Introduction
The field of ion-selective electrodes has been growing fast since the past tow decades[
1,
2,
3,
4,
5,
6,
7,
8]. These have been the subject of interest for analytical chemists as they provide accurate, rapid and low cost method of analysis. This technique is nondestructive, adaptable to very small sample volume and online monitoring is also possible by these devices[
9,
10,
11,
12,
13,
14,
15]. Some commercialized sensors for alkali and alkaline earth metals, halides, etc. are available. However, more efforts are required to develop ion-selective electrodes of commercial standards for heavy metal ions, which are toxic beyond a certain concentration level[
16].
Thorium is present in very small quantities in virtually all rock, soil, water, plants and animals. Where high concentrations occur in rock, thorium may be mined and refined, producing waste products such as mill tailings. If not properly controlled, wind and water can introduce the tailings into the wide environment[
17,
18]. If inhaled as a dust, some thorium may remain in the lungs for long periods of time, depending on its chemical form. If ingested, thorium typically leaves the body through face and urine within several days. The small amount of thorium left in the body will enter the blood stream and be deposited on the bones where it may remain for many years. Studies have shown that inhaling thorium dust causes an increased risk of developing lung cancer, and cancer of the pancreas. Bones cancer risk is also increased because thorium may be stored in bone[
19,
20,
21]. Hence the development of new methods for selective separation, concentration and determination of thorium[
22,
23,
24,
25] in submicron levels is still a challenging task.
In recent years, various methods for determining Th
4+ such as spectrophotometry[
26,
27,
28,
29], electrothermal atomic emission spectrometry[
30,
31], inductively coupled plasma atomic emission spectrometry (ICP-AES)[
32,
33], mass spectrometry (MS)[
34,
35,
36], neutron activation analysis[
37,
38], X-ray fluorescence[
39,
40,
41] and voltametric, and amperometric techniques[
42,
43], have been developed. However these methods require expensive instruments, well-controlled experimental conditions, and frequent maintenance and calibration. The potentiometric sensors, on the other hand have the advantages of easy and convenient fabrication, enhancing sensitivity, good selectivity and low coast and they are widely used in analytical chemistry. For these reasons, sensors are convenient, and an optical thorium sensors have been reported[
44,
45,
46]. However, literature survey showed that, only one potentiometric thorium electrode has been reported, in which zirconium phosphate was used as an electroactive material[
47]. Nevertheless, potentiometric sensors based on inorganic ion exchangers, generally have a lack of selectivity to primary ions.
The present paper deals with the development of a new thorium PVC membrane based on thorium-oxinate ionophore. This membrane was used in the preparation of a coated graphite rod thorium electrode. In order to optimized the response characteristics of the developed electrode, the used ionophore was prepared by tow different methods. The first one is the homogenous solution method, which produces a thorium precipitate with superior physical characteristics, and the second is a solid-solid reaction (free solvent) method[
48], in which two macroscopic (Th(NO
3)
4.5H
2O) and (8-Hydroxyquinoline) solids interact directly to produce a solid thorium 8-Hydroxyquinolate ionophore without intervention of a liquid or vapor phase. Under the optimum conditions the sensitivity, reproducibility and stability offered by this simple electrode configuration are high enough to allow accurate determination of low levels of thorium. The selectivity coefficient measurements for many tested cations are negligibly small. The sensor is used to determine thorium content in a real monazite sand samples which give results in a good agreement with those obtained by the standard method[
49,
50,
51].
Experimental
Reagent and materials
All chemicals used were of analytical reagent grade unless otherwise stated; and doubly-deionized water was used throughout. Poly(vinyl chloride) powder, dioctylphthalate (DOP), tetrahydrofuran (THF), acetic acid, sodium acetate and ammonium acetate were obtained from Aldrich Chemical Co. Thorium nitrate (pentahydrate) was a highly purified product (Code 103, Lindsay Chemical Co., West Chicago, Illinois,U.S.A.). 8-Hydroxyquinoline (oxine) was obtained from Fluka AG(Buchs, Switzerland). A freshly prepared thorium nitrate solution of 10-1 mol l-1 was prepared by dissolving 5.88 g thorium nitrate in 100 ml of acetic acid-sodium acetate buffer solution (0.05 mol l-1 pH 3.65 ). A stock 0.2 mol l-1 8-hydroxyquinoline solution was prepared by dissolution 2.5 g of the reagent in 6 ml of glacial acetic acid and diluting with water to 100 ml.
