Amperometric Metronidazole Sensor Based on the Supermolecular Recognition by Metalloporphyrin Incorporated In Carbon Paste Electrode
Abstract
:Introduction
Experimental
Materials and Reagents
Synthesis of T(o-glu)PPH2

Synthesis of MnT(o-glu)PPCl
Apparatus
Sample Preparation
Preparation of Mnt(O-Glu)TTCl Modified Electrode
Renewal of the Sensor Surface
Measurement with Prepared Sensor
Results and Discussion
Selection of Active Materials for Recognition Element in Electrode
| Electrode* | Modifier | Working range (M/L) | Slope(μA/decade) |
|---|---|---|---|
| 1# | FeTPPH2 | 2.6 ×10-8—1.3 ×10-3 | 27.5 ± 1.8 μA |
| 2# | T(o-glu)PPH2 | Sluggish response | ------ |
| 3# | MnT(o-glu)PPCl | 5.8× 10-8—2.9 ×10-3 | 25.2 ± 1.3 μA |
Response Characteristics of the MTZ Sensor
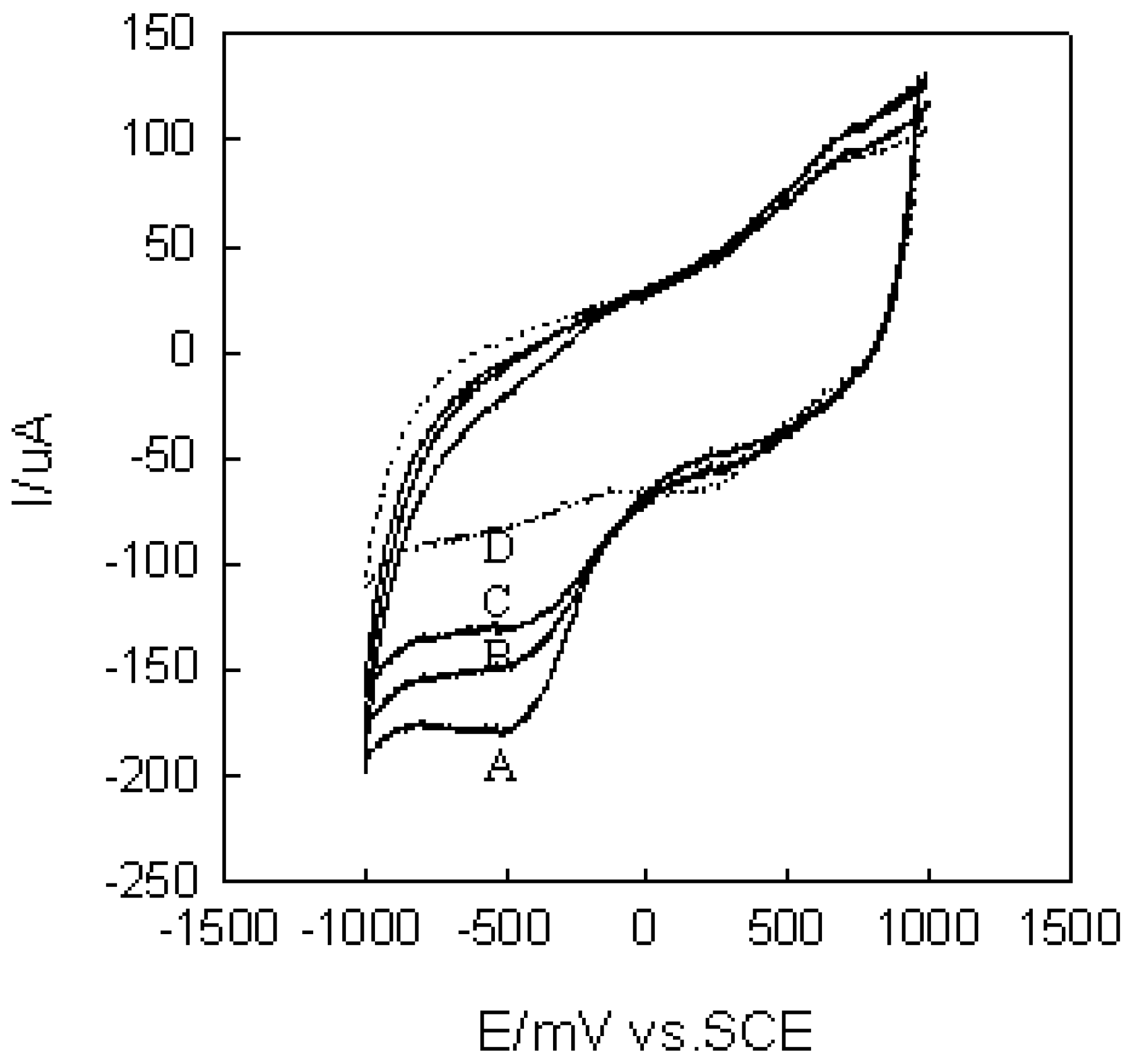
Selectivity
| Interferent | Current ratio a |
|---|---|
| Hexacyanoferrate(II) | 0.98 |
| Ascorbic acid | 1.00 |
| Albendazole | 1.00 |
| o-aminophenol | 0.99 |
| Fe3+ | 1.01 |
| Cu2+ | 1.03 |
| imidazole | 1.12 |
| NO3- | 1.00 |

