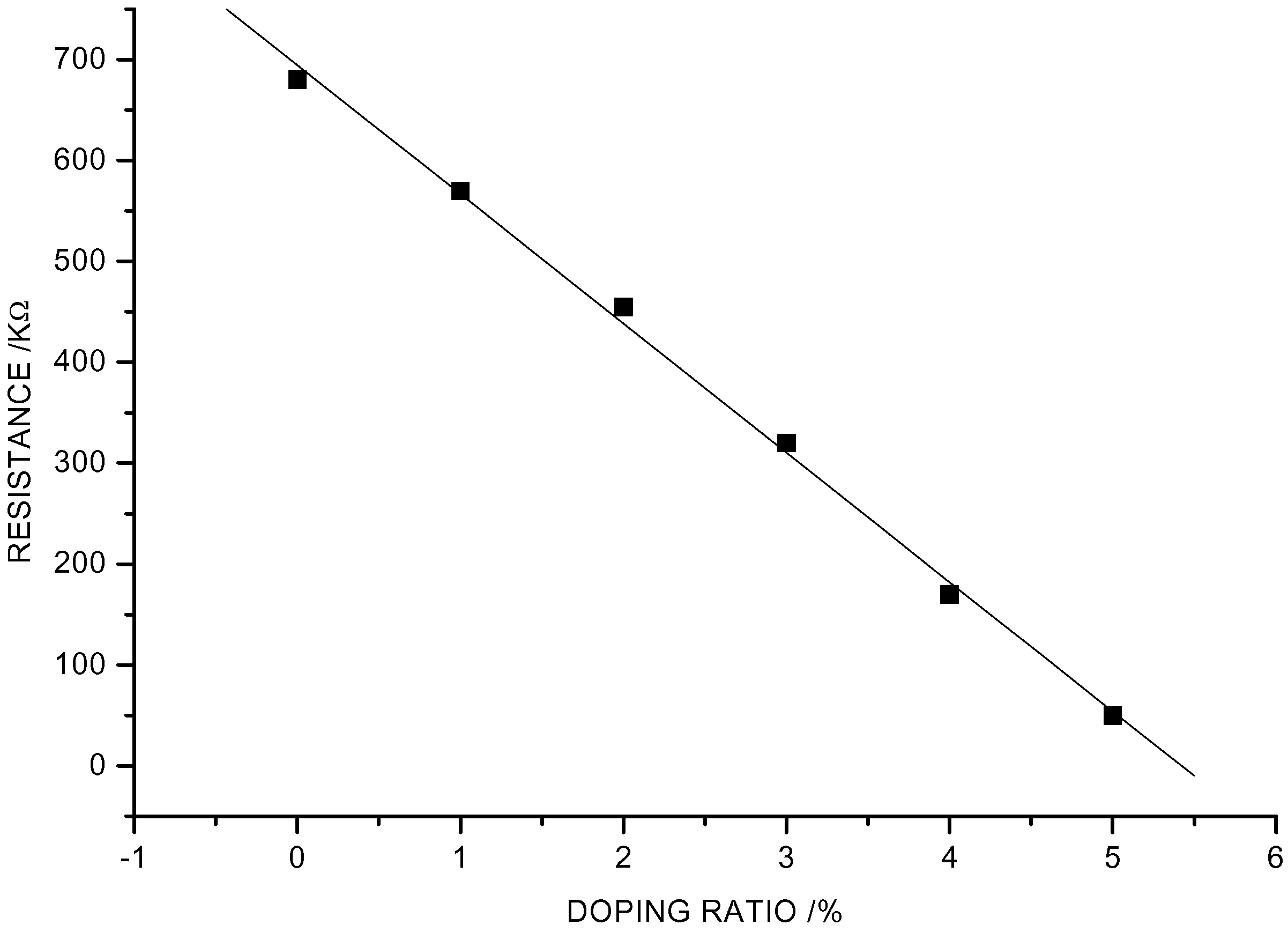
R-T and T-S Characteristics of Ag– Doped CuO-BaTiO3
| Doping substances | Operating temperature (°C) | Rg(MΩ) | Ra(MΩ) | Rg/Ra |
|---|---|---|---|---|
| / | 420 | 5.65 | 4.48 | 1.26 |
| Au | 440 | 12.7 | 10.7 | 1.19 |
| Ag | 430 | 0.54 | 0.34 | 1.59 |
| Pt | 490 | 5.71 | 4.53 | 1.26 |
| Pd | 540 | 10.3 | 7.46 | 1.38 |
| CeO2 | 530 | 0.38 | 0.29 | 1.31 |
| MgO | 580 | 0.97 | 0.77 | 1.26 |
| SrO | 580 | 1.23 | 0.83 | 1.48 |
| La2O3 | 600 | 2.58 | 1.70 | 1.52 |
| ZnO | 550 | 4.83 | 2.91 | 1.66 |
| Fe2O3 | 440 | 1.41 | 1.50 | 0.94 |
| Bi2O3 | 640 | 9.78 | 16.03 | 0.61 |