Apparatus and Conditions
The potential measurements were carried out at 25±1 °C using Hanna pH/mV meter (model 8417) with coated graphite rod PVC based thorium electrode in conjunction with a single- junction Ag/AgCl reference electrode containing 10 % (w/v) potassium chloride in its compartment. A combined glass pH electrode (Hanna HI 1131B) was used for all pH measurements. Freshly prepared 10-1 mol l-1 thorium nitrate solution was diluted to 1x10-2 – 1x10-8 mol l-1 using variable Epindorf micropipettes. The digestion of monazite samples was carried out by using a microwave oven (model 7165/195) O.I. Analytical, USA. Thorium content in the real monazite sand sample was measured spectrophotometrically by Shimadzu UV-VIS Spectrophotometer (model 1601), using 1cm Quartz cells. The absorbances were measured in the range from 400 to 800 nm., at 25 °C in air- conditioned laboratory. The cell were carefully cleaned with dilute HNO3 acid washed with distilled water and then rinsed with the used solution. The out-put of the spectrophotometer gives a spectrum which represent the absorbace against the wavelength in nm.
Thorium oxinate coated graphite rod based sensor
Thorium 8-hydroxyquinolate ionophore was prepared by two different methods; the first one is the homogenous method (PFHS), in which 5 ml of a 2 mol l-1 acetic acid solution were added to 25 mg of thorium nitrate in a 250 ml of doubly-deionized water, and the resulting solution was added to 15 ml of 0.2 mol l-1 8-hydroxyquinoline. The resulting mixture was then heated to 90 °C , and a 50 ml of ammonium acetate solution was added with continuous stirring. The solution was cooled to room temperature before filtering. The second method includes a solid-solid reaction, in which a total of 25 mg of Th(NO3)4.5H2O and 100 mg of 8-hydroxyquinoline were properly grounded together at 25±1 °C for 10 h. The obtained orange yield was washed by 2 mol l-1 acetic acid, to remove the excess from 8-hydroxyquinoline. Infrared analysis data of the two ionophore individually prepared by the two mentioned methods agreed with the formation of 8-hydroxyquinolate complex. These two ionophores were used as sensing materials in the preparation of two different membrane coating mixtures. These mixtures were - in turn- used in the preparation of two different graphite rod based electrodes.
The electrode coating mixture was prepared by mixing a 10 mg portion of the prepared thorium 8-hydroxyquinolate, Th(C9H6NO)4.2H2O ionophore with 350 mg of dioctylphthalate, 190 mg PVC and 6 ml THF in a 25 ml glass beaker. This mixture was transferred to a small tube (3 ml). A graphite rod (0.5 cm diameter and 2 cm long) used as a conducting substrate was dipped in the membrane coating mixture and, after evaporation of the solvent, the procedure was repeated until a uniform layer of the membrane was obtained on the rod. The electrodes were initially conditioned by soaking in a 1x10-3 mol l-1 thorium nitrate solution overnight. When not in use these electrodes were stored in a similar solution and between measurements the electrodes were washed by doubly distilled water.
Characterization and analytical application of the electrode
The electrode potential was measured at 25±1 °C with thorium nitrate solution covering the range 1x10-1 – 1x10-8 mol l-1, by transferring 10 ml of these solutions to 25 ml glass beakers, followed by immersing the thorium-oxinate based sensor in conjunction with a Ag/AgCl reference electrode in each solution. The dilute solutions series were freshly prepared, from the stock immediately before measurements and were stirred during measurements. The potential readings were recorded after stabilization (±0.5 mV) and the mV values were plotted as a function of the logarithm of thorium ion concentration.
The proposed electrodes were used in the determination of thorium content in the real monazite sand samples by direct potentiometric method using a calibration graph performed at the same day. Standard measurements of thorium content in these samples were also performed by arzenazo III spectrophotometric method[
49,
50,
51] for comparison.