Effect of pH

Regeneration, Stability and Repeatability of MTZ Sensor
Preliminary Analytical Application

| Sample a | Sensor method (mg/tablet) | UV-visible spectroscopy |
|---|---|---|
| 1# | 4.96±0.07 b | 4.93 |
| 2# | 5.01±0.05 | 4.99 |
| 3# | 4.97±0.04 | 4.98 |
Conclusion
Acknowledgment
References
- Edwards, D. I. J. Antimicrob. Chemother. 1993, 31, 9.
- Molav, A. Med. Clin. North Am. 1982, 66, 12.
- “British Pharmacopaeia”; 1988; Page 199.
- Sanyal, A.K. Analyst 1992, 1, 117.
- Bhoir, L.C.; Raman, B.; Sundaresan, M.; Bhagwat, A.M. Anal. Chim. Acta 1997, 354, 123.
- Smyth, W.F.; Chabala, E.D. Fresenius’ J. Anal. Chem. 1993, 345, 701.
- Wang, Z.H.; Zhou, H.X.; Zhou, S.P. Talanta 1993, 40, 1073.
- Chaniotakis, N.A.; Chasser, A.M.; Meyerhoff, M.E.; Groves, J.T. Anal. Chem. 1998, 60, 185.
- Yoon, J.; Shin, J.H.; Paeng, I.R.; Nam, H.; Cha, G.S.; Paeny, K.J. Anal. Chim. Acta 1998, 367, 175.
- Chaniotakis, N.A.; Park, S.B.; Meyerhoff, M.E. Anal. Chem. 1989, 61, 566. [PubMed]
- Guota, V.K.; Jain, A.K.; Singh, L.P.; Khurana, U. Anal. Chim. Acta 1997, 355, 33.
- Ammann, D.; Huser, M.; Krautler, B.; Schulthess, P.; Lindemann, B.; Halder, E.; Simon, M. Helv. Chim. Acta 1986, 69, 849.
- Chang, Q.L.; Meyerhoff, M.E. Anal. Chim. Acta 1986, 186, 81.
- Frey, H. H.; McNeil, C.J.; Keay, R.W.; Bannister, J.V. Electroanalysis 1998, 10, 480.
- Duony, B.; Arechabaleta, R.; Tao, N.J. J. Electroanal. Chem. 1998, 447, 63.
- Walker, F. A. J. Am. Chem. Soc. 1973, 95, 1150. [PubMed]
- Adler, A.D.; Lonyo, F.R.; Finarelli, J.D. J. Org. Chem. 1967, 32, 476.
- Zhang, X.-B.; Guo, C.-C.; Xu, J.-B.; Yu, J.-B. J.Mol.Catal.A 2000, 154, 31.
- Zuman, P.; Fijalek, Z.; Dumanovic, Z.; Suznjevic, D. Electroanalysis 1992, 4, 783.
- Dumanovic, D.; Ciric, J. Talanta 1973, 20, 525.
- Brett, A.M.O.; Srrano, S.H.P.; Gutz, I.; La-Scalea, M.A. Bioelectrochem.and Bioenerg. 1997, 42, 175.
- Zhi, Y.; Hu, J.B.; Wu, Z.D.; Li, Q.L. Analytical. Lett. 1998, 31, 429.
- Sample Availability: Available from the authors.
© 2003 by MDPI (http://www.mdpi.net). Reproduction is permitted for noncommercial purposes.
Share and Cite
Gong, F.-C.; Zhang, X.-B.; Guo, C.-C.; Shen, G.-L.; Yu, R.-Q. Amperometric Metronidazole Sensor Based on the Supermolecular Recognition by Metalloporphyrin Incorporated In Carbon Paste Electrode. Sensors 2003, 3, 91-100. https://doi.org/10.3390/s30400091
Gong F-C, Zhang X-B, Guo C-C, Shen G-L, Yu R-Q. Amperometric Metronidazole Sensor Based on the Supermolecular Recognition by Metalloporphyrin Incorporated In Carbon Paste Electrode. Sensors. 2003; 3(4):91-100. https://doi.org/10.3390/s30400091
Chicago/Turabian StyleGong, Fu-Chun, Xiao-Bing Zhang, Can-Cheng Guo, Guo-Li Shen, and Ru-Qin Yu. 2003. "Amperometric Metronidazole Sensor Based on the Supermolecular Recognition by Metalloporphyrin Incorporated In Carbon Paste Electrode" Sensors 3, no. 4: 91-100. https://doi.org/10.3390/s30400091