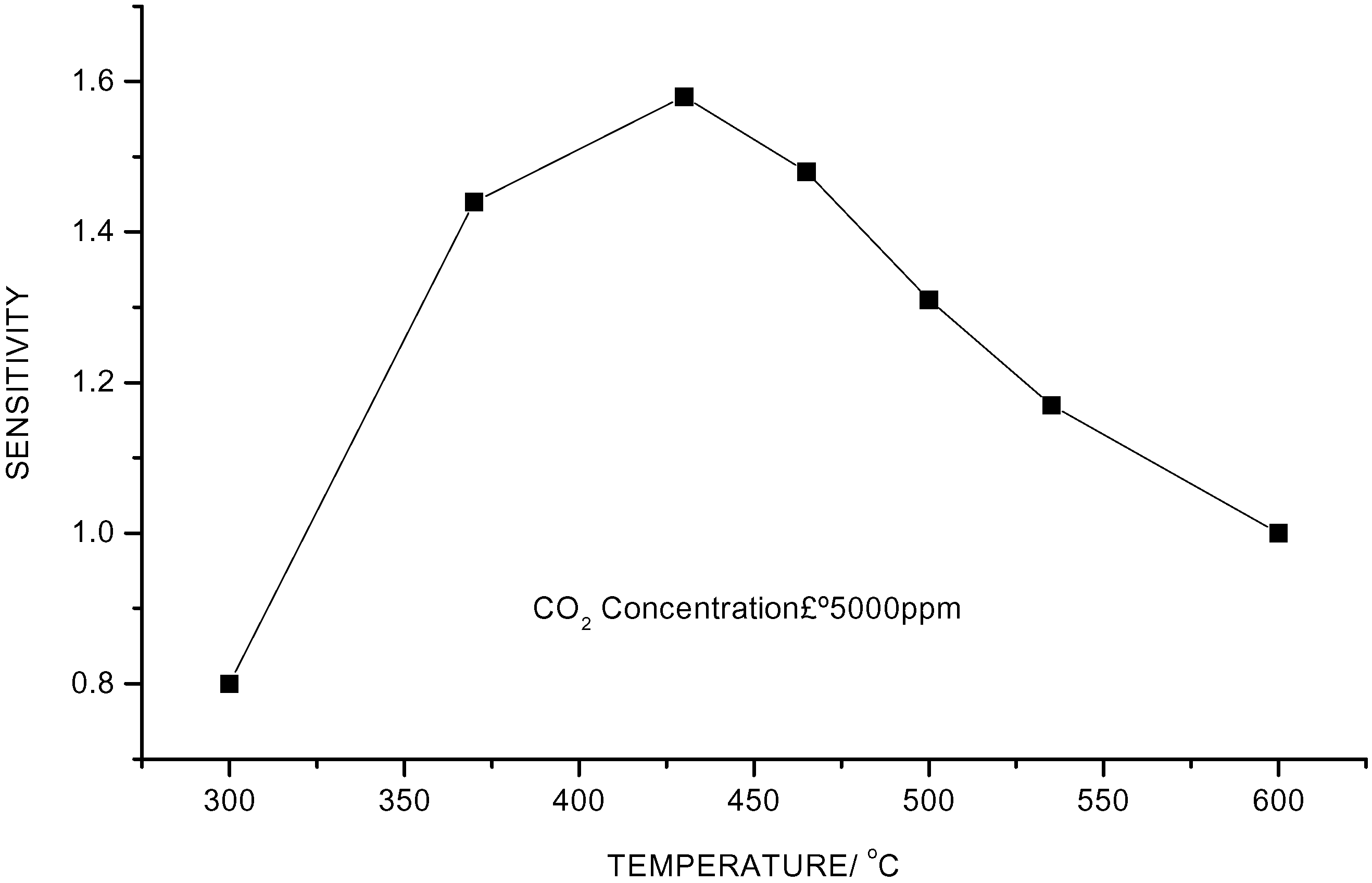

Influence of Ag– –Doping on CO2 Sensitivity

Influence of Sintering Temperature on CO2 Sensitivity
C-S Characteristics of Ag– Doped CuO-BaTiO3 Sensor

Selectivity of Ag- Doped CuO-BaTiO3 Sensor


Stability of the CuO-BaTiO3 Based CO2 Sensors
Conclusion
Acknowledgements
References
- Cote, R.; Bale, C.W.; Gauthier, M. J. Eletrochem. Soc. 1984, 131, 63–67.
- Ogata, T.; Fujitsu, S.; Koumoto, K.; Yanagida, H. J. Mater. Sci. Lett. 1986, 5, 285–286. [CrossRef]
- Maruyama, T.; Ye, X.; Saito, Y. Solid State Ionics 1987, 23, 113–117.
- Maruyama, T.; Sasaki, S.; Saito, Y. Solid State Ionics 1987, 23, 107–112.
- Maruyama, T.; Ye, X.; Saito, Y. Solid State Ionics 1987, 24, 281–287.
- Saito, Y.; Maruyama, T. Solid State Ionics 1988, 28-30, 1644–1647.
- Imanaka, N.; Kawasato, T.; Adachi, G. Chem. Lett. 1990, 497–500.
- Yao, S.; Shimizu, Y.; Miura, N.; Yamazoe, N. Chem. Lett. 1990, 2033–2036.
- Imanaka, N.; Kawasato, T.; Adachi, G. Chem. Lett. 1991, 13–16.
- Imanaka, N.; Kawasato, T.; Adachi, G. Chem. Lett. 1991, 1743–1746.
- Imanaka, N.; Murata, T.; Kawasato, T.; Adachi, G. Chem. Lett. 1992, 103–106. [CrossRef]
