Impedance Spectroscopy Technique for DNA Hybridization
Abstract
:Introduction
Experimental
Materials
Immobilization of DNA probe
Hybridization
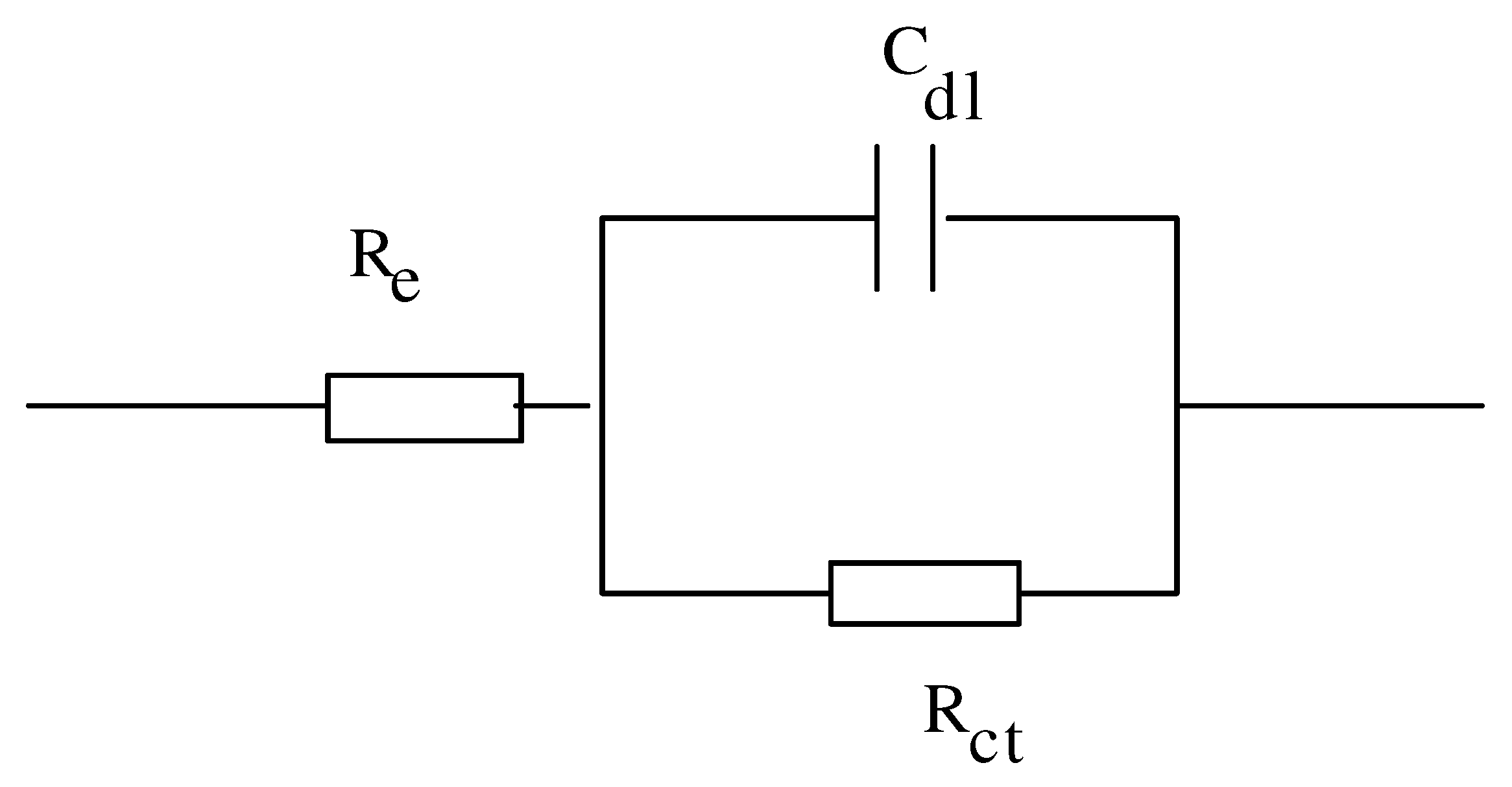
Impedance Spectroscopy
