Introduction
Organophosphates (OP) pesticides are potent neurotoxins that share structural similarities to chemical warfare agents such as soman, sarin and VX. Because of their inherent toxicity, there exists a strong interest in their rapid, reliable, and highly sensitive and selective determination. Biosensors, therefore, seem to be a promising strategy and have been an actively researched area. The mostly favoured area of such biosensors is the indirect detection, e.g. by the inhibition of the enzymes acetylcholinesterase (AChE) or butyrylcholinesterase (BuChE) [
1,
2]. This method, however, has several limitations. Analysis with inhibition-based sensors is a multi-step operation requiring the measurement of the response for the substrate (e.g., acetylcholine), followed by inhibition of the enzyme activity by OP and measurement of the remaining activity and ultimately regeneration, which in most cases is incomplete. This complicated multi-step protocol limits the application of such sensors for on-line monitoring. Additionally, because the enzymes AChE and BuChE are inhibited by many other chemicals such as heavy metals, carbamates, etc., the sensor is not selective.
A novel concept in determination of organophosphates is the use of the enzyme organophosphorus hydrolase (OPH). Since the organophosphates are substrates of the enzyme this allows a direct detection method [
3,
4,
5]. The present paper describes an OPH-based capacitive EIS biosensor for the direct determination of pesticides. In an earlier study we used the ion-selective electrode (ISE) in conjunction with OPH to demonstrate the principle of direct, selective and rapid determination of OP pesticides [
4]. However, the ISE-based version has drawbacks in terms of miniaturisation and mechanical stability, because it is built-up of a pH-glass electrode as the electrochemical transducer part. In contrast, the new, silicon-based type transducer is fully prepared by means of silicon planar technology, which yields a miniaturised, rugged and reliable chip design. In this work, fundamental studies and aspects of development of this capacitive enzyme biosensor will be presented and discussed: various pH-sensitive transducer materials, different immobilisation strategies, proper choice of buffer composition, and typical biosensor characteristics.
Experimental
Three different EIS-type structures of Al/p-Si/SiO
2/Al
2O
3, Al/p-Si/SiO
2/Ta
2O
5 or Al/p- Si/SiO
2/Si
3N
4, respectively, were fabricated from p-type Si (18-24 Ωcm, Wacker Chemitronic, <100> orientation) as pH-sensitive electrochemical transducer. The SiO
2 layer (30 nm) was grown by means of thermal oxidation. The pH-sensitive insulator materials Al
2O
3 and Ta
2O
5 (30-50 nm) were prepared by means of the PLD (pulsed laser deposition), whereas the LPCVD (low pressure chemical vapour deposition) technique was used to deposit the Si
3N
4 layer. The Al rear contact was deposited by means of electron beam evaporation. All experimental details of the fabrication of the EIS structures are given elsewhere [
6,
7,
8,
9,
10].
After preparation of the pH-sensitive EIS transducer structures, OPH (produced and purified according to the protocol described in [
3], specific activity of 587 U/mg protein) was immobilised on the pH-transducing materials by adsorption, cross-linking via glutaraldehyde and entrapment in nafion™. For all experimental details, see [
11]. A schematic of all fabrication steps is summarised in
Fig. 1, exemplarily shown for Ta
2O
5 as pH-sensitive material.
After immobilisation of the enzyme, the biosensors were mounted in a home-made measuring cell and stored in buffer solution in the refrigerator at 4°C. Prior to measurements, the respective biosensor was equilibrated at room temperature for at least two hours.
The hydrolytic reaction of the enzyme OPH is demonstrated in
Fig. 1 (bottom), where G1 represents phenoxy, thiol, cyanide or fluorine, G
2 and G
3 are standing for methyl to butyl and alkyl or phenyl, respectively, and X is an oxygen or a sulphur molecule. The catalytic hydrolysis of an OP molecule releases two H
+ ions. As a result, the hydrogen ion concentration increases (pH decrease) with increasing amount of the OP such as paraoxon. By using a weakly buffered test sample, this pH change can be detected by the underlying pH-sensitive material of the EIS biosensor (see site-binding theory in e.g., [
12,
13]). The resulting potentiometric response is recorded and correlated to the concentration of the analyte in the test sample.
