Introduction
Polyphenolic compounds are widespread in nature. They are important constituents of natural products, such as fruit and vegetables. Their role as antioxidants has caused great impact on prevention of cardiovascular diseases and some of them are claimed to prevent cancer. They are free radical scavengers, neutralizing oxygen reactive species and chelating metalic ions. However some polyphenols are pro-oxidants [
4,
5]. Besides, they are responsible for the organoleptic properties of wine and olive oil [
6].
Analytical methods mostly used in the qualitative and quantitative determination of polyphenols in fruit juices, teas, olive oil and red wine involve techniques of chromatographic separation, such as HPLC and GC – MS [
7,
8]. These methods are expensive and require several operations. Besides, they waste large amounts of eluents, not always environmental friendly.
A biosensor can be defined as a combination of a biological reconaissance of an analyte and transduction of that event to a measurable signal. The proportionality of the signal to the concentration of the analyte/analytes of interest allows its use for quantitative analysis. Some of the advantages of these recent tools, often quoted are: easy preparation, minimal preparation of sample required, selectivity, sensitivity, reproducibility, relatively low cost, rapid time of response, long half life, easy storage. The present work uses an amperometric biosensor, based on the measurement of current resulting from the reduction of an electroactive species, at a potential selectively optimized for the molecule under study.
Laccase Coriolus versicolor was the enzyme used in the present work. It was immobilized on a derivatized polyether sulfone membrane, by covalent attachment.
Biosensors using laccase have been said to give very stable amperometric response to phenolic compounds [
9].
Laccases are glicoproteíns which form dimers or tetramers, generally containing four copper ions in each monomer, bound to three redox centers, forming a trinuclear copper “cluster”. They are enzymes which belong to the family of multi copper oxidases, that catalyse the reactions of oxidation of various inorganic and aromatic, mainly phenolic, compounds, accompanied by the reduction of oxygen to water. As a consequence of the high preference for oxygen, as oxidant substrate, these enzymes show low specificity for reducing substrates. The oxidation of the reducing substrates involves the formation of free radicals upon the transfer of one electron to laccase. The radical, is then oxidized by laccase yielding either quinones resulting of a phenol or reacting to form polymers. [
9,
10,
11].
In our work we firstly studied the response of the laccase containing biosensor to catechol at an applied potential of –200mV. There was a definite answer of the biosensor thus prepared to catechol in acetate buffer pH 4.5. We then proceeded to test the response of the biosensor to polyphenol compounds existing in wine. No difference was observed between the response of the electrode to the polyphenols tested, only with the internal membrane on and with the additional enzyme containing membrane, at -200mV. When scanning the applied potentials it was observed that catechin responded at +100 and caffeic acid at -50 mV in 12% ethanol, acetate buffer pH 4.5 and 3.5. Tests with the other polyphenols: gallic acid, malvidin, quercetin, rutin, trans-resveratrol were done at potentials ranging from –500 to + 500mV. However we could not get a response which could be clearly ascribed to an actuation of the enzyme on the substrate and subsequent detection at the electrode for the other polyphenols tested.
Materials
Catechol was from Merck. (+)-Catechin hydrate was from Sigma, trans - 3,4- Dihydroxycinnamic acid (caffeic acid), Quercetin, gallic acid, rutin and trans-resveratrol were from Aldrich. Malvidin was from Extrasynthese.
Laccase from Coriolus Versicolor (EC 1.10.3.2), was from Fluka (0,83U/mg). Absolut ethanol, density 1,790Kg/L, was from Panreac Quimica AS.
ABTS (2,2’-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) was from Roche Diagnostics Corporation.
