Results and Discussion
Our investigations were devoted to development and investigation of corresponding technologies and regimes for obtaining of relatively thermally stable nano-sized SnO2 particles and thin films on its base.
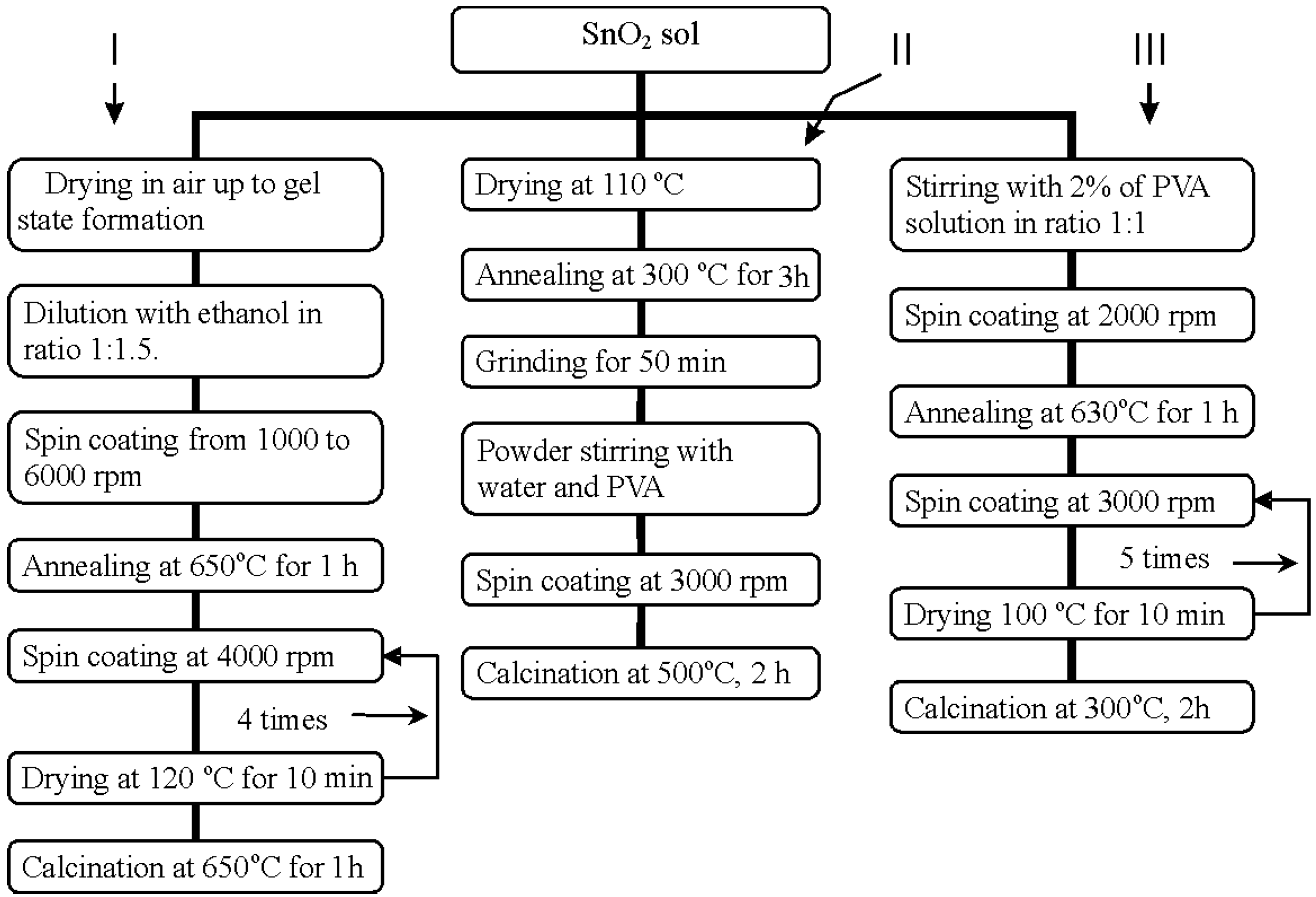
Tin oxide was obtained by two techniques: 1) tin chloride hydrolysis and 2) hydrolysis of sodium stannate with phosphoric acid. On the first technique, the 1.2M SnCl
4 solution added dropwise in the 1% ammonia aqueous solution at steady mixing. Resulting precipitate was repeatedly washed by distilled water to get rid of Cl
- ions. The resulting tin dioxide sol was divided on 3 parts. Different experiments were carried out with each of parts connected with change of substrates, material and size of previously deposited interdigital electrodes, sol deposition regimes, annealing temperatures etc (see
Fig. 1). Common demerit all these films is the fact that finally they prepared from suspense consisted of agglomerates of SnO
2 particles. As a result, it is difficult to obtain uniform film without abruptions and cracks by both dipping and spinning.