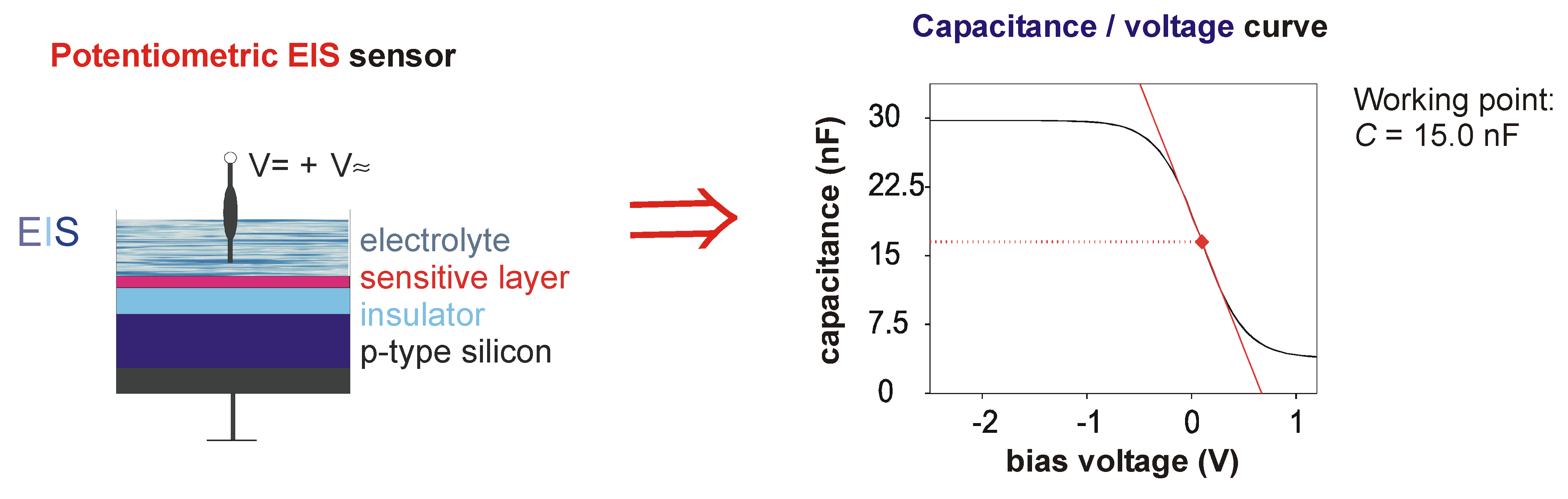
Figure 1.
Schematic of fabrication steps (a-c) of the capacitive EIS biosensor (upper part), and hydrolytical reaction of the enzyme OPH releasing H+ ions that can be detected by means of the pH-sensitive transducer surface (lower part).
Figure 1.
Schematic of fabrication steps (a-c) of the capacitive EIS biosensor (upper part), and hydrolytical reaction of the enzyme OPH releasing H+ ions that can be detected by means of the pH-sensitive transducer surface (lower part).
The measurement principle of the EIS biosensor is given in
Fig. 2.
Figure 2.
Measuring principle of the capacitive EIS biosensor with immobilised OPH layer (left side), and typical C/V (capacitance/voltage) curve for the determination of the working point of the biosensor (right side).
Figure 2.
Measuring principle of the capacitive EIS biosensor with immobilised OPH layer (left side), and typical C/V (capacitance/voltage) curve for the determination of the working point of the biosensor (right side).
In order to study the biosensor characteristics, the sensor chip was contacted on its front side by the electrolyte (test sample) and a reference electrode (Ag/AgCl) and on its rear side by a gold-plated pin. C/V measurements were performed with an impedance analyser (Zahner electric) at a d.c. voltage which was swept from -3 V to 0.5 V in steps of 100 mV and a superposed a.c. voltage with a frequency of 120 Hz and a signal amplitude of 20 mV. To record the dynamic sensor behaviour, the EIS biosensor was operated in the ConCap mode.
For pH measurements, 50 mM HEPES buffer, pH 5 to pH 10, containing 50 µM CoCl2 and 100 mM NaCl was used; for the paraoxon calibration measurements, 0.2 mM HEPES buffer, pH 9, containing 50 µM CoCl2 and 100 mM NaCl with concentrations of 0.5 µM to 500 µM paraoxon was utilised. All measurements were performed in a dark Faraday cage at room temperature. All chemical used are of analytical grade: (HEPES buffer (Fisher Scientific, Tustin, CA USA), BSA ( Sigma Chemicals, St. Louis, MO, USA), glutaraldehyde (Fisher Scientific, Tustin, CA USA), nafion (Aldrich Chemicals Co., Madison, WI, USA), paraoxon (Sigma Chemicals, St. Louis, MO, USA), pH buffer (Fisher Scientific, Tustin, CA USA)).
Results and Discussion
The performance characteristic of the biosensor is a function of various parameters, like the pH sensitivity of the pH-transducer layer, the buffer composition and concentration, the starting pH of the buffer, the temperature and the units of enzymes immobilised on the chip surface. As already discussed in [
11], the pH behaviour of the three investigated pH-sensing transducer materials Al
2O
3, Ta
2O
5 and Si
3N
4 show results which are in a good agreement with values found in literature. There is, however, a slight decrease in the original pH sensitivity on modification of the surface by OPH. Nevertheless, all three pH-sensitive materials are still well-suited as transducer layers for biosensor measurements. In contrast, the chosen immobilisation protocol does have a clear influence on the biosensor behaviour. Only the biosensors prepared using glutaraldehyde cross-linking and nafion entrapment strategies lead to a distinct dependence of the biosensor signal on the OP concentration, e.g. paraoxon, whereas for the adsorptively immobilised biosensors almost no signal dependence was achieved (for details, see [
11]).
