Experimental
ZnCr
2O
4 spinel was synthesized according to a previously described method [
4]. The precursor solution for atomization was prepared by dissolving the appropriate amounts of corresponding metal nitrates Zn(NO
3)
2x6H
2O and Cr(NO
3)
3x9H
2O in distilled water, in order to obtain 0.03mol/dm
3. Assuming that the precursor stoichiometry persisted in the final particles, a Zn:Cr = 1:2 cations ratio is chosen for the starting composition. The aerosol was produced by an ultrasonic generator (Gapusol RBI, 1.7 MHz) and introduced in a twin-zone tubular flow reactor with air as the carrier gas. The heated region was a 1.3 m long, 3.2 cm dia quartz tube, with increase–humped temperature profile and T
max=900°C in both reaction zones. Three charges of powders were collected with different droplet/particle residence time - after the first heated zone (powder charge 1), the second one (powder charge 2) and from the collecting chamber (powder charge 3). In the case of powder charge 1, the aerosol residence time was 3s at T
max. After the powder was trapped, hot air (700°C) blow over the powder sample for an additional 16 h. The residence time for powder charge 2 at T
max was 6s. Similarly as for the charge 1, after the powder charge 2 was trapped, hot air (400°C) blow over the powder sample for an additional 16 h. Powder charge 3 had a residence time of 9s at T
max. The temperature in the collecting chamber was 150°C. All the as-prepared samples were checked by powder XRD. As-prepared powders were additionaly thermaly treated at a temperature of 1000°C for 2 hours.
The investigation of precursors decomposition behavior was examined by differential scanning calorimetry (detector type SHIMADZU DSC-50) under a nitrogen atmosphere at a heating rate of 10°C/min.
Particle size distribution analysis was carried out with a Malvern Laser Master Sizer. 100mg of as-prepared powders were used for analysis, ultrasonically deagglomerated and suspended in referent solution (0.05% sodium hexametasulphate water solution).
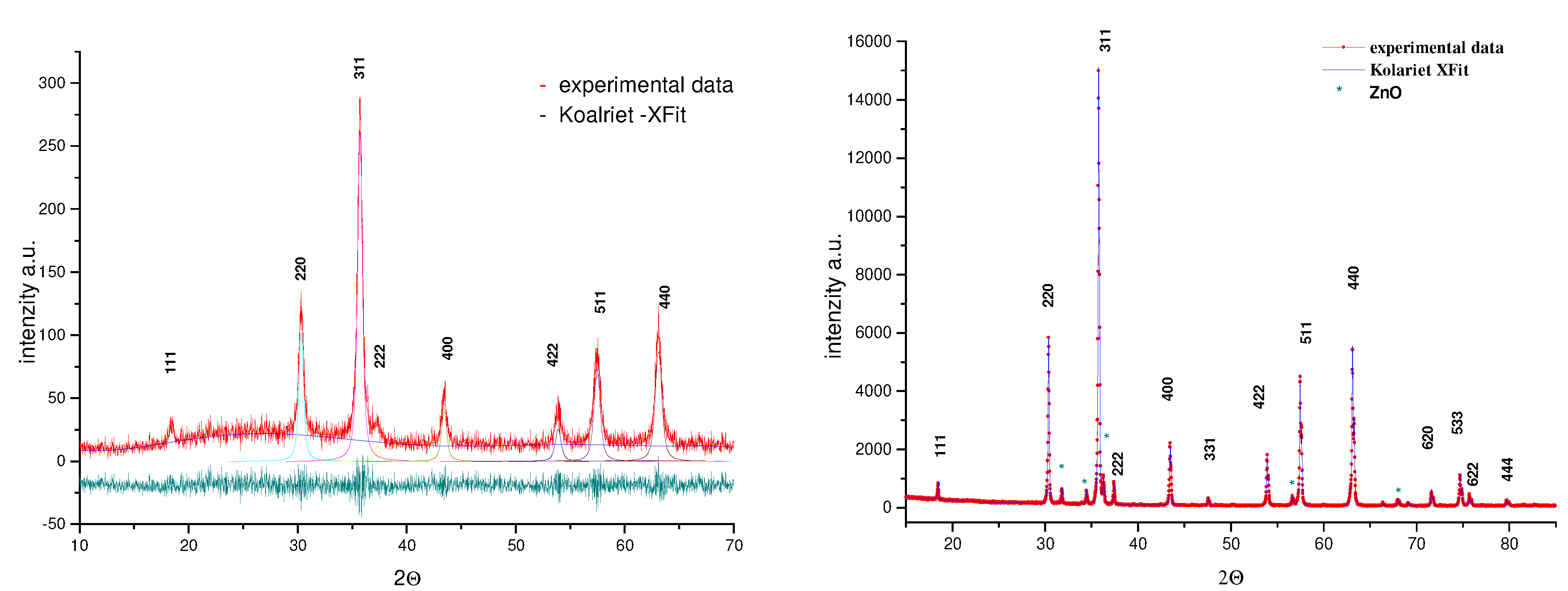
X-ray diffraction data of thermally treated powders were collected in the range 3°-70° (2θ), using a Philips PW 1710 automated diffractometer with CuK
α radiation and a graphite monochromator. Step-scanning was 0.02°/15s. All peak positions were used in the determination of precise microstrucural parameters. Structural refinements were carried out using the Rietveld program Koalariet-Xfit [
9]. Peak profiles were fited with a pseudo-Voight function and asymmetry parameters were considered for peaks below 70° (2θ). The background was refined with a polynomial function.