The procedure used for the digestion and separation of thorium from monazite sand sample was similar to that reported in the literature. After the digestion process, the separation of thorium from the sample was performed by raising the pH to pH 1 by the addition of a concentrated ammonium hydroxide solution this is followed by a 10 % of oxalic acid solution to precipitate thorium oxalate, the clean dried precipitate was then dissolved in 2 mol l-1 HNO3. The concentration of thorium in standard solutions of thorium nitrate were also determined using calibration graphs performed at the same conditions.
Electrode selectivity coefficient measurements
The potentiometric selectivity coefficient of thorium-oxinate based thorium sensors were determined by the separate solution method (SSM)[
52,
53]. The potential readings (mV) of two separate solutions; one containing only the thorium ion at the concentration level of 10
-3 mol l
-1, the other containing the interferent cations at the same concentration level were measured.
The selectivity coefficients
were calculated using the equation (1)[
54]:
Where
and
are the observed potentials (mV) for the same concentration of
ion and interfering ionic species, respectively;
is the activity of
,
and
are the charge number of thorium and the interfering ion B, respectively.
Response time measurements
The response time of the proposed electrodes was measured by the spiking calibration method, based on inducing a rapid changes of a stirred 1x10-6 mol l-1 thorium nitrate solution by injecting a more concentrated thorium nitrate solution of 1x10-1 mol l-1. The concentration of thorium was increased by adding aliquots of a standard solution of thorium nitrate to 10 ml of a stirred test solution, with a micropipet. The potential readings were recorded against time (min).
Gamma- irradiation of thorium-oxinate based electrode
Freshly prepared thorium-oxinate graphite rod based electrodes were irradiated using 60Co (Gamma chamber 4000A) at the National Center for Radiation Research and Technology, Cairo, Egypt with a dose rate of 12.5x103 Gy/min. (at January, 2003). The sensors were exposed to doses of 102, 104 and 106 Gy. Irradiated electrodes were potentiometrically calibrated after irradiated immediately.
Results and Discussion