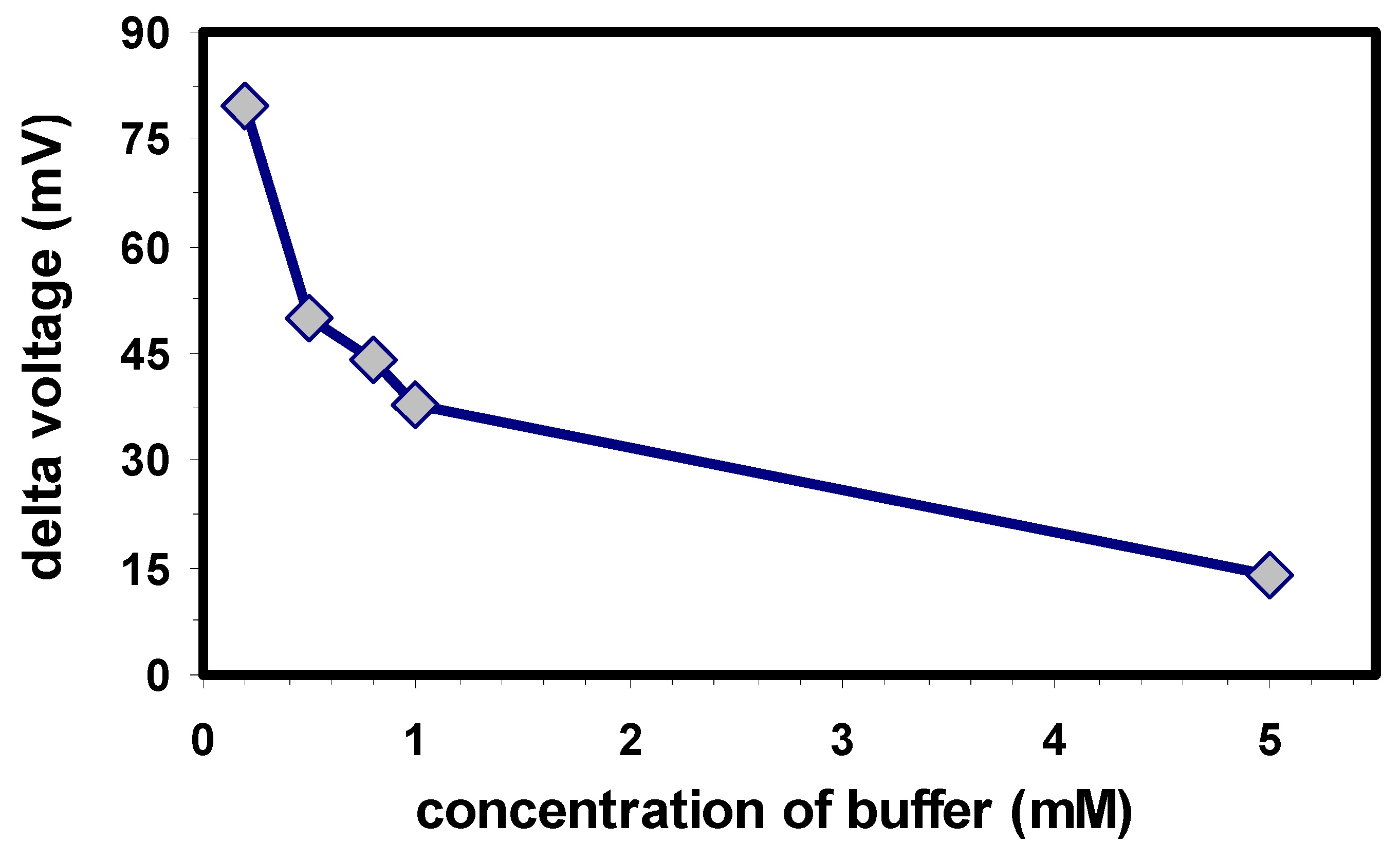
The measuring signal of the capacitive EIS biosensor is strongly influenced by the concentration of the buffering substances. H
+ ions that are released during the enzymatic cleavage of OP compounds are counteracted by buffering ions. Therefore, in this experiment, investigations have been carried out with a HEPES buffer within a concentration range from 0.2 to 5 mM.
Fig. 3 shows the inverse influence of the buffer concentration on the potential change of the sensor: the higher the buffer concentration, the smaller the biosensor signal due to counteracting buffer ions. The highest biosensor signal has been achieved with the buffer of the lowest molarity, here 0.2 mM. Lower buffer concentration (also tested, but not shown) resulted in drifting baselines and a rather unstable behaviour. Consequently, a buffer concentration of 0.2 mM has been used for the further experiments.