Buffers used were: PBS (Phosphate buffer saline) pH = 7.4, prepared with 9.9346g of NaCl from Riedel-deHaën with purity > 99.8%, 0.2460g KCl from Merck purity superior to 99.5%, 1.1356g of Na2HPO4 and 0.2448g KH2PO4, with purity superior to 99.9% and 99.7%, respectively, both supplied by Sigma and bidistilled Millipore water added up to 1L. Acetate buffer (0.1M, pH = 4.5) was prepared with 5.444g of sodium acetate trihydrated, from Sigma, having purity superior to 99.8%, and 3.43ml glacial acetic acid from Riedel-de-Haën, with purity of 99.8% and density 1.05kg/L - bidistilled Millipore water was added up to 1L.
Polyethersulphone membranes (Ultrabind, US450 0.45μm) were from Gelman and discs of 18.0 mm diameter were cut.
The electrode used for the amperometric determination was furnished by Universal Sensors (New Orleans, LA) and is formed by four Pt wires of 0.3mm diameter and a Ag/AgCl electrode. An Amperometric Biosensor Detector (ABD) from Universal Sensors (New Orleans, USA) was used to apply the required potential and measure the consequent current obtained, which was recorded on a Pharmacia Biotech recorder, REC 102.
Cyclic voltamograms of the buffers and substrates were obtained with a Voltalab 10, PGZ100 from Radiometer Analytical and three electrodes: the reference, was a saturated calomel electrode (SCE) from Radiometer Analytical; the working electrode was a Platinum disc with 5mm diameter and the secondary electrode was a Platinum helice.
UV-Vis spectra were obtained with a Schimadzu-UV-1603 spectrophotometer and quartz cells 1cm wide.
Preparation of the Biosensor
The amount of enzyme to be immobilized was chosen after testing the amount which was retained on the membrane. The choice was done after repeated washing of a membrane, where an excess of enzyme was firstly immobilized (0.3mg in 30μL of acetate buffer) and analysing the presence of enzyme in the washing solution (acetate buffer) by adding 12μL ABTS 50 mM to 1mL of the washing solution. The UV-Vis spectra of the solutions were then run. It was concluded that 0.1 mg was the amount of laccase that was retained on the membrane disc. A solution of the enzyme was than prepared in acetate buffer, and 30 μL (containing 0.1 mg Laccase) were dropped on the polyethersulphone membrane disc with a micropipette. The enzyme was allowed to react with the membranes for 2h at room temperature (ca 22°C). The membranes were then used.
Amperometric Detection
The membrane side containing the enzyme was applied on top of the internal membrane of the electrode stem in contact with the platinum electrode and the biosensor was dipped in 5ml of buffer. Then, a voltage was applied from an amperometric biosensor detector (ABD) (US), the background current was allowed to reach a steady value and 50μl of the substrate were added to the solution, under stirring. The transient current-time was registered until the system was stable again. (It was observed that the background current could strongly influence the results, mainly if the electrode was used at negative applied potentials after having been subjected to large positive potentials (Pt positive relative to Ag,AgCl) – the large background current observed then, could not be lowered by polishing with alumina 0.05μm pore, neither by washing with Nitric acid). So, one electrode was used when negative potentials were applied and another one for the positive potentials.
Initially a potential of –200mV was applied, because it is often referred in the literature as the potential of choice for biosensor phenolics determination [
6,
12,
13,
14,
15], though –400mV has been used [
6] for the detection of rutin. Our laccase containing biosensor was sensitive to catechol, at pH 4.5 at the referred applied potential: -200mV. However, other phenolic compounds tested did not give any definite answer at that potential and the laccase containing membrane. A scanning of the applied potential was done in order to get a response from (+)-catechin, caffeic acid, gallic acid, malvidin, quercetin, rutin and
trans-resveratrol. A large cathodic current was observed for (+)-catechin, at 100mV, and for caffeic acid, at –50 mV.
