Characterization of All Solid State Hydrogen Ion Selective Electrode Based on PVC-SR Hybrid Membranes
Abstract
:Introduction
Experimental
Reagents
Fabrication of Hydrogen Ion Selective Membrane Electrodes
| Entry Number | PVCa | DOAa | TDDAa | KTpClPBb | SRa | Slope | Selectivity, logK | ||
|---|---|---|---|---|---|---|---|---|---|
| Na+ | K+ | Ca2+ | |||||||
| 1 | 33.0 | 66.0 | 1.0 | 50.0 | 0.0 | 52.2 | -10.1 | -9.9 | -11.1 |
| 2 | 0.0 | 21.5 | 1.0 | 50.0 | 77.0 | 59.9 | -9.7 | -9.5 | -11.3 |
| 3 | 31.5 | 62.0 | 1.0 | 50.0 | 5.0 | 53.9 | -10.0 | -9.7 | -11.1 |
| 4 | 29.0 | 59.5 | 1.0 | 50.0 | 10.0 | 55.1 | -9.8 | -9.7 | -11.1 |
| 5 | 26.3 | 57.2 | 1.0 | 50.0 | 15.0 | 53.8 | -10.0 | -9.8 | -11.3 |
| 6 | 23.5 | 51.5 | 1.0 | 50.0 | 15.0 | 49.2 | -9.8 | -10.0 | -11.4 |
Apparatus and EMF Measurements
Results and Discussion
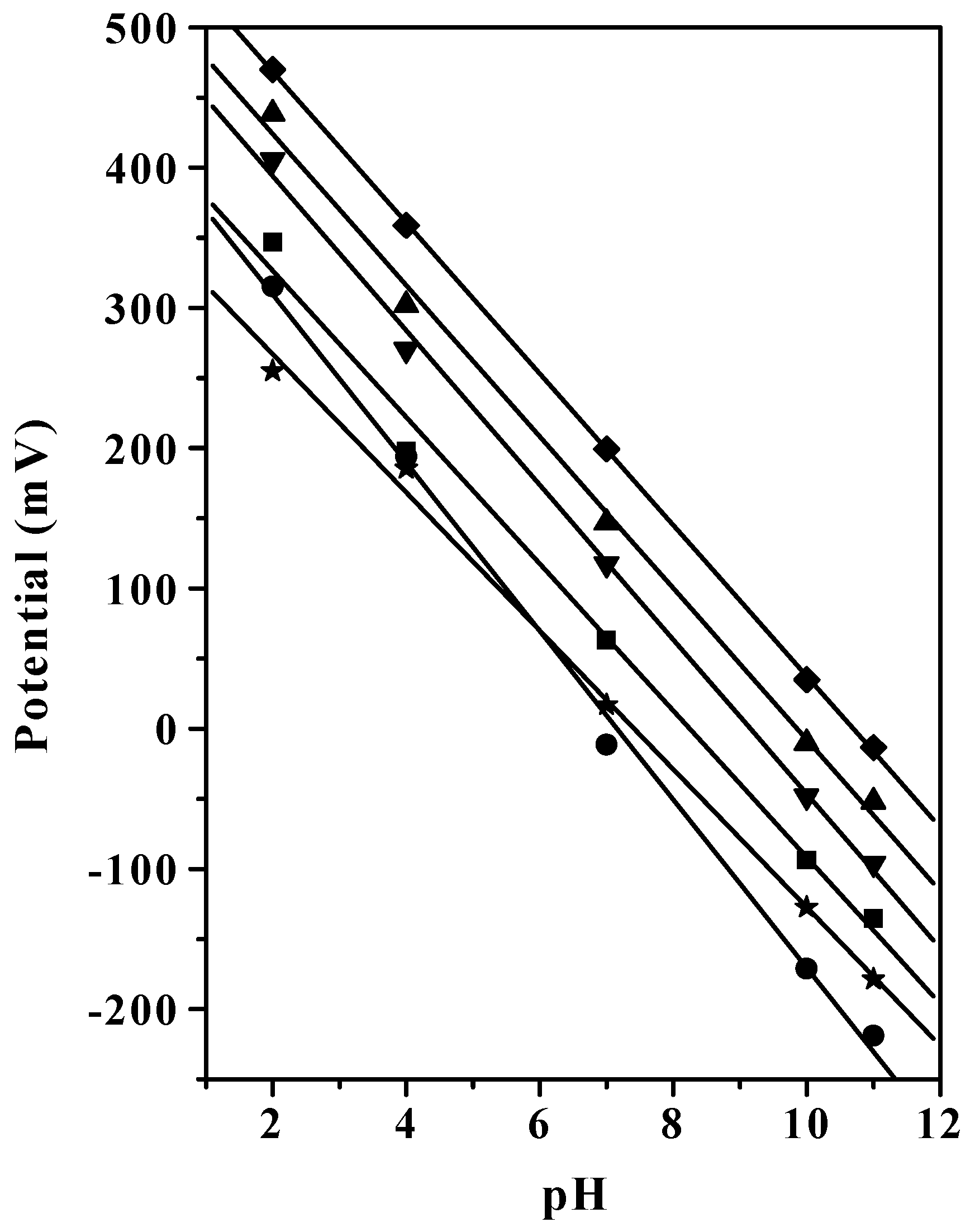
Potentiometric Response of PVC-SR Electrodes