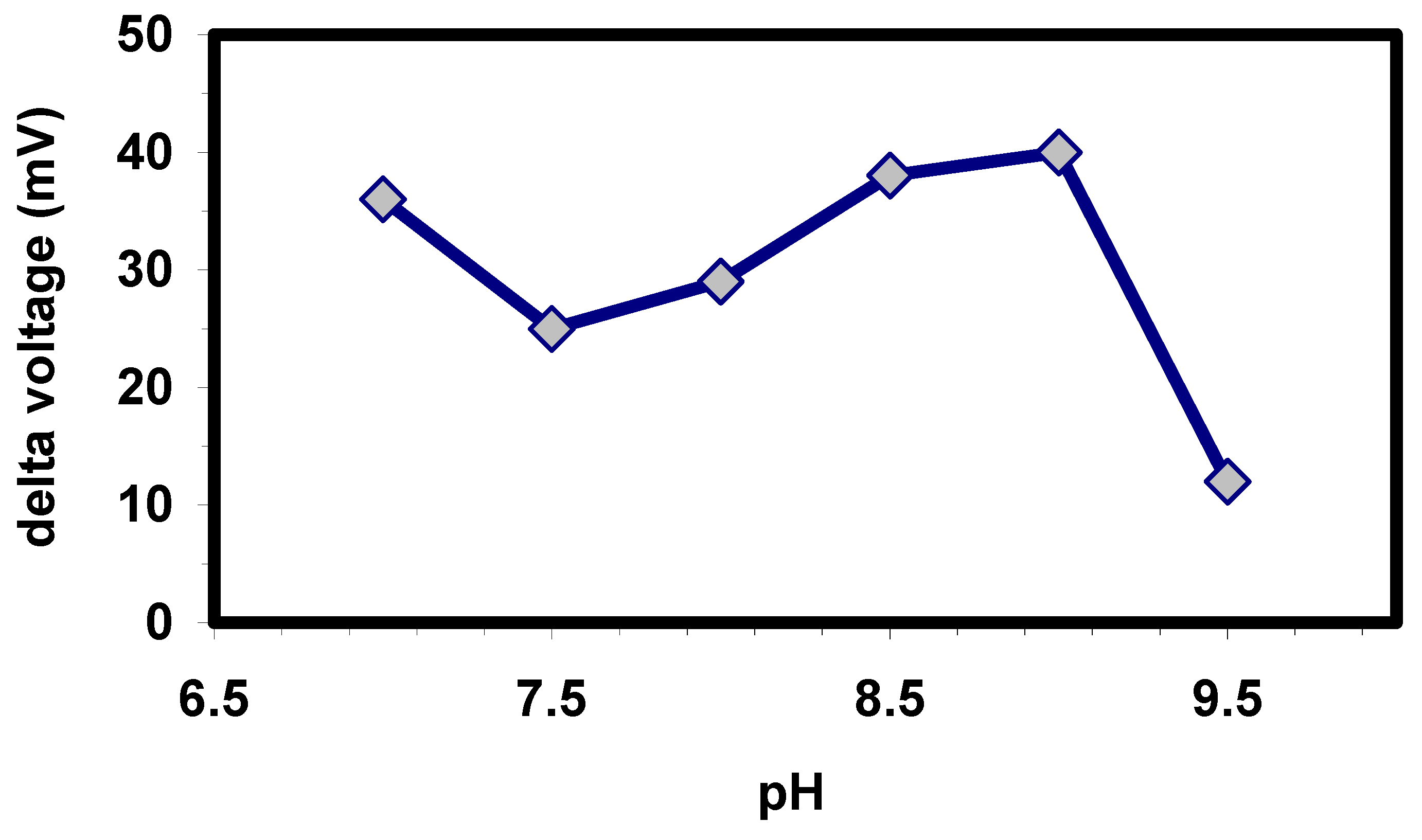
Besides the buffer concentration, also the starting pH of the buffer during the enzymatic hydrolysis has an influence on the biosensor signal. The pH profile of the OPH-modified capacitive EIS sensor is presented in
Fig. 4. As can be seen from the figure, the highest hydrolytic rates have been obtained at pH 9. This value gives a difference of about 0.5 units between the optimum of the free and immobilised enzyme, but is in good correlation with experiments, which have been performed with OPH-based ISEs [
3].
Figure 3.
Effect of the HEPES buffer concentration on the response of the EIS biosensor; measuring conditions: pH 9, 50 µM CoCl2, 100 mM NaCl, 100 µM paraoxon.
Figure 3.
Effect of the HEPES buffer concentration on the response of the EIS biosensor; measuring conditions: pH 9, 50 µM CoCl2, 100 mM NaCl, 100 µM paraoxon.
Figure 4.
Effect of pH of the HEPES buffer (0.2 mM) on the signal amplitude of the EIS biosensor at 50 µM paraoxon.
Figure 4.
Effect of pH of the HEPES buffer (0.2 mM) on the signal amplitude of the EIS biosensor at 50 µM paraoxon.