- Yao, S.; Hosohara, S.; Shimizu, Y.; Miura, N.; Futata, H.; Yamazoe, N. Chem. Lett. 1991, 2069–2072.
- Wu, X.; Shimizu, Y.; Egashira, M. J. Electrochem.Soc. 1989, 136, 2892–2895. [CrossRef]
- Ishihara, T.; Kometani, K.; Hashida, M.; Takita, Y. Chem. Lett. 1990, 1163–1166.
- Ishihara, T.; Kometani, K.; Hashida, M.; Takita, Y. J. Electrochem. Soc. 1991, 138, 173–176. [CrossRef]
- Shimizu, Y.; Komori, K.; Egashira, M. J. Electrochem. Soc. 1989, 136, 2256–2260. [CrossRef]
- Sample Availability: Available from the authors.
© 2002 by MDPI (http://www.mdpi.net). Reproduction is permitted for noncommercial purposes.
Share and Cite
Jiao, Z.; Chen, F.; Su, R.; Huang, X.; Liu, W.; Liu, J. Study on the Characteristics of Ag Doped CuO-BaTiO3 CO2 Sensors. Sensors 2002, 2, 366-373. https://doi.org/10.3390/s20900366
Jiao Z, Chen F, Su R, Huang X, Liu W, Liu J. Study on the Characteristics of Ag Doped CuO-BaTiO3 CO2 Sensors. Sensors. 2002; 2(9):366-373. https://doi.org/10.3390/s20900366
Chicago/Turabian StyleJiao, Zheng, Feng Chen, Run Su, Xingjiu Huang, Wei Liu, and Jinhuai Liu. 2002. "Study on the Characteristics of Ag Doped CuO-BaTiO3 CO2 Sensors" Sensors 2, no. 9: 366-373. https://doi.org/10.3390/s20900366