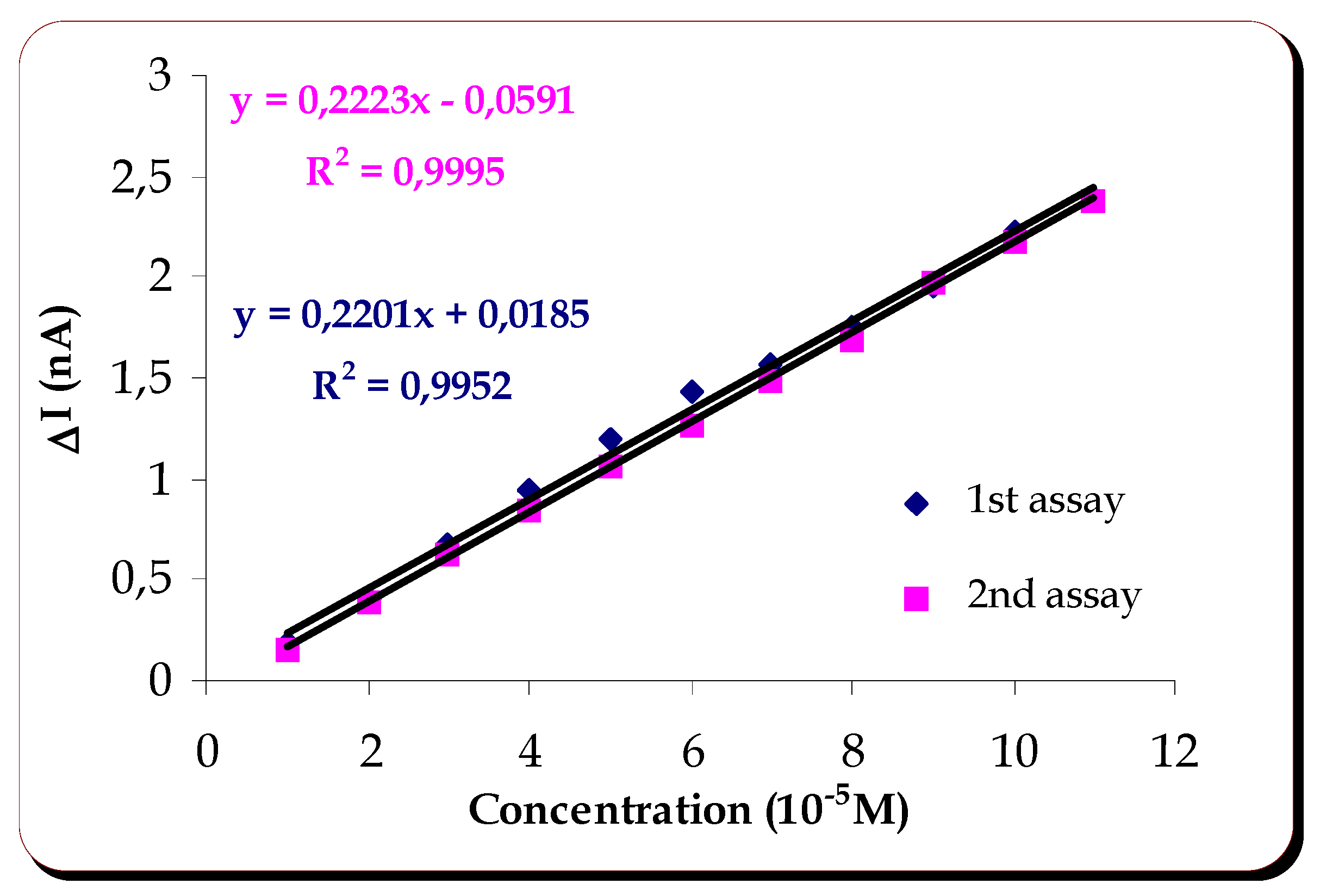
Response of the Laccase Biosensor to Catechol, at pH 4.5
A cathodic current was observed when catechol was added to acetate buffer where the biosensor had been previously stabilized, at an applied potential of -200mV. The reproducibility and linearity of response were then studied. An answer of
0.28±0.028nA (5 assays) was obtained for catechol 10
-5 mol dm
-3. The linearity of the response is shown on
figure 1.