Figure 1.
Flow chart for different modifications of SnO2 thin films preparation by SnCl4 hydrolysis.
Figure 1.
Flow chart for different modifications of SnO2 thin films preparation by SnCl4 hydrolysis.
In accordance with second technique, the 1M solution of H
3PO
4 at continuous mixing was added to Na
2SnO
3 aqueous solution until neutralization of solution (pH=7) [
5]. In this case, absolutely transparent very stable sol was obtained. As distinct from [
5], the solution does not cut even at centrifuge rate 14000 rpm. As a consequence, purification of that sol from phosphates by centrifuging was impossible. The solution was purified by means of electro dialysis in rectangular cell divided on three sections with ion-exchange membranes. Purified solution was filled into middle cell, whereas periodically replenished distilled water was in outermost cells. The process of electrodyalysis was accompanied by continuous stirring. At the result of sharp decrease in the electrolyte concentration during the process of the purification, the most part of SnO
2 precipitated near anode as a transparent gel. Therefore the sol particles are charged negative. Only about 0.1% of SnO
2 remained in the solution. Addition of ammonium to purified sol up to 1% concentration gives rise to the peptization of the sediment. At that, the solution with about 10%-concentration having exceptional steadiness with time (coagulation during fourth month was not observed) are obtained.
Acquired sol in mixture with the same quantity of 2% polyvinyl alcohol spin-on coated onto alumina and other substrates at 4000 rpm. The specimens dried in drying oven at the temperature of 100°C for 10 min whereupon the following layer deposited. This procedure repeated five times. After that, different samples were exposed to annealing at the various temperatures in the range from 500 to 750°C. As a result, homogeneous film without cracks with wholly satisfactory adhesion to substrate was obtained.
The investigations by the transmittance electron microscopy (TEM) Tesla BS 500 with 7 Å - resolution were carried out. The surface morphology of specimens obtained by the both above-mentioned methods is studied. The specimens were selected