Thorium nitrate readily reacts with 8-hydroxyquinoline to form thorium 8-hydroxyquinolate (thorium-oxinate) complex by two different methods, the first one is homogenous solution method (PFHS) which is a convenient method for the preparation of this complex in relatively large crystalline and better physical form than that obtained by a direct method of addition of 8-hydroxyquinoline. The second is the solid-solid reaction method, in which the two previous component capable of a chemical reaction are grounded in the absence of a solvent “solvent free”.
A chemical reaction thus occurs in the solid phase, that proceed rapidly and to a high degree of completion between two solid reactants actually occur in the solid state. Instead, a liquid or a melt phase, which imbues the individual molecules with the required mobility reactive collision, intervenes, allowing rapid reaction between the two solid reagents. These reactions should therefore be classified together with classical liquid / liquid and liquid solid system that react in the absence of an added solvent. The elemental analysis data of the ionophore agree with the composition of Th(C9H6NO)4.2H2O. The composition of this ionophore obtained from the two previous methods is identical.
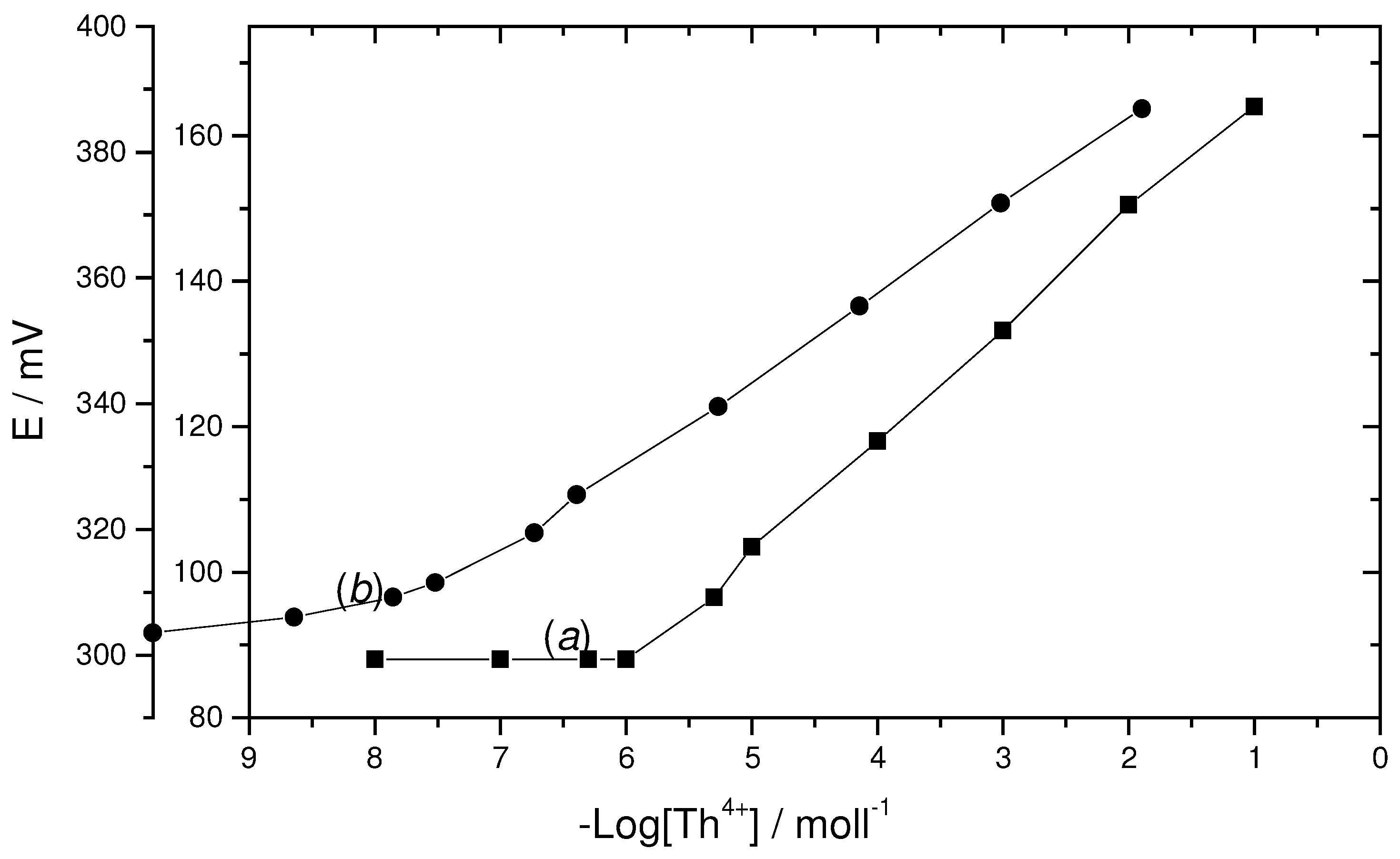
Calibration curve and statistical data
Two different thorium-oxinate [Th(C
9H
6NO)
4.2H
2O] coated graphite rod based electrodes were individually prepared. The electrochemical performance characteristics of these electrodes were evaluated according to IUPAC recommendations, and the results are given in
Table 1. The potential responses of the electrodes show a linear response to the concentration of Th
+4 ion in the range 5x10
-6 to 1x10
-1 mol l
-1 with a cationic slope of 15.5±0.5 mV per concentration decade (
Figure 1). The limit of detection, as determined from the intersection of the two extrapolated segments of the calibration graph was 1.6x10
-6 mol l
-1. Although the developed two electrodes have nearly, similar behavior, the solid-solid reaction based ionophore electrode provides the best response characteristics (i.e; slope of 16±0.5 mV and detection limit 5x10
-7). The standard deviation of 10 replicate measurements is ±0.5 mV. The coated graphite rod electrodes could be used for at least 2 months without any measurable change in potential, provided that the electrodes were stored in 1x10
-3 mol l
-1 thorium ion solution when not in use. Moreover the membrane can be easily regenerated daily by dipping the peeled graphite rod in the stock solution of membrane coating mixture.
Table 1.
Potentiometric response characteristics of the coated graphite rod based thorium electrodes.
Table 1.