Compositional homogeneity and morphology of obtained particles were determined in accordance to scanning/transmission electron microscopy (SEM S-4500), TEM (JEOL JEM-200) and HORIBA X-ray Microanalyzer EMAX-7000. For that purpose, powder samples were sputtered with Au ( Au depth layer 150 A made by Quick auto Coater JFC 1500). Qualitative and quantitative sample analysis were proceeded in accordance to spot (analyzing area 100A, beam depth 50 nm) as well as square analysis. Mapping of the constitutive elements was proceeded for the purpose of inner particle uniformity confirmation.
Results and Discussion
Thermal analysis of mixed precursor salts shows that precursor decomposition is complete at a temperature below 350°C [
4]. Having in mind specific mechanisms of particle formation during the spray pyrolysis process, as well as the fact that spinel phase formation during conventional solid-state reaction process occurs at temperatures higher than 1000°C [
8], a temperature of 900°C is chosen as a maximum temperature for the synthesis process.
Particle size measurements, performed for all investigated samples, show the same value of mean particle size, estimated to be 470nm, indicating that residence time did not affect a broadening of the particle size distributions [
4].
From the SEM micrographs on
Fig.1 is easy to notice morphology changes that occurred with increasing residence time. Having in mind that powders from charges 1 and 2 were exposed to
in situ additional thermal treatment at different temperatures (since they were trapped in tubular furnace after first and second zone during synthesis) powder from charge 3 is characterized with the shortest residence time (9s on T
max, 38s in tubular furnace).
The main feature of particles presented in
Fig.1c is their composite structure and the presence of primary crystallites visible on their surface. Two types of particles are setting apart. While some of them have visually uniform surface composition, others are characterized by compositional segregation limited to the level of primary grains. For the powders from charge 1, shown in
Fig.1a (3s on T
max, additional 16h on 700°C), characteristic smooth surface of the individual irregular shaped particles indicating dense particle formation with a crust evolved during the melting of the precursor. Particle bonding and neck formation denote initial interparticle sintering. Slightly agglomerated particles from charge 2,
Fig.3b (6s on T
max, additional 16h on 400°C), exhibit the coexistence of both smooth, dense particles, as well as composite ones, having complex inner crystallite structure.
Fig.1.
Scanning electron micrographs of the as-prepared ZnCr2O4 powders: a) charge 1; b) charge 2; c) charge 3
Fig.1.
Scanning electron micrographs of the as-prepared ZnCr2O4 powders: a) charge 1; b) charge 2; c) charge 3
EMAX mapping for as-prepared samples showed uniform distribution of the constitutive elements inside the examined particles: zinc, chromium, oxygen and nitrogen [
4]. The results obtained mean that during spray pyrolysis each particle is formed through volume precipitation. This is proved also by TEM analysis,
Fig.1. The primary crystallites visible on
Fig.1 ranged from 10 to 60nm, which is in accordance with diffraction line broadening analysis(
Table 1.).
Chemical composition determined by semiquantitative EMAX analysis indicates similar stoichiometry for all examined particles, with the mean Zn/Cr ratio at 0.68. The value obtained denotes the solution concentration ratio (0.5) is not fully achieved in the resulting particles, while the presence of nitrogen should be the consequence of the high reactivity of synthesized powders and also surface chemisorption. Considering the obtained particle stoichiometry, no clear evidence about the influence of the particle residence time on the Zn/Cr concentration ratio was established.
