Polypyrrole-Wrapped Carbon Nanotube Composite Films Coated on Diazonium-Modified Flexible ITO Sheets for the Electroanalysis of Heavy Metal Ions
Abstract
:1. Introduction
2. Experimental
2.1. Material and Reagents
2.2. In Situ Diazonium Modification of ITO Electrode
2.3. Purification and Deposition of CNTs on Diazonium-Modified ITO
2.4. Preparation of PPy Films on ITO Surface
2.5. Spectroscopic and Electrochemical Characterization of PPy-Modified Electrodes
2.6. Morphological Characterization of PPy-Modified Electrodes
3. Results and Discussion
3.1. Preparation of PPy Films on Bare ITO, NH2-ITO and CNT/NH2-ITO Electrodes
3.2. Structural Characterization of Modified Flexible ITO Sheets
3.2.1. Electrochemical Properties
3.2.2. Raman Study of Modified Flexible ITO Sheets
3.2.3. Morphology of Modified Flexible ITO Sheets
3.2.4. XPS Surface Analysis
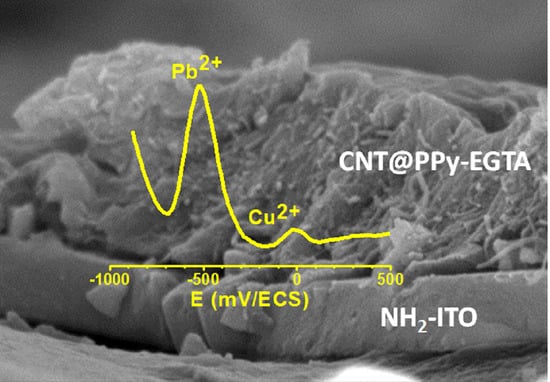
3.3. Electroanalytical Application of PPy/CNT/NH2-ITO Electrodes
3.4. Density Functional Theory Calculations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Iijima, S.; Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 1993, 364, 603–737. [Google Scholar] [CrossRef]
- Al-Harbi, E.A.; Abdelrahman, M.H.; El-Kosasy, A.M. Ecofriendly Long Life Nanocomposite Sensors for Determination of Carbachol in Presence of Choline: Application in Ophthalmic Solutions and Biological Fluids. Sensors 2019, 19, 2357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George, J.; Abdelghani, A.; Bahoumina, P.; Tantot, O.; Baillargeat, D.; Frigui, K.; Bila, S.; Hallil, H.; Dejous, C. CNT-Based Inkjet-Printed RF Gas Sensor: Modification of Substrate Properties during the Fabrication Process. Sensors 2019, 19, 1768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Yang, T.; Dong, Y.; Wang, X. A Room Temperature VOCs Gas Sensor Based on a Layer by Layer Multi-Walled Carbon Nanotubes/Poly-ethylene Glycol Composite. Sensors 2018, 18, 3113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Du, S.; Onodera, T.; Yatabe, R.; Tanaka, M.; Okochi, M.; Toko, K. An SPR Sensor Chip Based on Peptide-Modified Single-Walled Carbon Nanotubes with Enhanced Sensitivity and Selectivity in the Detection of 2,4,6-Trinitrotoluene Explosives. Sensors 2018, 18, 4461. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Zhang, H.; Zhou, L.; Huang, X.; Yu, C. Polypyrrole-Coated Zinc Ferrite Hollow Spheres with Improved Cycling Stability for Lithium-Ion Batteries. Small 2016, 12, 3732–3737. [Google Scholar] [CrossRef]
- Chen, S.; Zhitomirsky, I. Strategies to Optimize the Capacitive Behavior of PolypyrroleElectrodes. Mater. Manuf. Process. 2016, 31, 2017–2022. [Google Scholar] [CrossRef]
- Chen, S.; Zhitomirsky, I. Influence of Additives on Performance of Polypyrrole-Carbon Nanotube Supercapacitors. Mater. Manuf. Processes 2016, 31, 1246–1252. [Google Scholar] [CrossRef]
- Bharti, M.; Singh, A.; Samanta, S.; Aswal, D.K. Conductive polymers: Creating their niche in thermoelectric domain. Prog. Mater. Sci. 2018, 93, 270–310. [Google Scholar]