Figure 1.
Linearity of response of the laccase containing biosensor to catechol.
Figure 1.
Linearity of response of the laccase containing biosensor to catechol.
Figure 1 shows that there is linearity of response from 1 to 11x 10
-5 mol dm
-3 . The sensitivity of this method is very similar for the two assays done with the same membrane, its value being
0.0216mAM-1.
Study of the Response of the Biosensor to Catechin in:
a) aqueous acetate buffer pH 4.5
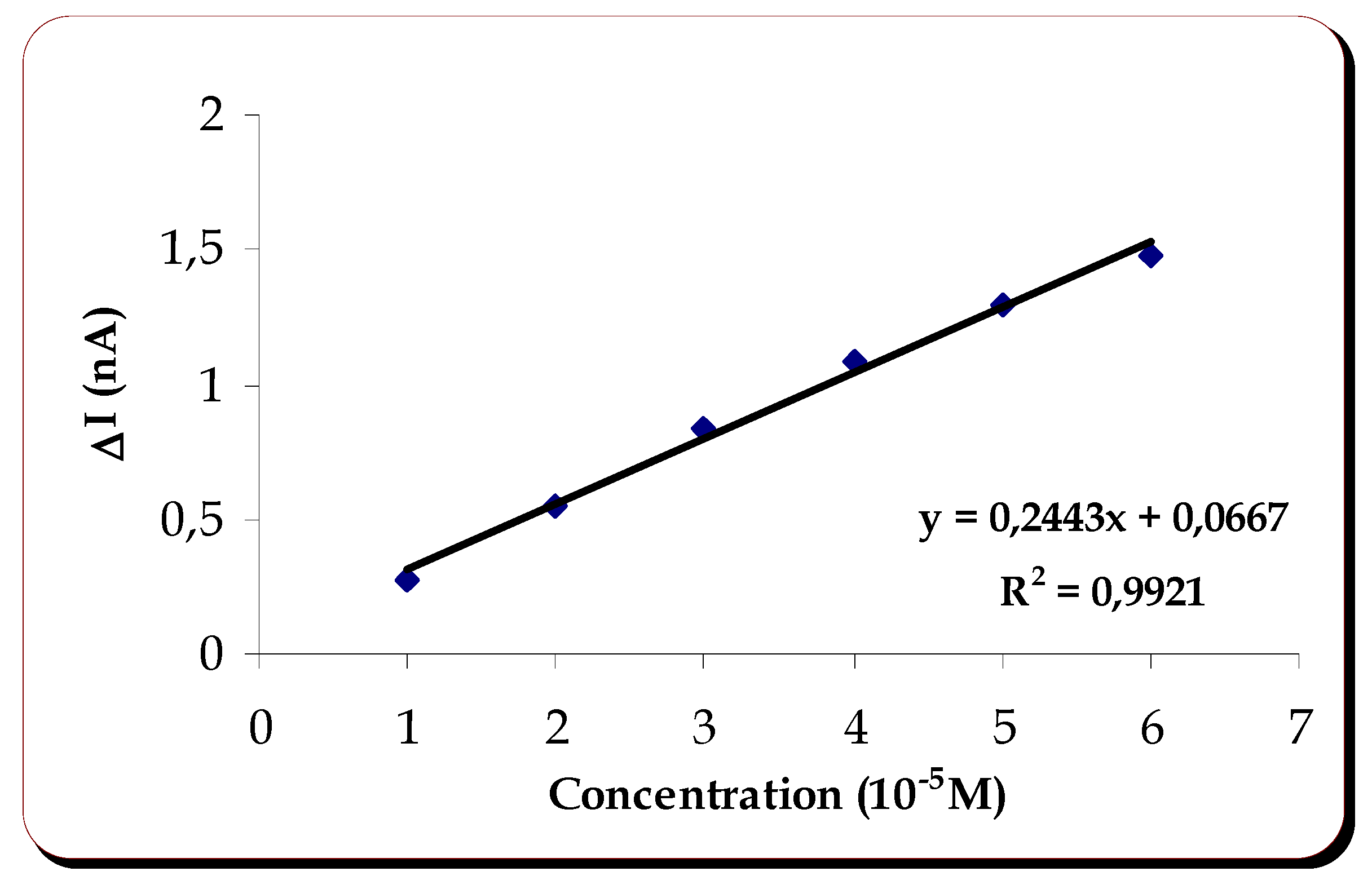
Catechin is one of the polyphenol of interest in red wine. We started its study, testing the response of the biosensor with immobilized laccase, at -200mV . After verifying that the response of the biosensor, in the presence of this substrate, was pratically zero, at this applied potential, it was necessary to study the variation of the current at different applied potentials. A definite cathodic current was observed at an applied potential of +100mV.