Together with SEM/EMAX morphological analysis, XRD data have also allowed a better characterization of the chemical homogeneity of the as-prepared powders. A typical X-ray diffraction pattern of as-prepared powders is presented on
Fig.2a. Spinel phase (ZnCr
2O
4) formation is confirmed in all investigated samples due to the presence of diffraction lines indicated on the JCPDS cards 22-1107 (8.327 Å). Slightly broadening of diffraction lines may be attributed to small crystallite effects, crystal defects or chemical heterogeneity of the samples. In accordance with the procedure based on Koalariet-Xfit program [
9], results of structural refinement obtained for as-prepared spinel powders are present in
Table 1. Considering Zn
2+ and Cr
3+ cation radii the spinel phase with higher unit-cell parameter (
a) should be richer in Zn. It is not possible to establish the influence of the primary crystallite size and microstains change versus particle residence time by comparison the attained structural parameters given in
Table 1, but the same behavior in changes is observed.
Table 1.
Values of lattice parameters (a), average crystallite size (D) and crystal lattice microstrains (ε) in as-prepared ZnCr2O4 particles
Table 1.
Values of lattice parameters (a), average crystallite size (D) and crystal lattice microstrains (ε) in as-prepared ZnCr2O4 particles
| Powder charge | a [Å] | D [nm] | <ε2(L)>1/2 [%] |
| 1 | 8.332±0.001 | 33.3±3.1 | 0.551±0.068 |
| 2 | 8.338±0.001 | 22.6±1.2 | 0.125±0.054 |
| 3 | 8.335±0.001 | 44.1±6.4 | 0.638±0.081 |
Determined cation ratio in as-prepared spinel powders at 0.68 confirmed presumptions that the residence time of each droplet/particle is insufficient to fully establish the stoichiometry dictated by the precursor solution. The condition which have ensured further diffusion of Cr
3+ cations into previously formed spinel crystal lattice include an additional thermal treatment of as-prepared powders. The powder diffraction data of the ZnCr
2O
4 samples annealed at 1000°C shown in
Fig.2b, reveal a well-crystallized cubic spinel phase (98.1 wt%). Also, a small amount (1.9 wt%) of ZnO phase (JCPDS cards 36-1451) was detected. The occupancy values of particular cation sites in the spinel crystal lattice (Zn
2+-0.9951; Cr
3+-0.9593) correspond to the spinel phase with the cation Zn/Cr ratio of 0.52. Estimated values of structural refinement such as unit-cell parameters (
a,
b), average crystallite size (D) and crystal lattice microstrains (ε) of obtained phases after heating are presented in
Table 2.
The presence of ZnO as an accompanying phase in the process of spinel synthesis, is known. Although, non-stoichiometry of spinel compound due to the dissolution of excess oxide which containing three valent ions (in our case Cr
2O
3) is more typical [
8]. In addition, there is also the possibility of forming Schottky defects and three kinds of Frenkel defect at high temperatures. Stoichiometric ZnCr
2O
4 has a face-centered cubic arrangement of oxygen ions space group Fd3m, in which the Cr
3+ ions occupy half of the octahedral and the Zn
2+ ions fill one-eighth of the tetrahedral
Table 2.
Structural parameters of obtained phases ZnCr2O4 and ZnO in powder charge 1 after additional heating
Table 2.
Structural parameters of obtained phases ZnCr2O4 and ZnO in powder charge 1 after additional heating
| Phase | Unit-cell parameter, Å | D, nm | ε, % |
| ZnCr2O4 | 8.330±0.001 | 337.7±18 | 0.0647±0.003 |
| ZnO | a=b: 3.251±0.001
c: 5.207±0.001 | 388.1±13 | 0.0472±0.019 |
Fig.2.
Experimental and calculated diffraction profiles obtained for as-prepared (a) and thermally treated (b) ZnCr2O4 powder (charge 1)
Fig.2.