- Kotresh, S.; Ravikiran, Y.; Vijaya Kumari, S.; Chandrasekhar, T.; Ramana, C.V.; Thomas, S. Solution-based Spin Cast Processed Polypyrrole/Niobium Pentoxide Nanocomposite as Room Temperature Liquefied Petroleum Gas Sensor. Mater. Manuf. Processes. 2016, 31, 1976–1982. [Google Scholar] [CrossRef]
- Muliwa, A.M.; Leswifi, T.Y.; Onyango, M.S.; Maity, A. Magnetic Adsorption Separation (MAS) Process: An Alternative Method of Extracting Cr (VI) from Aqueous Solution using Polypyrrole Coated Fe3O4 Nanocomposites. Sep. Purif. Technol. 2016, 158, 250–258. [Google Scholar] [CrossRef]
- Bhaumik, M.; Agarwal, S.; Gupta, V.K.; Maity, A. Enhanced Removal of Cr (VI) from Aqueous Solutions using Polypyrrole Wrapped Oxidized MWCNTs Nanocomposites Adsorbent. J. Colloid Interface Sci. 2016, 470, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Sadki, S.; Schottland, P.; Brodie, N.; Sabouraud, G. The mechanisms of pyrrole electropolymerization. Chem. Soc. Rev. 2000, 29, 283–293. [Google Scholar]
- Wu, T.M.; Lin, S.-H. Characterization and electrical properties of polypyrrole/multiwalled carbon nanotube composites synthesized by in situ chemical oxidative polymerization. J. Polym. Sci Part B. 2006, 44, 1413–1418. [Google Scholar] [CrossRef]
- Park, S.J.; Kwon, O.S.; Jang, J. A high-performance hydrogen gas sensor using ultrathin polypyrrole-coated CNT nanohybrids. Chem. Commun. 2013, 49, 4673–4675. [Google Scholar] [CrossRef]
- Lu, Y.; Li, T.; Zhao, X.; Li, M.; Cao, Y.; Yang, H.; Duan, Y.Y. Electrodeposited polypyrrole/carbon nanotubes composite films electrodes for neural interfaces. Biomaterials 2010, 31, 5169–5181. [Google Scholar] [CrossRef]
- Hamouma, O.; Oukil, D.; Omastovà, M.; Chehimi, M.M. Flexible paper@carbon nanotube@polypyrrole composites: The combined pivotal roles of diazonium chemistry and sonochemical polymerization. Colloids Surf. A 2018, 538, 350–360. [Google Scholar] [CrossRef]
- Karim, M.R.; Lee, C.J.; Chowdhury, A.M.S.; Nahar, N.; Lee, M.S. Radiolytic synthesis of conducting polypyrrole/carbon nanotube composites. Mater. Lett. 2007, 61, 1688–1692. [Google Scholar] [CrossRef]
- Rahman, G.M.M.; Guldi, D.M.; Cagnoli, R.; Mucci, A.; Schenetti, L.; Vaccari, L.; Prato, M. Combining single wall carbon nanotubes and photoactive polymers for photoconversion. J. Am. Chem. Soc. 2005, 127, 10051–10057. [Google Scholar] [CrossRef]
- Cochet, M.; Maser, W.K.; Benito, A.M.; Callejas, M.A.M.; Martinez, T.; Benoit, J.M.; Schreiber, J.; Chauvet, O. Synthesis of a new polyaniline/nanotube composite:“in-situ” polymerisation and charge transfer through site-selective interaction. Chem. Commun. 2001, 20, 1450–1451. [Google Scholar] [CrossRef]
- Bayrakçeken, H.; Naktiyok, J.; Özer, A.K.; Yurtcan, A.B. Investigation of the Thermal Behavior of Polypyrrole/Carbon Nanotube Composites and Utilization as Capacitive Material or Support for Catalysts. Chem. Eng. Commun. 2017, 204, 916–925. [Google Scholar] [CrossRef]
- Cai, H.; Xu, Y.; He, P.G.; Fang, Y.Z. Indicator Free DNA Hybridization Detection by Impedance Measurement Based on the DNA-Doped Conducting Polymer Film Formed on the Carbon Nanotube Modified Electrode. Electroanalysis 2003, 15, 1864–1870. [Google Scholar] [CrossRef]