Resultas and discussion
DOT immobilization


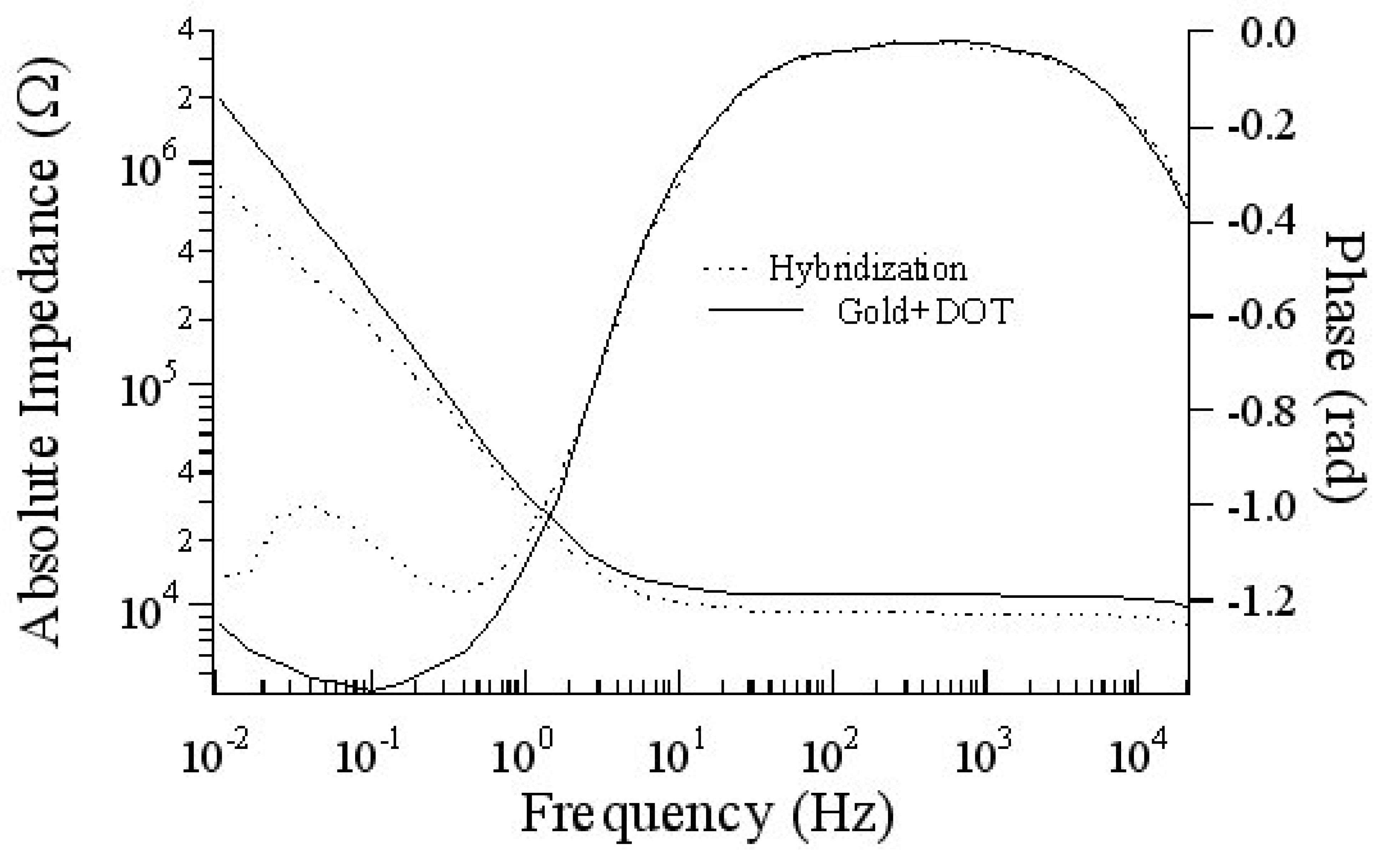
Specific interaction
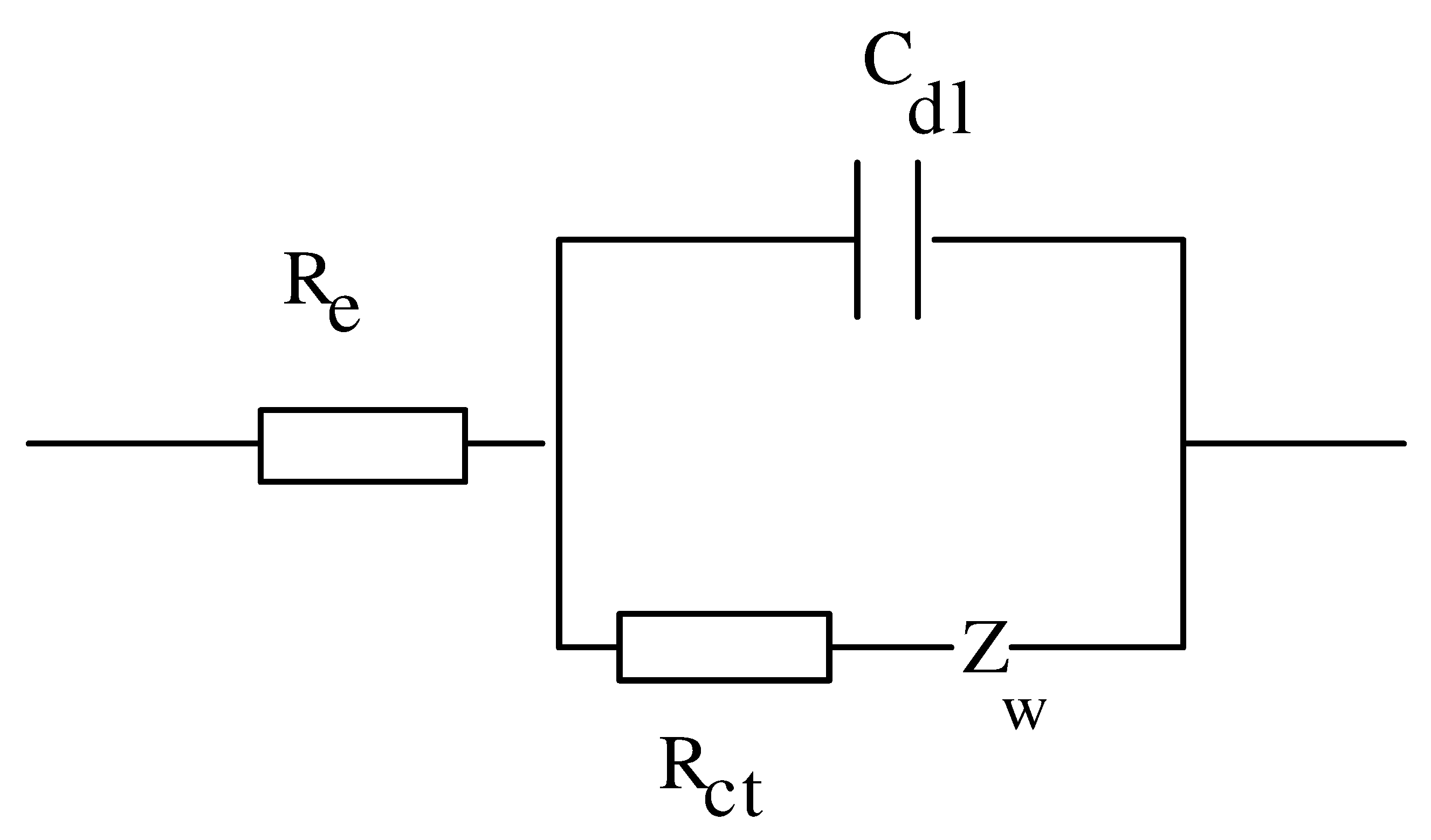
Hybridization
Conclusion
Acknowledgements
References
- Muratsugu, M.; Ohta, F.; Miya, Y.; Hosokawa, T.; Hosokawa, S.; Kamo, N.; Ikeda, H. J. Anal. Chem. 1993, 65, 2933–2937. [CrossRef] [PubMed]
- Streeghorn, C.; Skladalt, P. J. Biosensors &Bioelectronics 1997, 12, 19–27.