Experimental and calculated diffraction profiles obtained for as-prepared (a) and thermally treated (b) ZnCr2O4 powder (charge 1)
sites enclosed within the anion sublattice. With this distribution, the theoretical calculated lattice parameter of the crystal is 8.340Å. The variation in the experimental values, in the range 8.321-8.359Å [
8,
10,
11], may indicate formation of a non-stoichiometric and defective material. It is shown that atomistic methods for simulation and description of the perfect and defect spinel ZnCr
2O
4 crystal lattice based on the calculation of the formation energies of an ideal crystal, as well as set of isolated defects and also defects cluster formation could be used for the interpretation of this variation [
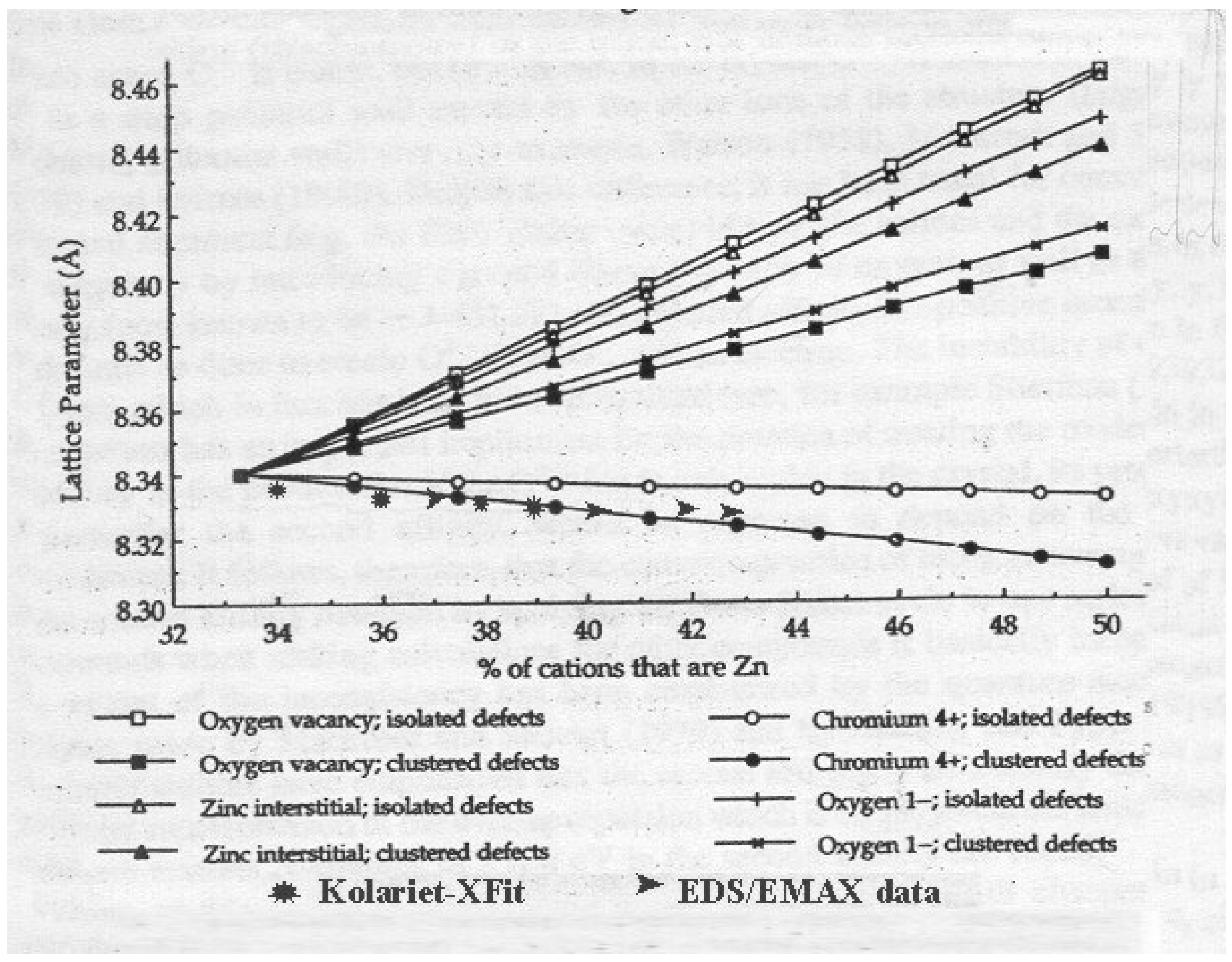
8]. According to this model, the different defect reactions associated with ZnO solution give rise to different combinations of defects. Two of them lead to a decrease of lattice parameter, while others result in an incense of the spinel unit cell, see
Fig.3. Together with the predicted lattice parameters from model [
8] the experimental data presented in this study are shown on the same figure (lattice parameters from Kolariet –Xfit stucture refinement* and Zn content determined through ►EDS/EMAX anlysis) indicating that clustered Cr
4+ defects are the dominant defects present in prepared spinel structures of nanophased powders.
Fig.3.
Predicted spinel lattice parameters due zinc excess presence [
8] shown together with the experimental data presented in this study (* Kolariet-Xfit; ►EDS/EMAX)
Fig.3.