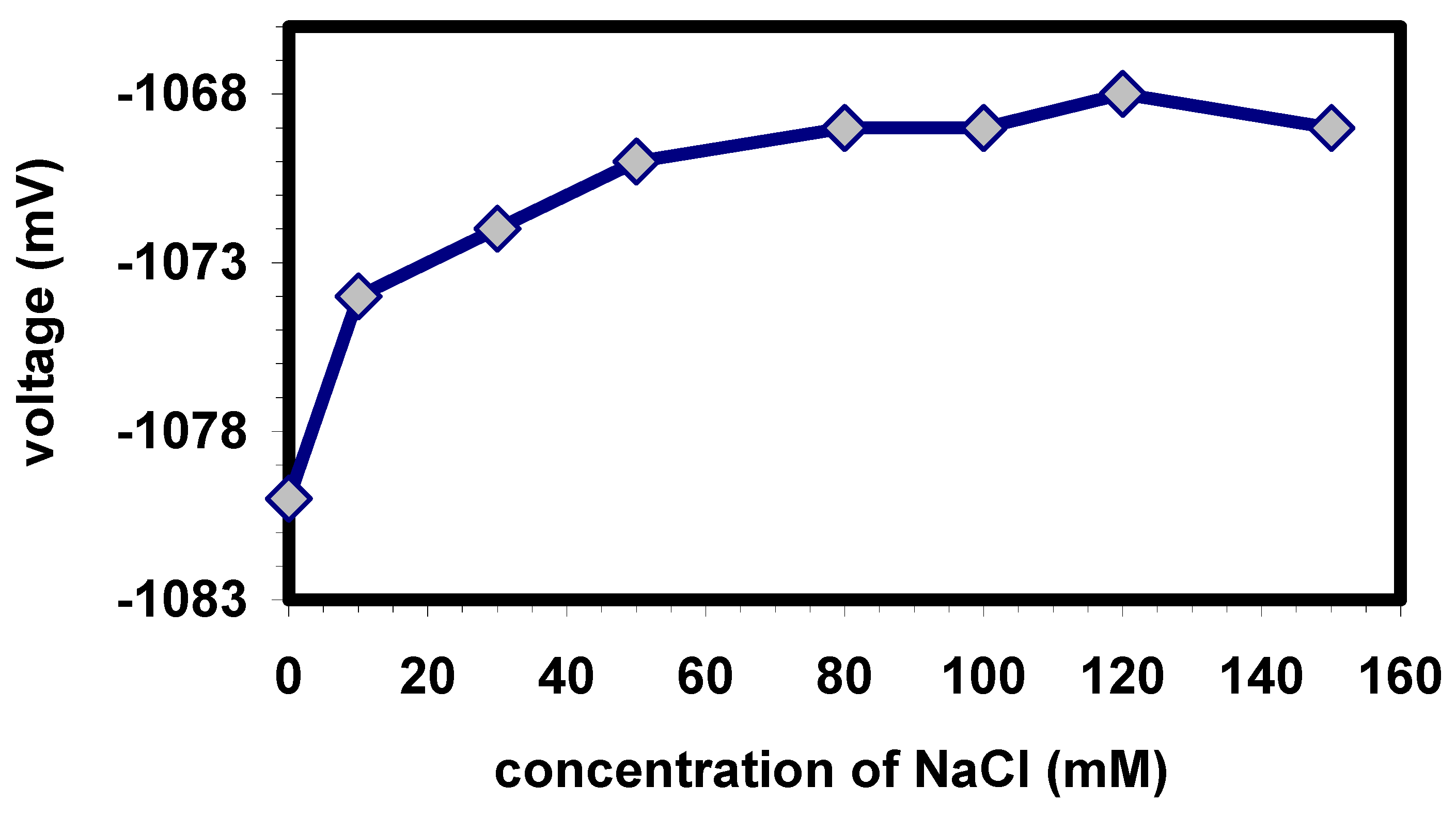
Fig. 5 shows the effect of conductivity (varying NaCl concentration) of the buffer on the potential of the biosensor. For potentiometric measurements, as like in this work by means of a capacitive field-effect sensor, the measuring buffer requires a certain amount of a background salt. Sodium chloride addition to the measuring buffer provided the conductivity that is necessary for the reference electrode and the chosen measurement set-up: the capacitance is evaluated by means of an impedance measurement from both the real part (ohmic resistance) and the imaginary part (capacitance). To exactly determine the capacitance, therefore, the resulting voltage signal has to be stable and independent of the conductivity, i.e. the concentration of the added NaCl. As shown in
Fig. 5, a nearly stable signal was obtained for NaCl concentrations higher than 80 mM. A NaCl concentration of 100 mM was used in future experiments.
Figure 5.
Effect of conductivity of the HEPES buffer (1 mM) on the signal amplitude of the EIS biosensor at 50 µM paraoxon.
Figure 5.
Effect of conductivity of the HEPES buffer (1 mM) on the signal amplitude of the EIS biosensor at 50 µM paraoxon.