- Ketterer, T.; Stadler, H.; Rickert, J.; Bayer, E.; Gopel, W. J. Sensors and Actuators B 2000, 65, 73–75. [CrossRef]
- Okahata, Y.; Kawas, M.; Niikura, K.; Ohtake, F.; Furusawa, H.; Ebara, Y. J. Anal. Chem. 1998, 70, 1288–1296. [Google Scholar] [CrossRef] [PubMed]
- Caruso, F.; Rodda, E.; Neil Furlong, D. J. Anal. Chem. 1997, 69, 2043–2049. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.C.; Huang, L.Q.; Fong, S.; Li, Y. J. Biosensors &Bioelectronics. 2001, 16, 85–95. [Google Scholar]
- Okahata, Y.; Matsunobu, Y.; Ijiro, K.; Mukae, M.; Amurakami; Makino, K. J. Am. Chem. Soc. 1992, 114, 8299–8300. [Google Scholar] [CrossRef]
- George, G. Guilbault and Bertol Hock Anal. Letters. 1995, 28, 749–764. [Google Scholar]
- Marie, R.; Jensenius, H.; Thaysen, J.; Christensen, C.B.; Boisen, A. Ultramicroscopy. 2002, 91, 29–36. [Google Scholar]
- Koblinger, C.; Drost, S.; Aberl, F.; Wolf, H.; Koch, S.; Woias, P. J. J. Biosensors &Bioelectronics. 1991, 7, 397–404. [Google Scholar]
- Zilberman, G.; Tsionsky, V.; Gileadi, E. Electrochimica Acta. 2000, 45, 3473–3482. [Google Scholar]
- MacDonald, J.R. Impedance spectroscopy; John Wiley & Sons: New York, 1987. [Google Scholar]
- Hillebrandt, H.; Wiegand, G.; Sackmann, E. Langmuir. 1999; 15, 8451–8459. [Google Scholar]
- Wiegand, G. Fundamental principles of the electric properties of supported lipid membranes investigated by advanced methods of impedance spectroscopy. Ph.D. thesis, Shaker verlag, Technishe Universitat of Muenchen, Germany, 1999. [Google Scholar]
- Hillebrandt, H.; Abdelghani, A.; Abdelghani-Jacquin, C.; Aepfelbacher, M.; Sackmann, E. Appl Phys A. 2001, 73, 5, 539–546. [Google Scholar]


Sample Availability: Available from the authors. |
© 2003 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Hleli, S.; Abdelghani, A.; Tlili, A. Impedance Spectroscopy Technique for DNA Hybridization. Sensors 2003, 3, 472-479. https://doi.org/10.3390/s31000472
Hleli S, Abdelghani A, Tlili A. Impedance Spectroscopy Technique for DNA Hybridization. Sensors. 2003; 3(10):472-479. https://doi.org/10.3390/s31000472
Chicago/Turabian StyleHleli, S., A. Abdelghani, and A. Tlili. 2003. "Impedance Spectroscopy Technique for DNA Hybridization" Sensors 3, no. 10: 472-479. https://doi.org/10.3390/s31000472