At this potential the variation of the current upon injection of catechin was zero when only the internal membrane was on the electrode, whereas a reduction current of -0,26nA was observed when the laccase containing membrane was applied.
The study of linearity and reproducibility of response were then done at that potential.
Five assays were done to get the values of
-0.26±0.0342nA. The linearity of response is shown on
figure 2.
Figure 2.
Linearity of response of the laccase containing biosensor to catechin.
Figure 2.
Linearity of response of the laccase containing biosensor to catechin.
The response of the biosensor to catechin was linear between 1 and 6x10-5 M. The sensitivity of the method was 0.02443mAM-1.
b) Study of catechin in 12% ethanol, acetate buffer
In order to approach the real samples, red wine, where the biosensor is intended to be used, the effect of ethanol on catechin solution 10
-3M was studied. Thus, the response to catechin and corresponding reproducibility was obtained in aqueous acetate buffer and in 12% ethanol, acetate buffer, for the same laccase membrane. The values are presented on
Table 1.
It was verified that ethanol had only a slight influence on the biosensor response to catechin, as table I evidences for a 12% ethanol, acetate solution. Previous trials with catechol had shown that no answer to catechol was observed when ethanol, at the same percentage as used for catechin, was mixed to acetate buffer. This is an important finding, since ethanol is always present in wine, where the biosensor is intended to be used.
Table 1.
Response of the electrode base system to catechin in aqueous and 12% ethanol, acetate buffer.
Table 1.
Response of the electrode base system to catechin in aqueous and 12% ethanol, acetate buffer.
| | ΔI/nA | ΔI/nA |
|---|
| Substrate | Internal Membrane | Laccase Membrane |
|---|
| Catechin 10-5M in Acetate buffer | 0.02 | -0.29±0.021 |
| Catechin 10-5M in 12% Ethanol, Acetate buffer | 0.01 | -0.27±0.014 |
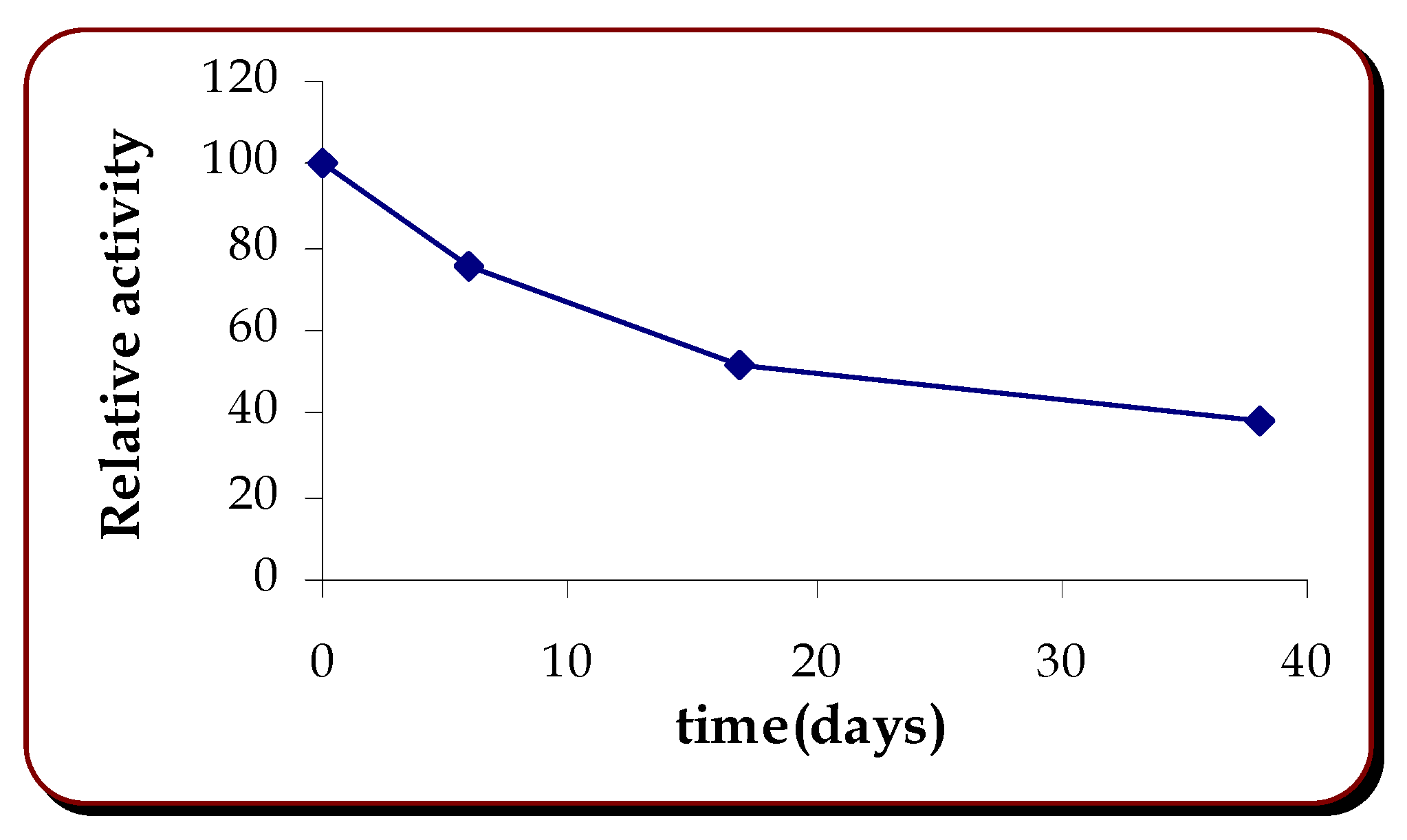
Stability of the Biosensor
Long term stability was studied for membranes stored at 4°C. Response to catechin, at different days of storage was found to give a half life of 15 days, with a response of 38% of the initial one on day 38.
Figure 3.
Stability of the biosensor.
Figure 3.
Stability of the biosensor.
Figure 4.
cyclic voltammograms of (+)-catechin 10-3 M in acetate buffer pH 4.5 (10mV/s); (+)-catechin 10-2 M in acetate buffer pH 4.5 (10mV/s); (+)-catechin 10-2 M in acetate buffer pH 4.5 (20mV/s).Ipc1 = -23.50μA/cm2 , Epc1 = 200.2 mV ; Ipa2 = 94.95μA/cm2, Epa2 = 559.2 mV; Ipc2 = -10.95μA/cm2, Epc2 = 120.5 mV; Ipa3 = 2.74μA/cm2, Epa3 = 584.0 mV, Ipc3 = -21.25μA/cm2, Epc3 = 143.5 mV.