after various technological stages – after deposition of 1 and 5 SnO2 layers on the substrates of alumina, silica and sapphire with following calcinations at different temperatures.
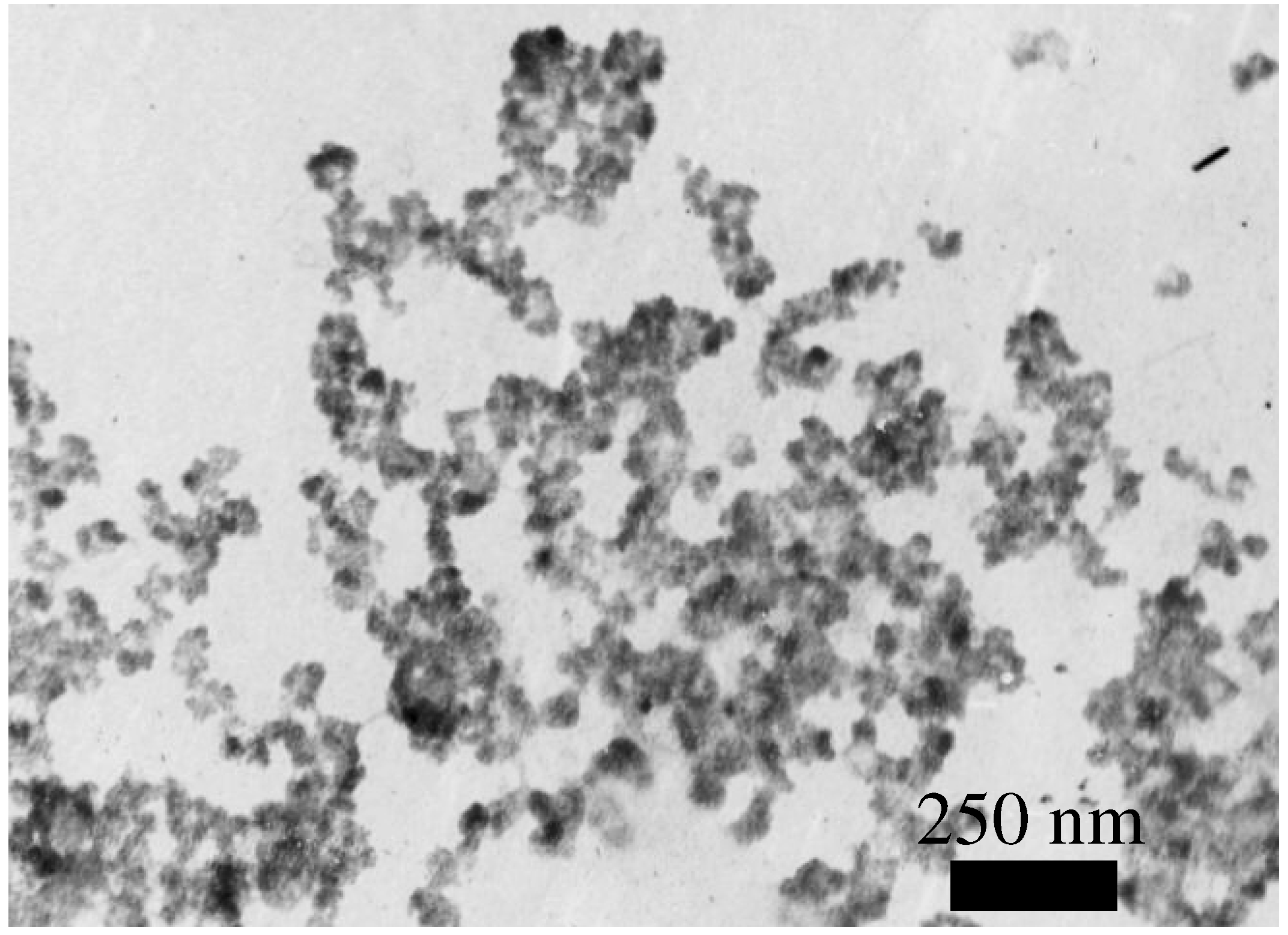
In
Fig. 2 TEM images of SnO
2 particles of powder obtained by tin chloride (IV) hydrolysis in the ammonium aqueous solution are shown. It can be seen that the particles are join together in agglomerations, which isolated islands are formed at the deposition on the substrate. At the second case, this is not observed (see
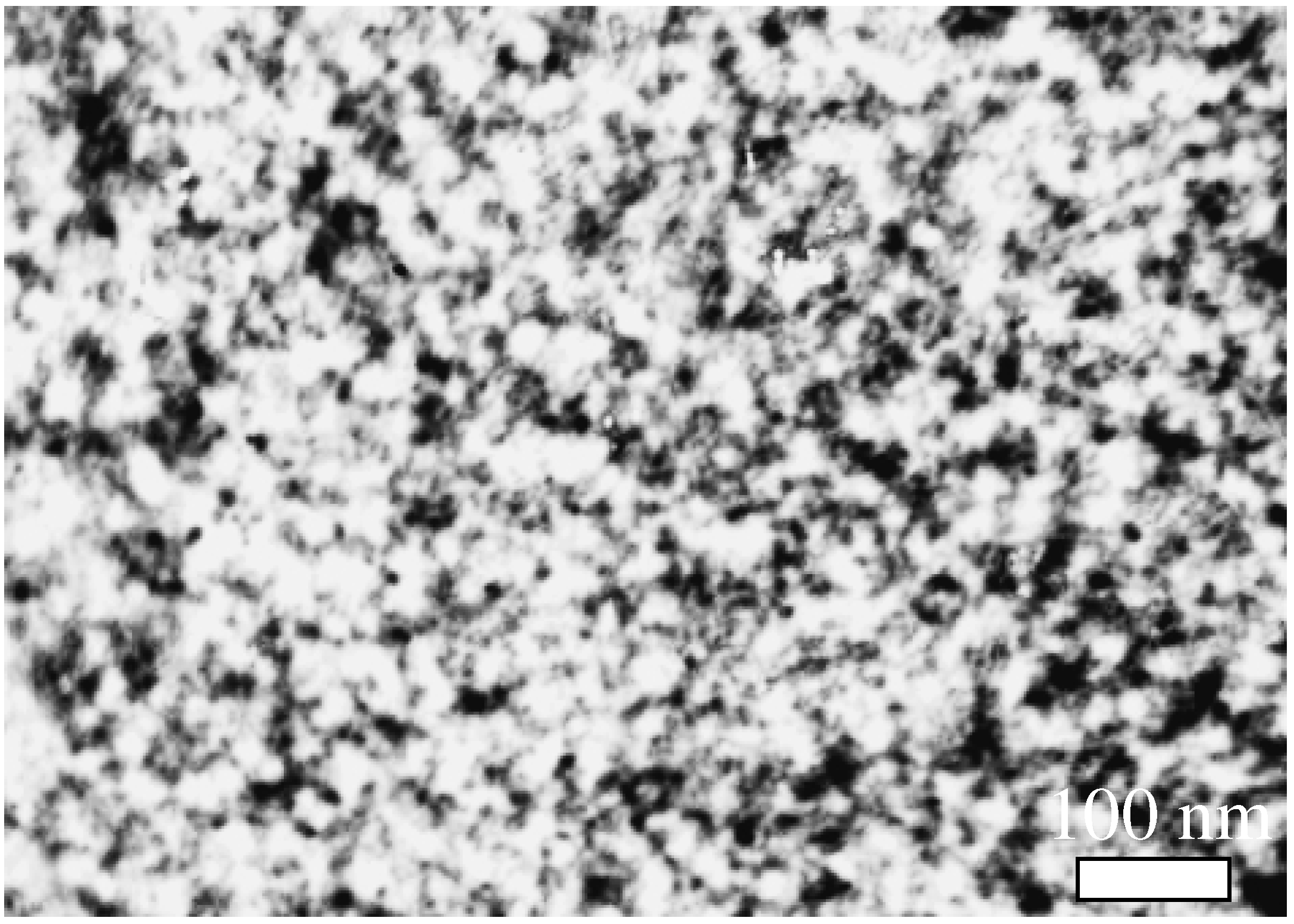
Fig. 3, where it is seen particles with average size 6-8 nm).
Figure 2.
TEM image of SnO2 particles of powder obtained by the SnCl4 hydrolysis methods.
Figure 2.
TEM image of SnO2 particles of powder obtained by the SnCl4 hydrolysis methods.
Figure 3.
TEM image of SnO2 film obtained by the hydrolysis of sodium stannate on glass substrate (G-250 sample). Minimal grain size ~6 HM.
Figure 3.
TEM image of SnO2 film obtained by the hydrolysis of sodium stannate on glass substrate (G-250 sample). Minimal grain size ~6 HM.