- Gupta, S.; Murthy, C.N.; Ratna, P.C. Recent advances in carbon nanotube based electrochemical biosensors. Int. J. Biol. Macromol. 2018, 108, 687–703. [Google Scholar] [CrossRef] [PubMed]
- Cheraghi, S.; Taher, M.A.; Karimi-Maleh, H.; Faghih-Mirzaeide, E. A nanostructure label-free DNA biosensor for ciprofloxacin analysis as a chemotherapeutic agent: An experimental and theoretical investigation. New. J. Chem. 2017, 41, 4985–4989. [Google Scholar] [CrossRef]
- Arulraj, A.D.; Sundaram, E.; Vasantha, V.S.; Neppolian, B. Polypyrrole with a functionalized multi-walled carbon nanotube hybrid nanocomposite: A new and efficient nitrite sensor. New J. Chem. 2018, 42, 3748–3757. [Google Scholar] [CrossRef]
- Eom, G.; Oh, C.; Moon, J.; Kim, H.; Kim, M.K.; Kim, K.; Seo, J.-W.; Kang, T.; Lee, H.J. Highly sensitive and selective detection of dopamine using overoxidized polypyrrole/sodium dodecyl sulfate-modified carbon nanotube electrodes. J. Electroanal. Chem. 2019, 848, 113295. [Google Scholar] [CrossRef]
- Oularbi, L.; Turmine, M.; Rhazia, M.E. Preparation of novel nanocomposite consisting of bismuth particles, polypyrrole and multi-walled carbon nanotubes for simultaneous voltammetric determination of cadmium(II) and lead(II). Synth.Met. 2019, 253, 1–8. [Google Scholar] [CrossRef]
- Lo, M.; Diaw, A.K.D.; Gningue-Sall, D.; Aaron, J.-J.; Oturan, M.A.; Chehimi, M.M. The role of diazonium interface chemistry in the design of high performance polypyrrole-coated flexible ITO sensing electrodes. Electrochem. Commun. 2017, 77, 14–18. [Google Scholar] [CrossRef]
- Lo, M.; Pires, R.; Diaw, K.; Gningue-Sall, D.; Oturan, M.A.; Aaron, J.J.; Chehimi, M.M. Diazonium salts: Versatile molecular glues for sticking conductive polymers to flexible electrodes. Surfaces 2018, 1, 5. [Google Scholar] [CrossRef] [Green Version]
- Perruchot, C.; Chehimi, M.M.; Delamar, M.; Cabet-Deliry, E.; Miksa, B.; Slomkowski, S.; Khan, M.A.; Armes, S.P. Chemical deposition and characterization of thin polypyrrole films on glass plates: role of organosilane treatment. Colloid Polym. Sci. 2000, 278, 1139. [Google Scholar] [CrossRef]
- Lo, M.; Diaw, A.K.D.; Gningue-Sall, D.; Aaron, J.-J.; Oturan, M.A.; Chehimi, M.M. Tracking metal ions with polypyrrole thin films adhesively bonded to diazonium-modified flexible ITO electrodes. Environ. Sci. Pollut. Res. 2018, 25, 20012–20022. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Pintauro, P.N. Selective removal of heavy metals from contaminated kaolin by chelators. Water Air Soil Pollut. 1996, 87, 73–91. [Google Scholar] [CrossRef]
- Heitzmann, M.; Bucher, C.; Moutet, J.-C.; Pereira, E.; Bernabé, L.R.; Royal, G.; Saint-Aman, E. Complexation of poly(pyrrole-EDTA like) film modified electrodes: Application to metal cations electroanalysis. Electrochim. Acta 2007, 52, 3082–3087. [Google Scholar] [CrossRef]