Figure 4.
cyclic voltammograms of (+)-catechin 10-3 M in acetate buffer pH 4.5 (10mV/s); (+)-catechin 10-2 M in acetate buffer pH 4.5 (10mV/s); (+)-catechin 10-2 M in acetate buffer pH 4.5 (20mV/s).Ipc1 = -23.50μA/cm2 , Epc1 = 200.2 mV ; Ipa2 = 94.95μA/cm2, Epa2 = 559.2 mV; Ipc2 = -10.95μA/cm2, Epc2 = 120.5 mV; Ipa3 = 2.74μA/cm2, Epa3 = 584.0 mV, Ipc3 = -21.25μA/cm2, Epc3 = 143.5 mV.
Cyclic Voltammetry
Cyclic voltammetry of catechin solutions were done. It was observed that there was a large oxidation peak when concentrated catechin solution (10-2 M) was used at a scan rate of 10mV/s. This was an irreversible process and was not observed for more diluted solutions (10-3M), neither for scan rates larger than 10 mV/s.
In any case, whatever the processes occurring, it could be observed that reduction occurred at 100mV, and that could be checked on the biosensor.
It was observed that E
pa2 = 559.2 mV, the potential of the anodic peak obtained for (+)-catechin 10
-2 M in acetate buffer pH 4.5 (10mV/s) was close to the value reported for a solution of 1x10
-4 M (+)-catechin solution in 1+1 methanol-2.5% V/V acetic acid: 510 mV [
16]. Cyclic voltammograms of laccase containing solutions were not conclusive.
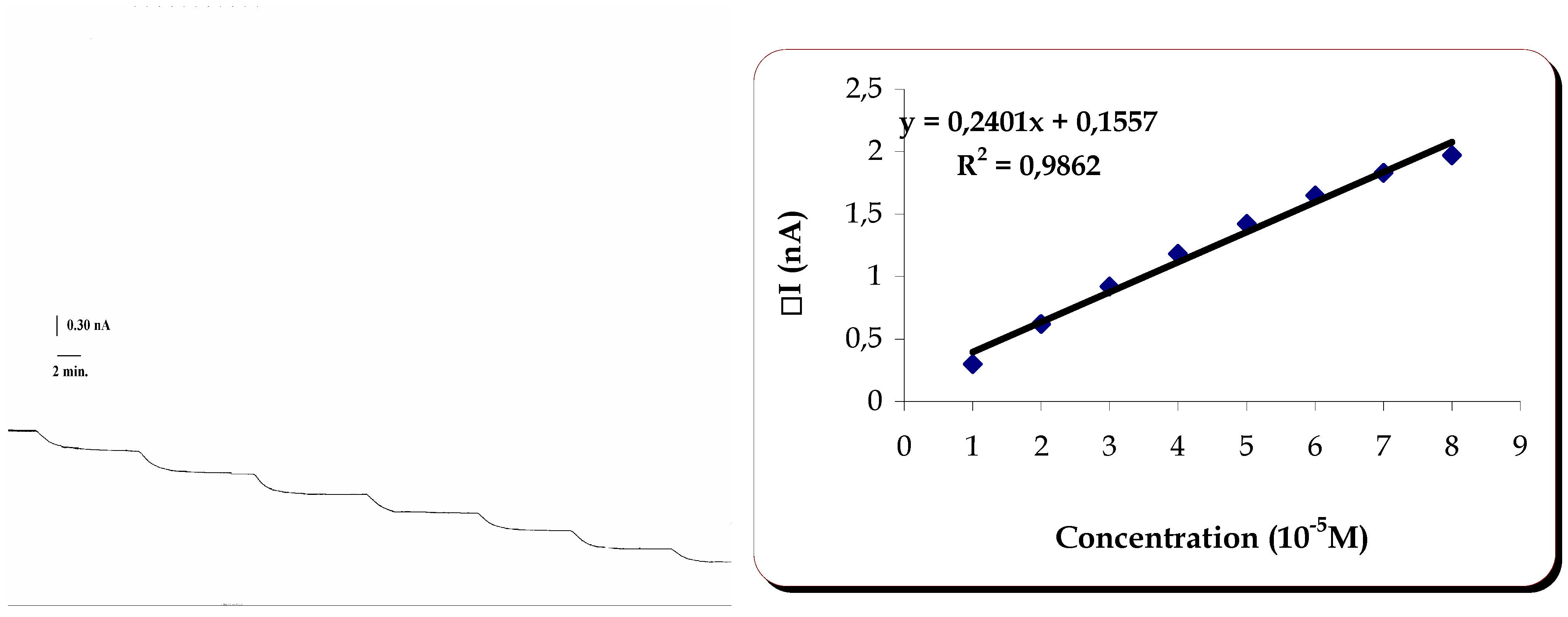
Response of the Laccase Biosensor to Caffeic Acid
Similarly to what was done to the previously mentioned substrates, tests of the biosensor were performed at different applied potentials with caffeic acid as substrate. It was found that the largest cathodic current for the response of the biosensor to caffeic acid was obtained when the applied potential was –50 mV.