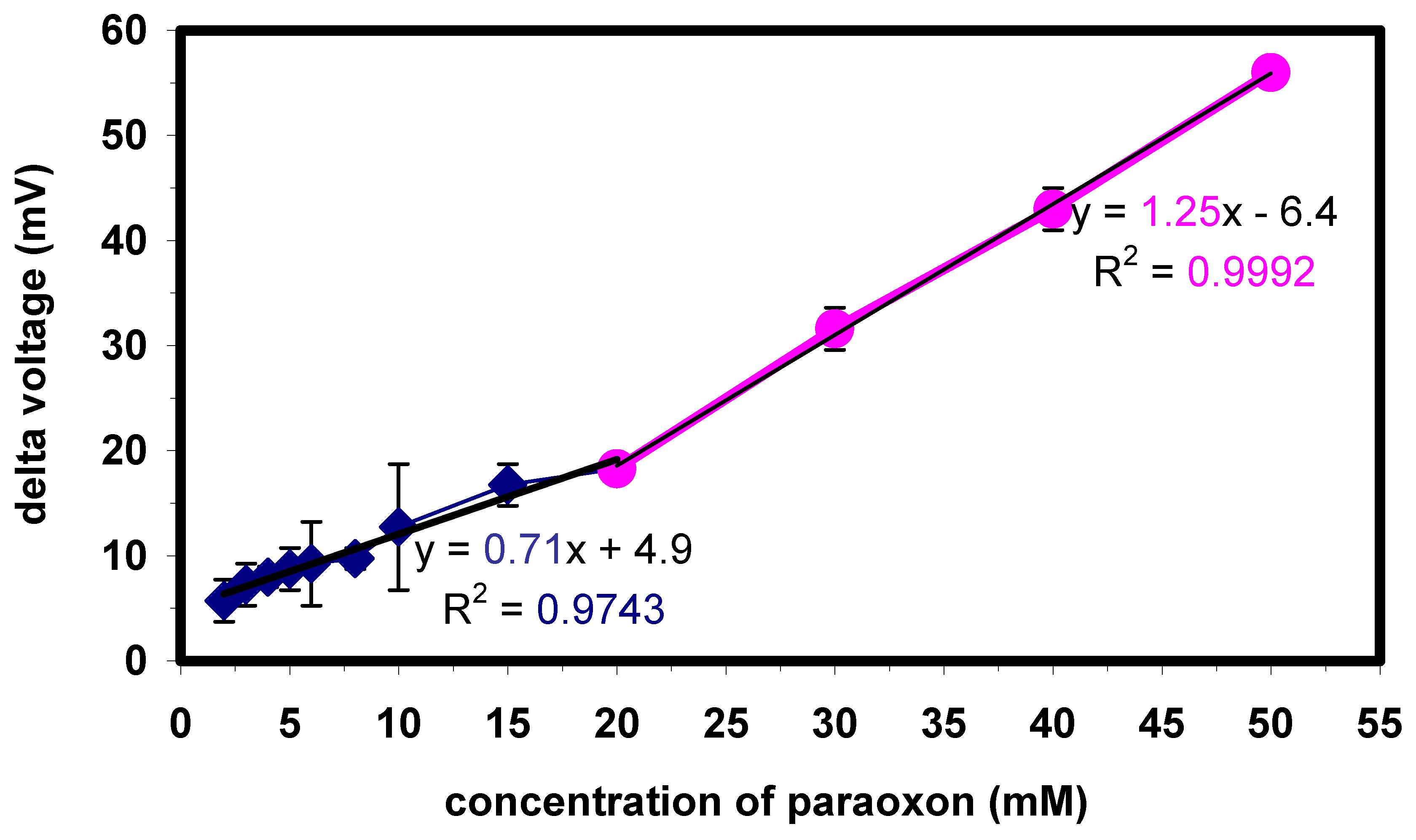
Figure 6.
Sensitivity characteristic of the enzyme biosensor with Ta2O5 as pH transducer. The calibration plot is split into two regions: between 2 and 20 µM (squares), the sensitivity towards paraoxon is 0.71 mV/µM; between 20 and 50 µM (circles), the sensitivity is 1.25 mV/µM.
Figure 6.
Sensitivity characteristic of the enzyme biosensor with Ta2O5 as pH transducer. The calibration plot is split into two regions: between 2 and 20 µM (squares), the sensitivity towards paraoxon is 0.71 mV/µM; between 20 and 50 µM (circles), the sensitivity is 1.25 mV/µM.
In
Fig. 6, a typical calibration plot of paraoxon is presented for an enzyme biosensor with Ta
2O
5 as pH-sensitive transducer layer. The plot has been calculated from the steady-state response of the biosensor after 3 minutes. Each measurement point represents the mean value from three independent single measurements. As can be seen from the diagram, the characteristic calibration plot can be divided into two linear regions for paraoxon: one spanning from 2-20 µM (squares) with an average sensitivity of 0.71 mV/µM, and the other one from 20-50 µM (circles) with an average sensitivity of 1.25 mV/µM. The lower detection limit (i.e., three times the standard deviation of the response obtained for a blank signal) of the presented silicon-based enzyme biosensor is 2 µM. The sensitivities towards paraoxon are similar for all three types of pH-sensitive transducer materials investigated so far [
11]. The presented capacitive field-effect biosensors do not reach the nanomolar detection limit of AChE-based enzyme biosensors, but have a much faster response characteristic without any complicated measurement and renewal protocol. Measurement of lower concentration samples can be achieved by the addition of well-established solid-phase extraction protocol used in chromatography.