Predicted spinel lattice parameters due zinc excess presence [
8] shown together with the experimental data presented in this study (* Kolariet-Xfit; ►EDS/EMAX)
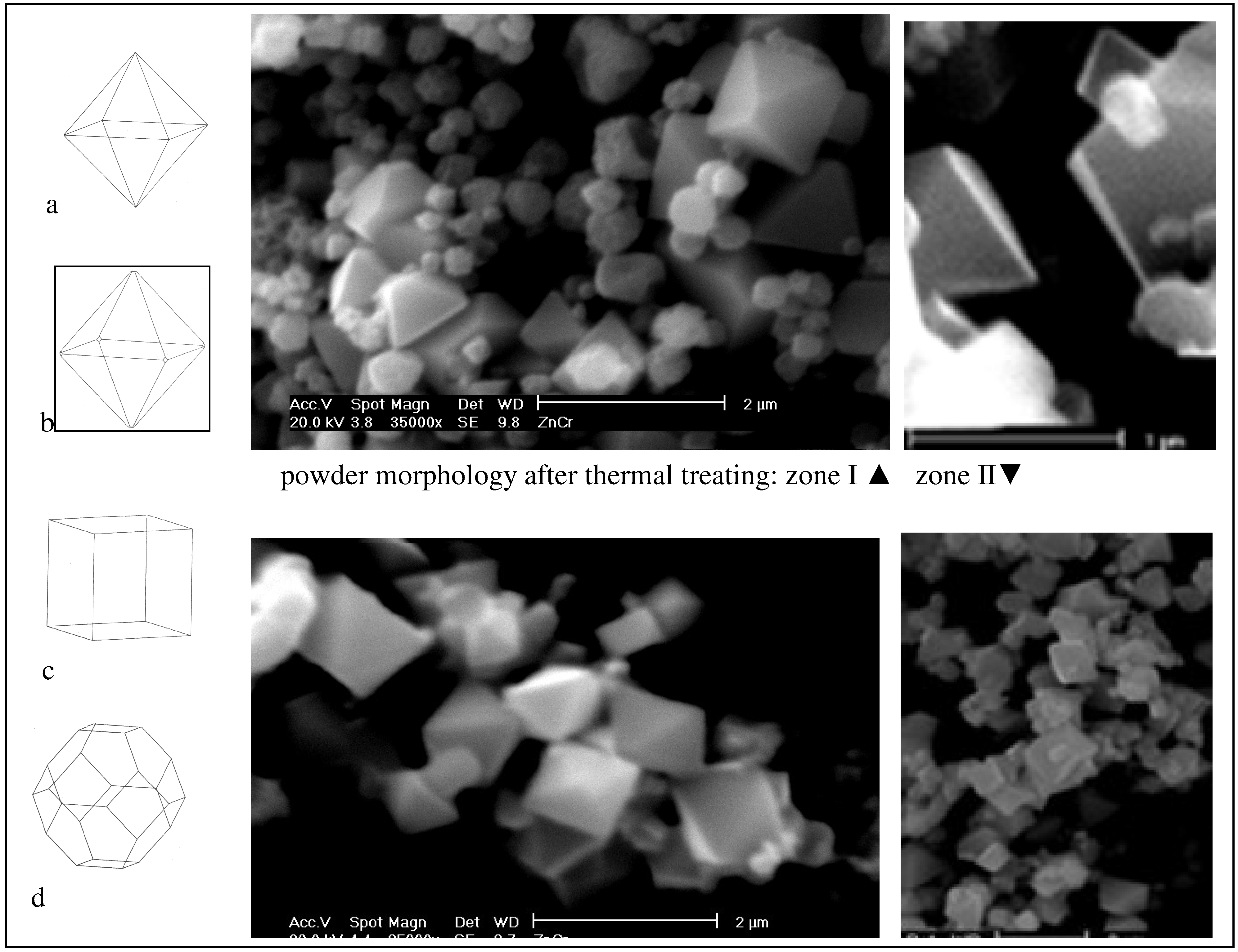
The same authors also used their description of the interactions between ions during spinel formation to predict the resultant crystal morphology of ZnCr
2O
4 [
7]. In this study crystallites are predicted to be essentially octahedral with the (111) surface dominating, stabilized by the presence of defects on surfaces. Shapes of the crystallites could be determined by two separate methods based on attachment and surface energies calculation, see
Fig.4a-d. SEM micrographs of powders after thermal treatment presented in the same figure confirms that the observed particle morphology is one of those predicted (
Fig.4b) that is associated with the calculation based on the growth of a highly defect surface structure. This relaxed growth mode was controlled by the slowest growing surface (111) in combination with tiny cups originated from (220) planes.
This significant microstructural and morphological change observed through the development of octahedral forms with the base under 1μm dominate the crystallite morphology. In addition, spherical particles and irregular shaped aggregates, which additionally densify during thermal treatment, are visible too,
Fig.4.