Figure 5 evidences the relative responses:
Figure 5.
Relative responses to caffeic acid of the internal and laccase membrane when applied to the electrode base, at different applied potentials.
Figure 5.
Relative responses to caffeic acid of the internal and laccase membrane when applied to the electrode base, at different applied potentials.
Linearity and reproducibility were then studied at that potential. They are shown on
Table 2 and
figure 6.
Table 2.
Response of the electrode base system to caffeic acid in 12% ethanol, acetate buffer.
Table 2.
Response of the electrode base system to caffeic acid in 12% ethanol, acetate buffer.
| | ΔI/nA | ΔI/nA |
|---|
| Substrate | Internal Membrane | Laccase Membrane |
|---|
| Caffeic acid 10-5M in 12% Ethanol, Acetate buffer | 0.00 | -0.30±0.0058 |
Figure 6.
Linearity of response of the laccase containing biosensor to caffeic acid.
Figure 6.
Linearity of response of the laccase containing biosensor to caffeic acid.
The response of the biosensor to caffeic acid was linear in the range 1.0 to 8.00×10-5M. The sensitivity of the method was 0.02401mAM-1.
An extensive study was done with the other polyphenols referred : gallic acid, malvidin, quercetin, rutin,
trans-resveratrol. Several applied potentials, as well as variation of the pH (3.0, 3.5, 4.5) were unsuccessful in providing a response of the biosensor with the laccase membrane, which would be a clear indication of a reduction, at the electrode, of the product of laccase driven oxidation of the relevant substrate. When testing the response of the biosensor at the negative potentials –300 and – 400mV, it was observed that there was an interference from the polyethersulfone membrane. At first we thought that a response could be obtained for rutin, at –400mV, similarly to what had been found before [
6], but we realized that there was a reaction between the substrate solution injected and the membrane, since the same response appeared when a blank polyethersulfone membrane was used.
Conclusions
The conditions to use enzymes appropriate to the detection of polyphenols existing in red wine in a biosensor were optimized. The required amount of laccase which was necessary to be immobilized on a derivatized polyethersulfone membrane was found and its combination with an amperometric transducer yielded a biosensor for (+)-catechin and caffeic acid at applied potentials of 100 and –50 mV, in aqueous and 12% ethanol acetate buffer. The linearity range was 1.0 to 6.0x10
-5 M, for catechin and 1.0 to 8.0x10
-5 M for caffeic acid, which covers the concentrations usually found in red wine [
17]. Sensitivities observed were 0.02443 mAM
-1 for catechin and 0.02401mAM
-1 for caffeic acid. No answer was obtained for gallic acid, malvidin, quercetin, rutin,
trans-resveratrol, which could be clearly ascribed to an actuation of the enzyme on the substrate and subsequent detection at the electrode. It is recommended that the base electrode is not used at negative applied potentials after having been subjected to positive applied potentials, since a strong interference of processes occurring in the films formed on the platinum surface would mask the laccase induced oxidation of the polyphenols.