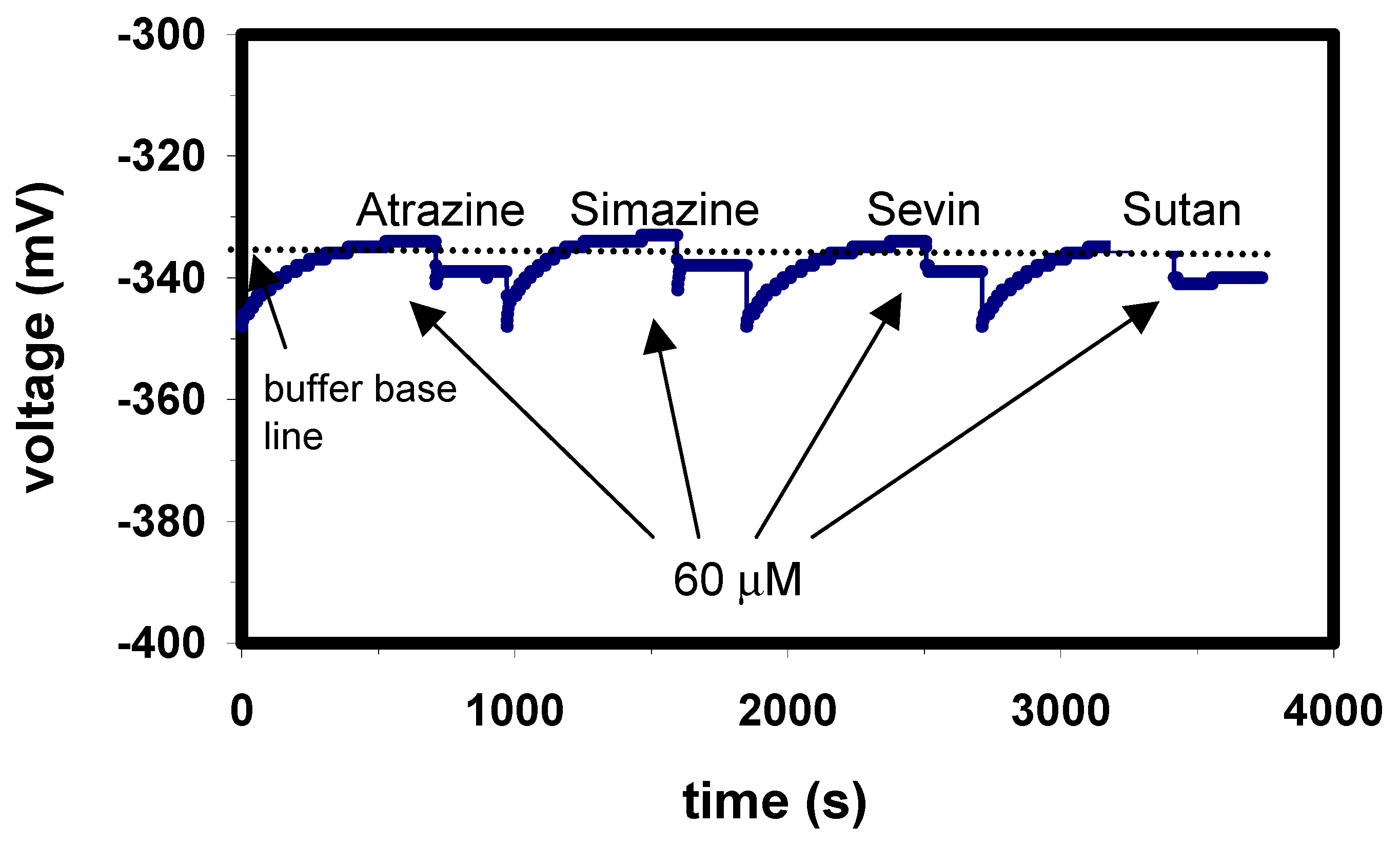
Figure 7.
Selectivity measurement of the enzyme biosensor with Ta2O5 as pH transducer. The ConCap plot is recorded for 60 µM atrazine, simazine, sevin and sutan.
Figure 7.
Selectivity measurement of the enzyme biosensor with Ta2O5 as pH transducer. The ConCap plot is recorded for 60 µM atrazine, simazine, sevin and sutan.