- Deshmukh, M.A.; Bodkhe, G.A.; Shirsat, S.; Ramanavicius, A.; Shirsat, M.D. Nanocomposite platform based on EDTA modified Ppy/SWNTs for the sensing of Pb(II) ions by electrochemical method. Front. Chem. 2018, 6, 451. [Google Scholar] [CrossRef] [Green Version]
- Bhakta, A.K.; Detriche, S.; Martis, P.; Mascarenhas, R.J.; Delhalle, J.; Mekhalif, Z. Decoration of tricarboxylic and monocarboxylic aryl diazonium functionalized multi-wall carbon nanotubes with iron nanoparticles. J. Mater. Sci. 2017, 52, 9648–9660. [Google Scholar] [CrossRef]
- Fu, X.-C.; Wu, J.; Nie, L.; Xie, C.-G.; Liu, J.-H.; Huang, X.-J. Electropolymerized surface ion imprinting films on a gold nanoparticles/single-wall carbon nanotube nanohybrids modified glassy carbon electrode for electrochemical detection of trace mercury(II) in water. Anal. Chim. Acta. 2012, 720, 29–37. [Google Scholar] [CrossRef]
- Tasis, D.; Tagmatarchis, N.; Bianco, A.; Prato, M. Chemistry of carbon nanotubes. Chem. Rev. 2006, 106, 1105–1136. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Salmi, S.; Dahoumane, S.A.; Mekki, A.; Carbonnier, B.; Chehimi, M.M. Functionalization of nanomaterials with aryldiazonium salts. Adv. Colloid Interface Sci. 2015, 225, 16–36. [Google Scholar] [CrossRef]
- Yu, S.S.C.; Tan, E.S.Q.; Jane, R.T.; Downard, A.J. An electrochemical and XPS study of reduction of nitrophenyl films covalently grafted to planar carbon surfaces. Langmuir 2007, 23, 11074–11082. [Google Scholar] [CrossRef]
- Pilan, L.; Raicopol, M.; Pruna, A.; Branzoi, V. Polyaniline/carbon nanotube composite films electrosynthesis through diazonium salts electroreduction and electrochemical polymerization. Surf. Interface Anal. 2012, 44, 1198–1202. [Google Scholar] [CrossRef]
- Liu, Y.-C.; McCreery, R.L. Reactions of Organic Monolayers on Carbon Surfaces Observed with Unenhanced Raman Spectroscopy. J. Am. Chem. Soc. 1995, 117, 11254–11259. [Google Scholar] [CrossRef]
- Song, H.; Cai, K.; Wang, J.; Shen, S. Influence of polymerization method on the thermoelectric properties of multi-walled carbon nanotubes/polypyrrole composites. Synt. Met. 2016, 211, 58–65. [Google Scholar] [CrossRef]
- Afzal, A.; Abuilaiwi, F.A.; Habib, A.; Awais, M.; Waje, S.B.; Atieh, M.A. Polypyrrole/carbon nanotube supercapacitors: Technological advances and challenges. J. Power Sources 2017, 352, 174–186. [Google Scholar] [CrossRef]
- Bensghaïer, A.; Truong, S.L.; Seydou, M.; Lamouri, A.; Leroy, E.; Matej, M.; Forro, K.; Beji, M.; Pinson, J.; Omastova, M.; et al. Efficient Covalent Modification of Multiwalled Carbon Nanotubes with Diazotized Dyes in Water at Room Temperature. Langmuir 2017, 33, 6677–6690. [Google Scholar]
- Hicks, M.; Wong, Z.Y.; Scurr, D.J.; Silman, N.; Jackson, S.K.; Mendes, P.M.; Aylott, J.W.; Yi, Z.; Rawson, F.J. Tailoring the Electrochemical Properties of Carbon Nanotube Modified Indium Tin Oxide via in Situ Grafting of Aryl Diazonium. Langmuir 2017, 33, 4924–4933. [Google Scholar] [CrossRef] [PubMed]
- Boukerma, K.; Omastová, M.; Fedorko, P.; Chehimi, M.M. Surface properties and conductivity of bis (2-ethylhexyl) sulfosuccinate-containing polypyrrole. Appl. Surf. Sci. 2005, 249, 303–314. [Google Scholar] [CrossRef]