Potentiometric response characteristics of the coated graphite rod based thorium electrodes.
| Parameter | Homogenous method based ionophore | solid-solid reaction based ionophore |
| Slope (mV/decade) | 15.5±0.5 | 16.0±0.5 |
| Lower limit of | | |
| detection, mol l-1 | 1.6x10-6 | 5x10-7 |
| Response time, s | 30 | 30 |
| Working pH range | 3-5 | 3-5 |
| Linear range, moll-1 | 5x10-6 – 1x10-1 | 1x10-6 – 1x10-1 |
| Life time (month) | 2 | 2 |
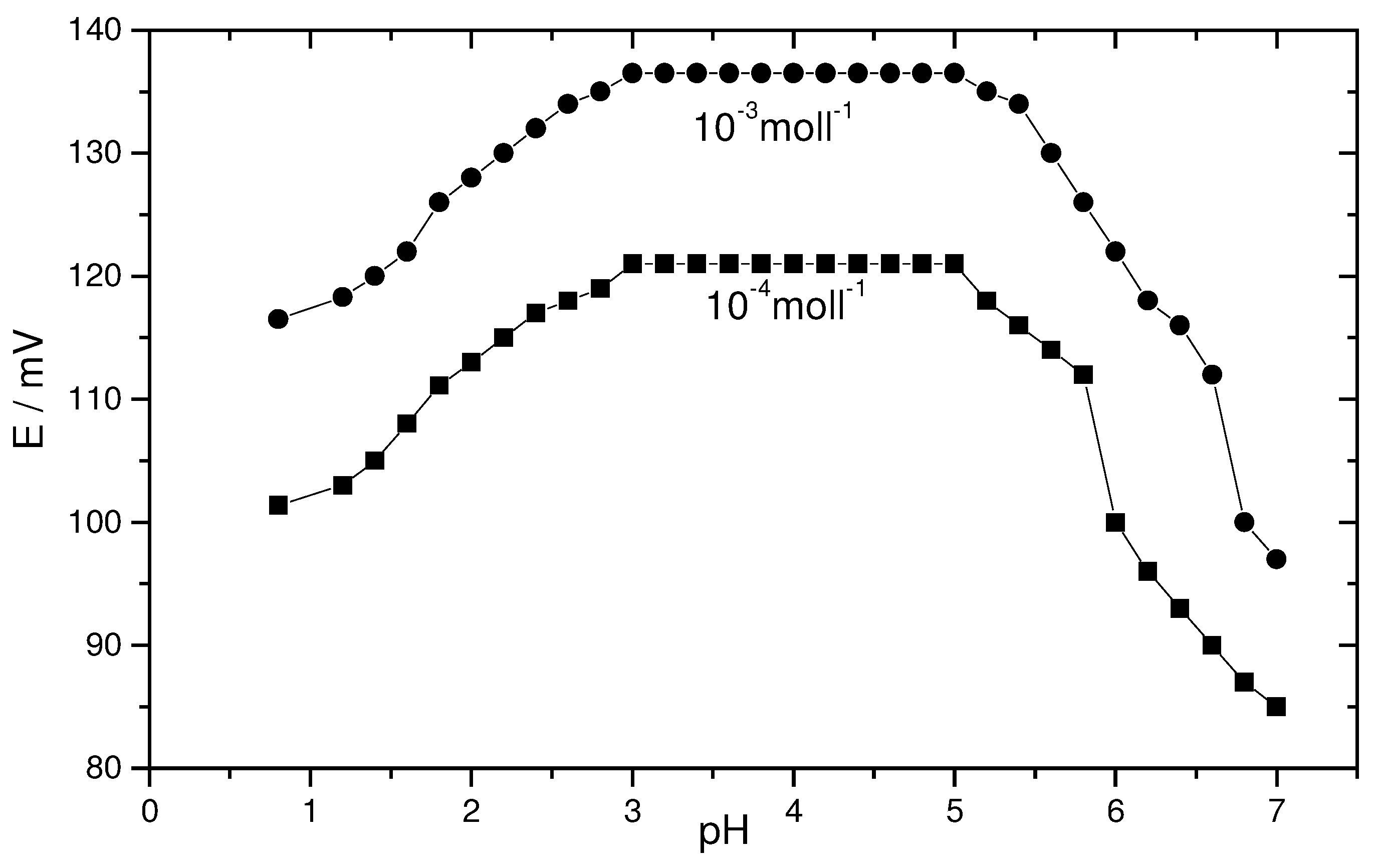
Effect of pH on electrode response
The pH effect of the test solutions on the potential response of the proposed two electrodes was investigated over the pH range 1-7 for 1x 10
-3 and 1x10
-2 mol l
-1 of Th
+4 ion solutions. The data obtained show that, the two electrodes gave similar pH plots, therefore, only one pH graph is plotted for simplicity. As shown in
figure 2, the potential was independent of the pH in the range 3 - 5, at a pH value higher than 5, the potential decreases due to the precipitation of thorium hydroxides in solution, while at pH lower than the mentioned range, the potential difference falls sharply which may be due to the dissolution of the electrode surface in the strong acidic medium.