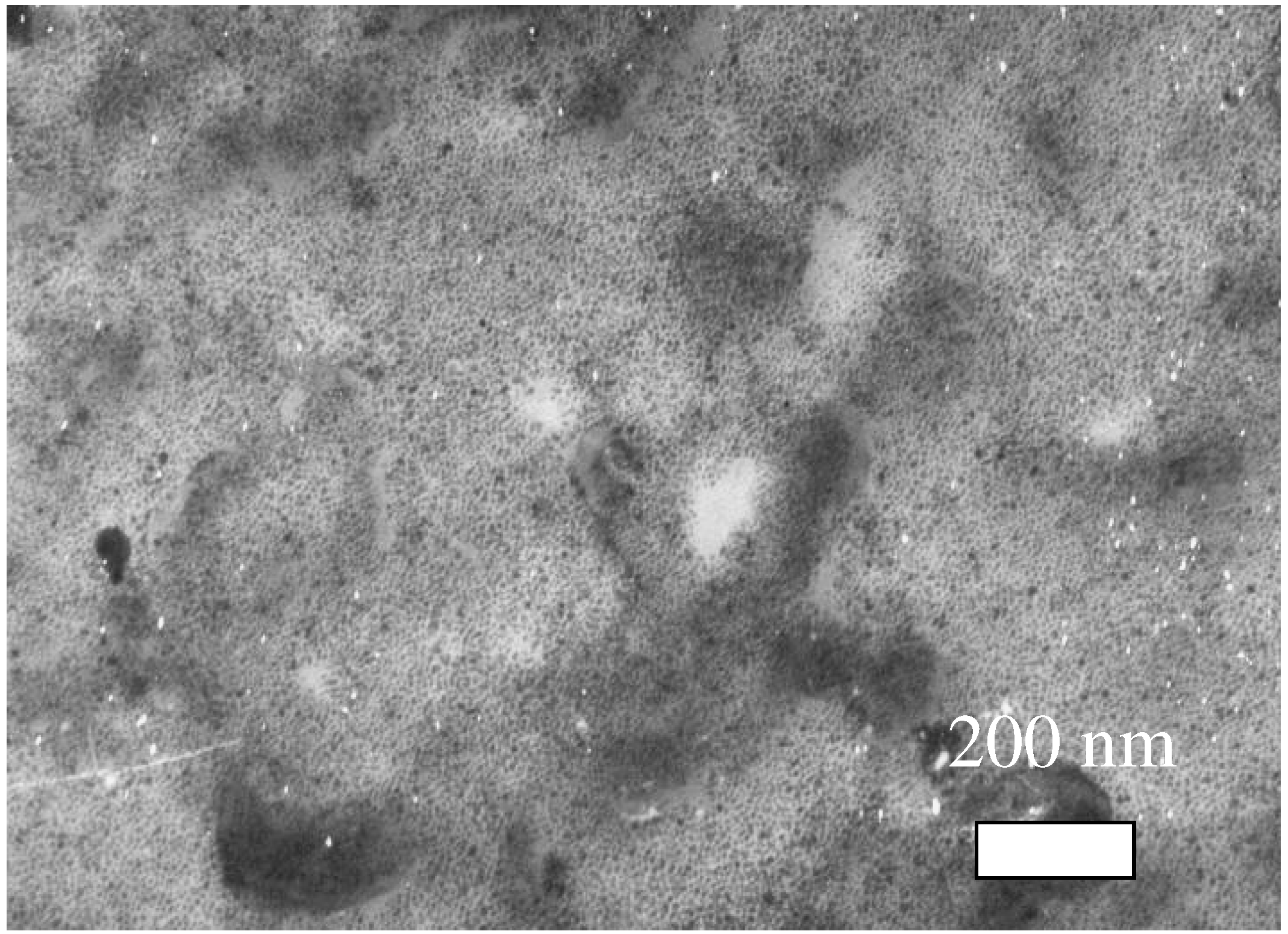
Figure 4.
TEM image of SnO2 film (Sph-500 sample). Minimal grain size ~4 nm.
Figure 4.
TEM image of SnO2 film (Sph-500 sample). Minimal grain size ~4 nm.
The solid-core pictures of the SnO
2 nano-sized particles, which were obtained due to an interaction between Na
2SnO
3 and H
3PO
4, appear in
Fig 3 and
Fig 4, where the sol spin coated 1 time but on the alumina substrate – 5 times (
Fig. 5). The investigated samples were selected according to the calcinations temperatures as well as by materials for substrates. The letters in the samples designations indicate on the substrates, but numbers – on the calcinations temperature.
Figure 5.
TEM image of SnO2 film (A-750 sample). Minimal grain size ~5 nm.
Figure 5.
TEM image of SnO2 film (A-750 sample). Minimal grain size ~5 nm.
The analysis of these images shown that dimensions of SnO2 particles changed a little in dependence of selected calcinations temperature in the range of 500-750 °C and their dimensions keep about the same as after calcinations at 250 °C. The experiment with various substrates revealed that films deposition on all used substrates possible without any problems. The best adhesion had the films deposited on the silica substrates.