- Jlassi, K.; Singh, A.; Aswal, D.K.; Losno, R.; Benna-Zayani, M.; Chehimi, M.M. Novel, ternary clay/polypyrrole/silver hybrid materials through in situ photopolymerization. Colloids Surf. A 2013, 439, 193–199. [Google Scholar] [CrossRef]
- Chamjangali, M.A.; Kouhestani, H.; Masdarolomoor, F.; Daneshinejad, H. A voltammetric sensor based on the glassy carbon electrode modified with multi-walled carbon nanotube/poly(pyrocatecholviolet)/bismuth film for determination of cadmium and lead as environmental pollutants. Sens. Actuators B 2015, 216, 384–393. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Trucks GW Schlegel HB Scuseria GE Robb MA Cheeseman JR Fox DJ Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Moore, C.E. “National Standard Reference Data Series,” National Bureau of Standards, No. 34; Washington, DC, 1970. Phys. Chem. Ref. Data 1974, 3, 771–779, Dasent, pp. 44–47. [Google Scholar]
- Barone, V.; Cossi, M.; Tomasi, J. A New definition of cavities for the computation of solvation free energies by the polarizable continuum model. J. Chem. Phys. 1997, 107, 3210–3221. [Google Scholar] [CrossRef]











| Sample | Mean | Standard Deviation | Median | Minimum | Maximum |
|---|---|---|---|---|---|
| (nm) | (nm) | (nm) | (nm) | (nm) | |
| CNT/NH2-ITO | 18.0 | 2.6 | 17.9 | 11.0 | 24.8 |
| PPy/CNT/NH2-ITO | 27.0 | 4.8 | 26.1 | 19.8 | 46.5 |
| formed by CV for 1 cycle | |||||
| PPy/CNT/NH2-ITO | 35.6 | 5.9 | 34.6 | 25.1 | 63.0 |
| formed by CV for 5 cycles | |||||
| PPy/CNT/NH2-ITO | 175.0 | 20.1 | 176.5 | 126.3 | 233.0 |
| formed by CV for 10 cycles |
| System | ∆E(+2)f(g) | ∆Esolv | ∆E(+2)f(solv) | dX-O (Å) | dX-N (Å) |
|---|---|---|---|---|---|
| (kJ/mol) | (kJ/mol) | (kJ/mol) | |||
| EGTA | - | −127.8 | − | - | - |
| Cu2+ | - | −1288.8 | − | - | - |
| Pb2+ | - | −1038.8 | − | - | - |
| [EGTA-Cu]2+ | −741.9 | −790.9 | −116.4 | 2.047 (2.238) | 2.135 (3.574) |
| [EGTA-Pb]2+ | −802.4 | −738.8 | −374.6 | 2.452 (2.662) | 2.854(2.903) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo, M.; Seydou, M.; Bensghaïer, A.; Pires, R.; Gningue-Sall, D.; Aaron, J.-J.; Mekhalif, Z.; Delhalle, J.; Chehimi, M.M. Polypyrrole-Wrapped Carbon Nanotube Composite Films Coated on Diazonium-Modified Flexible ITO Sheets for the Electroanalysis of Heavy Metal Ions. Sensors 2020, 20, 580. https://doi.org/10.3390/s20030580
Lo M, Seydou M, Bensghaïer A, Pires R, Gningue-Sall D, Aaron J-J, Mekhalif Z, Delhalle J, Chehimi MM. Polypyrrole-Wrapped Carbon Nanotube Composite Films Coated on Diazonium-Modified Flexible ITO Sheets for the Electroanalysis of Heavy Metal Ions. Sensors. 2020; 20(3):580. https://doi.org/10.3390/s20030580
Chicago/Turabian StyleLo, Momath, Mahamadou Seydou, Asma Bensghaïer, Rémy Pires, Diariatou Gningue-Sall, Jean-Jacques Aaron, Zineb Mekhalif, Joseph Delhalle, and Mohamed M. Chehimi. 2020. "Polypyrrole-Wrapped Carbon Nanotube Composite Films Coated on Diazonium-Modified Flexible ITO Sheets for the Electroanalysis of Heavy Metal Ions" Sensors 20, no. 3: 580. https://doi.org/10.3390/s20030580