Figure (1).
Potentiometric calibration response of the coated graphite rod based thorium electrodes. a:homogenous method-based ionophore and b: solid-solid reaction ionophore.
Figure (1).
Potentiometric calibration response of the coated graphite rod based thorium electrodes. a:homogenous method-based ionophore and b: solid-solid reaction ionophore.
Selectivity properties of the electrode
Figure (2).
Effect of pH on the potentiometric response of the coated graphite rod-based thorium electrode.
Figure (2).
Effect of pH on the potentiometric response of the coated graphite rod-based thorium electrode.
Selectivity is the most important characteristic that defines the nature of a device and the range to which it may be successfully employed. The selectivity coefficient values of the proposed two electrodes were determined against a number of interfering rare earth cations, alkali, alkaline earth cations and heavy metal ions using the separate solution method[
52,
53] with fixed 10
-3 mol l
-1 test solution of both thorium and interferent ions. The obtained data presented in
Table 2 show that, the proposed two electrodes exhibit a comparable and good selectivity coefficient values for thorium ion over most of the tested common species. Fortunately, these values are superior to those reported for the other thorium ion-selective electrode[
47]. It is also worth mentioned that the potentiometric response of the proposed electrodes were found to be insensitive to the nature of the anions used.
Table 2.
Potentiometric selectivity coefficients () of the coated graphite rod based thorium electrode.
Table 2.
Potentiometric selectivity coefficients () of the coated graphite rod based thorium electrode.
| Interferent Cations | Selectivity coefficients () |
| Homogenous method based ionophore | solid-solid reaction based ionophore |
| | | |
| Mn2+ | 3.7x10-4 | 2.9x10-4 |
| NH4+ | 2.7x10-4 | 1.7x10-4 |
| Co2+ | 3.0x10-4 | 8.0x10-4 |
| Cd2+ | 2.3x10-4 | 1.1x10-4 |
| Ni2+ | 5.7x10-4 | 2.3x10-4 |
| Cr3+ | 9.0x10-4 | 8.3x10-4 |
| Cu2+ | 2.0x10-4 | 3.9x10-4 |