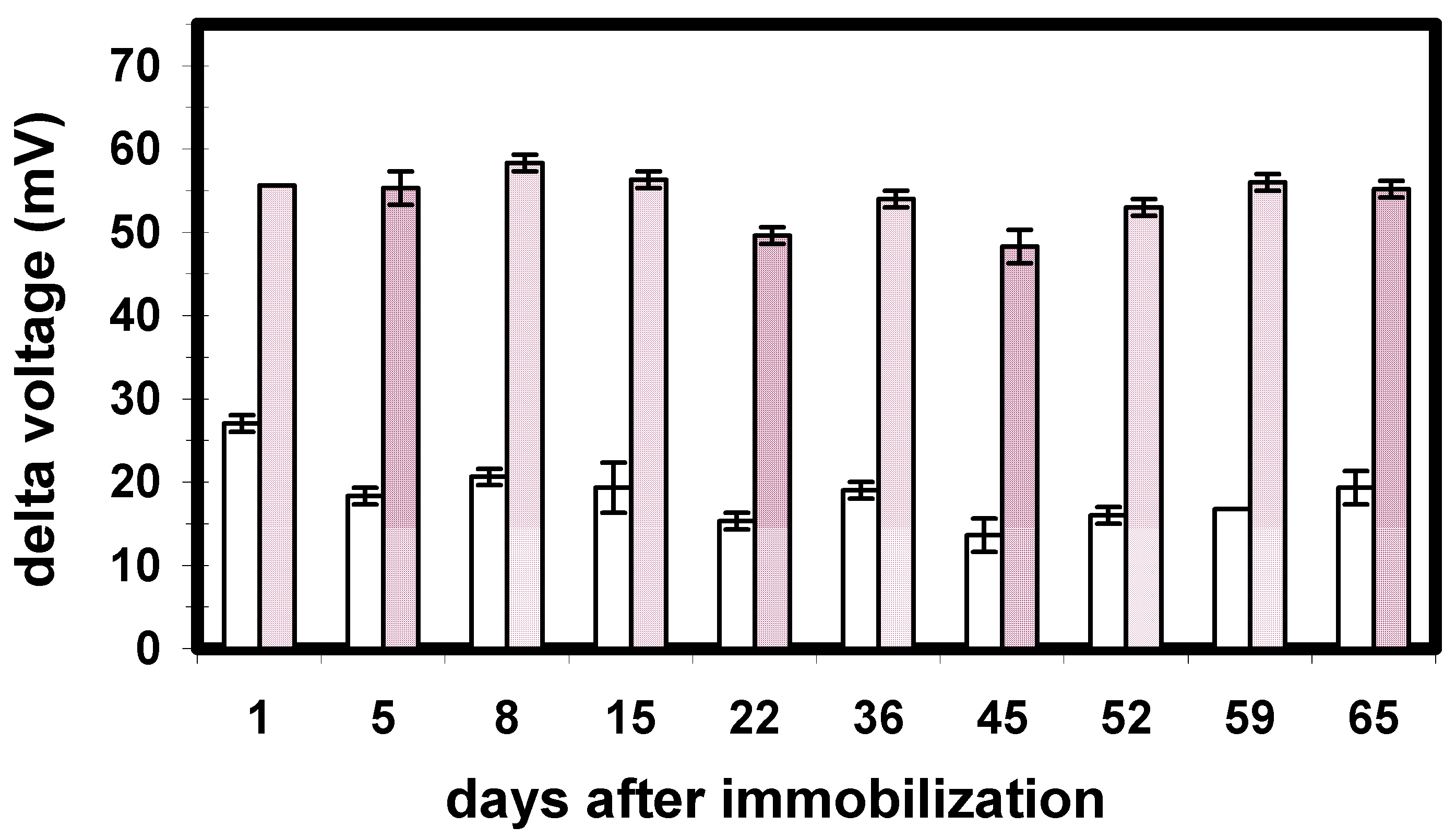
Figure 8.
Long-term stability of the enzyme biosensor with Ta2O5 as pH transducer. The signals of 15 µM paraoxon (small bars) and 50 µm paraoxon (large bars) are plotted over time.
Figure 8.
Long-term stability of the enzyme biosensor with Ta2O5 as pH transducer. The signals of 15 µM paraoxon (small bars) and 50 µm paraoxon (large bars) are plotted over time.
Like paraoxon, other organophosphates such as parathion, methyl parathion or diazinon can be hydrolysed by the enzyme OPH, and consequently detected by the capacitive EIS biosensor. In our study, for the two investigated pesticides paraoxon and parathion, similar sensitivities were achieved. In contrast, as expected non-OPs such as atrazine, simazine, sevin and sutan did not interfere even at concentration as high as 60 µM (
Fig. 7).
For practical applications, like in on-line process monitoring, the developed biosensor must have a certain long-term stability, i.e. a multiple use should be possible. To study the long-term stability, the signals of 15 µM (grey bars) and 50 µM (hatched bars) paraoxon have been recorded over a measuring period of more than 2 months. As can be seen from Fig. 8, where each bar is an average of three measurements, there was no significant decrease of the biosensor signal for a 77 day period. This implies a high stability of the developed capacitive biosensor. A similar behaviour has been obtained also for the biosensors with Al2O3 and Si3N4 as pH-sensitive material.