| Zn2+ | 9.7x10-4 | 1.0x10-4 |
| Ca2+ | 8.0x10-4 | 1.5x10-4 |
| Mg2+ | 7.4x10-4 | 1.9x10-4 |
| Sn2+ | 4.9x10-4 | 1.4x10-4 |
| K+ | 2.6x10-4 | 2.2x10-4 |
| Na+ | 3.0x10-4 | 8.0x10-4 |
| Fe3+ | 4.6x10-4 | 2.0x10-4 |
| Pb2+ | 4.0x10-4 | 2.2x10-4 |
| UO22+ | 4.0x10-4 | 1.8x10-4 |
| Nd3+ | 2.2x10-4 | 6.9x10-4 |
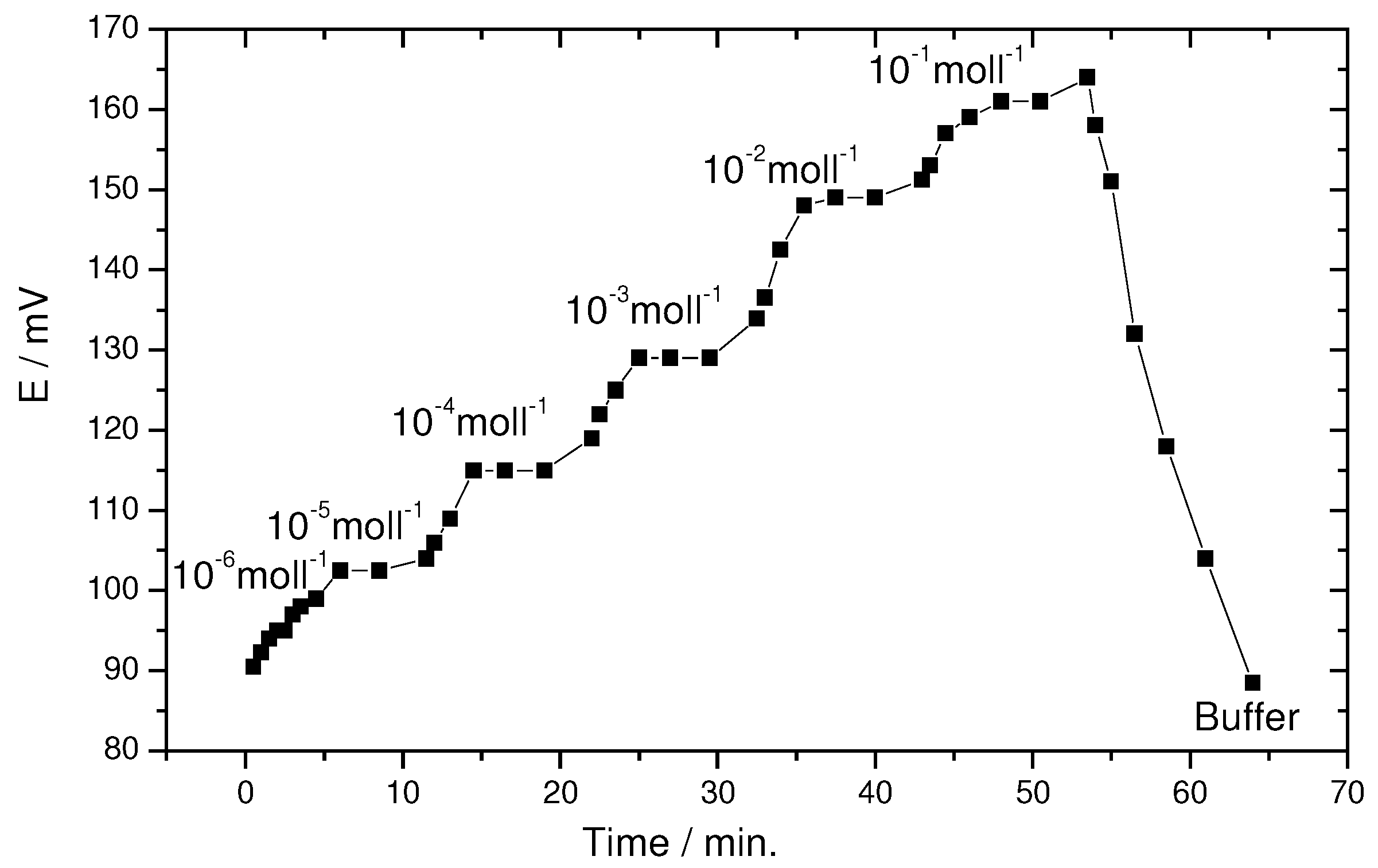
Dynamic response time and stability of the electrode
The response time and stability measurements have been performed for the two proposed electrodes. The results obtained provide that the two electrodes have a similar behavior. Therefore, the response time of only one electrode was reported for simplicity. In this study, the response time was recorded by changing the Th
4+ ion concentration in the test solution, over a concentration range of 1x10
-1 – 1x10
-6 mol l
-1. The average time required for the electrode to reach a potential within ±1 mV of the final equilibrium value after successive immersion of the electrode in a series of Th
4+ ions solutions, each having 10-fold difference in concentration was found to be 30 s. The potential response versus time traces is shown in
figure 3. The results that obtained are indicative of a rapid diffusion in the achievement of equilibrium between the aqueous layer and the membrane sensor, and rapid complex formation and exchange of ions in the membrane[
55]. The proposed sensor shows a good reproducibility and stability of the potential over a period of at least 2 months without any measurable divergence in the calibration slope, provided that the sensor was stored in 1x10
-3 mol l
-1 thorium ion solution when not in use. After this period, the electrode response becomes sluggish and decreases by about 25% after every 15 days. The response time and calibration curve have clearly indicated that the sensitivity and reproducibility of the membrane are reliable.
Figure (3).
Dynamic response time of the coated graphite rod based thorium electrode.
Figure (3).
Dynamic response time of the coated graphite rod based thorium electrode.
Effect of Gamma Radiation
The stability of thorium-oxinate based electrodes towards gamma radiation was demonstrated by irradiation of a set of three electrodes under investigation, at doses of 10
2, 10
4 and 10
6 Gy. The results obtained (
Figure 4 and
Figure 5) revealed that the potentiometric calibration response of thorium-oxinate based electrodes before and after gamma irradiation at doses up to 10
4 Gy was differed. All the potentiometric response characteristics as well as the initial potential of the two electrodes were significantly affected by irradiation up to 10
6 Gy.
Analytical applications
Figure (4).
The effect of gamma radiation on the potentiometric calibration response of homogenous method ionophore based electrode.
Figure (4).
The effect of gamma radiation on the potentiometric calibration response of homogenous method ionophore based electrode.
Figure (5).
The effect of gamma radiation on the potentiometric calibration response of solid-solid reaction ionophore based electrode
Figure (5).
The effect of gamma radiation on the potentiometric calibration response of solid-solid reaction ionophore based electrode
The proposed
ion-selective electrodes were found to work well under the laboratory conditions. In order to investigate the reliability of the developed two thorium electrodes, they were successfully applied to the determination of thorium content in real monazite sand and standard samples. The real samples were properly digested, treated and analyzed by the direct potentiometric method using a calibration graph performed at the same day. The thorium content of the tested real and standard samples were also determined by a standard technique UV-VIS arzenazo III[
49,
50,
51] for comparison. The results obtained presented in
Table 3 and
Table 4, provide that the developed two thorium electrodes have a similar and good reliability. The average recovery was 97.0 - 93.4 %, and the mean standard deviation was 1.5 % (n=8).
Table 3.
Potentiometric recovery studies on homogenous method ionophore-based coated graphite rod thorium electrode.
Table 3.
Potentiometric recovery studies on homogenous method ionophore-based coated graphite rod thorium electrode.
| Sample | Conc. of Th4+ / mol l-1 | Recovery, % |
| Reference methoda | (ISE) b |
| | | | |
| S1 | 3.62x10-3 | 3.52x10-3 | 97.2 |
| S2 | 2.71x10-3 | 2.62x10-3 | 96.6 |
| S3 | 1.80x10-3 | 1.75x10-3 | 97.2 |
| S4 | 9.05x10-4 | 8.75x10-4 | 96.6 |
| S5 | 7.24x10-4 | 6.95x10-4 | 95.9 |
| S6 | 5.40x10-4 | 5.29x10-4 | 97.8 |
| S7 | 3.62x10-4 | 3.54x10-4 | 97.7 |
| S8 | 1.80x10-4 | 1.75x10-4 | 97.2 |
| S9 | 9.05x10-5 | 8.78x10-5 | 97.0 |
| | | | |
| T1 | 2.71x10-3 | 2.65x10-3 | 97.7 |
| T2 | 2.72x10-3 | 2.64x10-3 | 97.0 |
| Average recovery = 97.0 % |
Table 4.
Potentiometric recovery studies on solid-solid reaction ionophore coated graphite rod thorium electrode.
Table 4.
Potentiometric recovery studies on solid-solid reaction ionophore coated graphite rod thorium electrode.
| Sample | Conc. of Th4+ / mol l-1 | Recovery, % |
| Reference methoda | (ISE) b |
| | | | |
| S1 | 3.62x10-3 | 3.42x10-3 | 94.47 |
| S2 | 2.71x10-3 | 2.55x10-3 | 94.09 |
| S3 | 1.80x10-3 | 1.69x10-3 | 93.9 |
| S4 | 9.05x10-4 | 8.35x10-4 | 92.26 |
| S5 | 7.24x10-4 | 6.66x10-4 | 91.9 |
| S6 | 5.40x10-4 | 4.99x10-4 | 92.4 |
| S7 | 3.62x10-4 | 3.44x10-4 | 95.02 |
| S8 | 1.80x10-4 | 1.68x10-4 | 93.3 |
| S9 | 9.06x10-5 | 8.35x10-5 | 92.16 |
| | | | |
| T1 | 2.71x10-3 | 2.56x10-3 | 94.46 |
| T2 | 2.72x10-3 | 2.55x10-3 | 93.7 |
| | Average recovery = 93.4% |