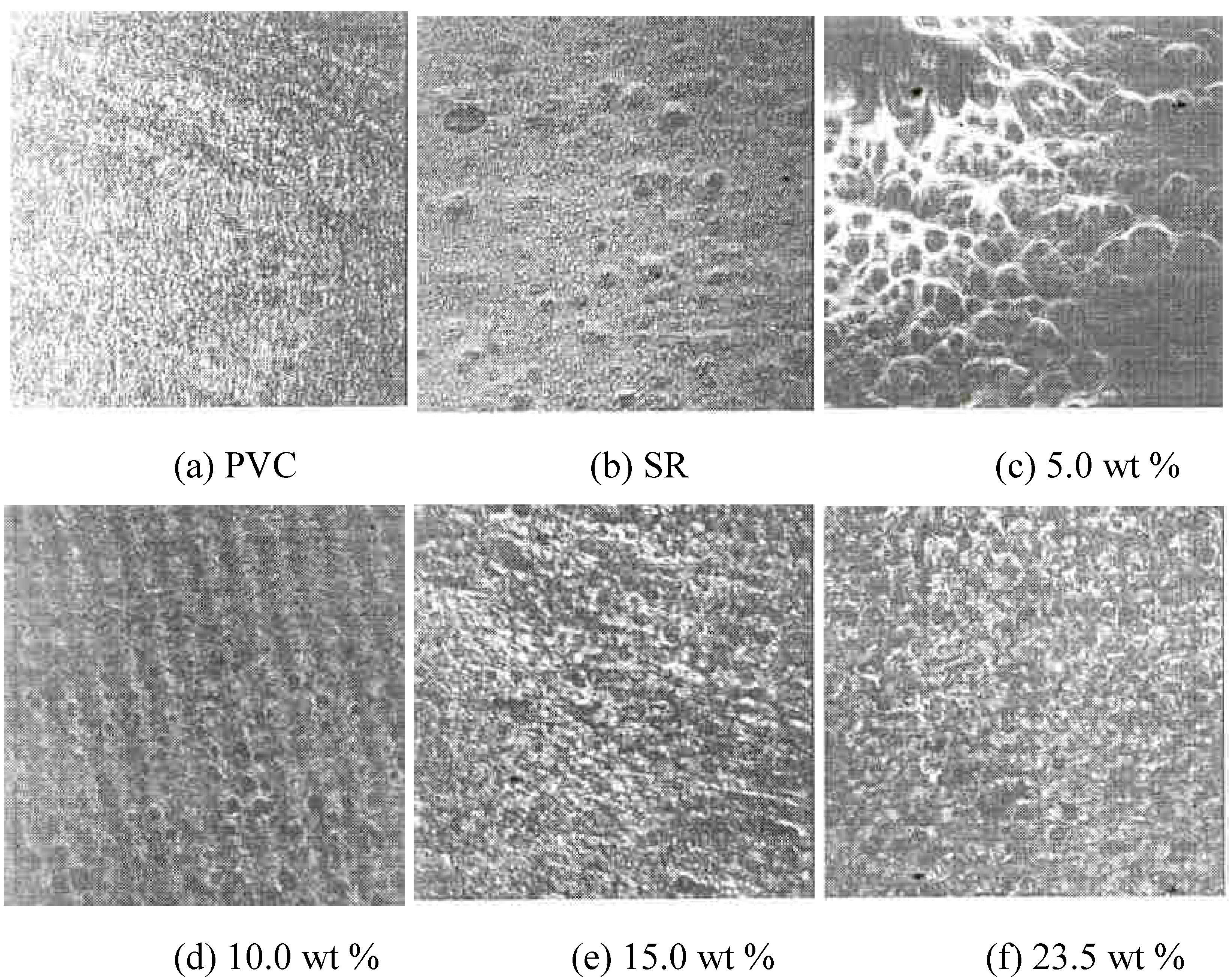
The Surface Morphorlogy According to SR Contents
Impedance Spectra of PVC-SR Membrane Coated Electrode

| R1 (Ω) | CPE1 | CPE2 | X2d | |||
|---|---|---|---|---|---|---|
| Y0b (Ω-1) | n | Y0b (Ω-1) | n | |||
| PVC | 4.2×104 | 9.1×10-9 | 0.84 | 1.0×10-5 | 0.67 | 4.9×10-4 |
| 5.0 wt % | 1.5×105 | 2.8×10-10 | 0.84 | 7.4×10-6 | 0.75 | 5.1×10-4 |
| 10.0 wt % | 3.5×105 | 3.3×10-10 | 0.83 | 4.6×10-6 | 0.71 | 5.7×10-4 |
| 15.0 wt % | 8.0×105 | 5.7×10-10 | 0.79 | 4.6×10-6 | 0.58 | 3.6×10-4 |
| 23.5 wt % | 2.8×106 | 3.5×10-10 | 0.83 | 2.8×10-6 | 0.55 | 4.7×10-4 |
Selectivity Coefficients and Real Sample Analysis

Conclusion
Acknowledgment
References
- Ammann, D.; Lanter, F.; Steiner, R. A.; Schulthess, P.; Shijo, Y.; Simon, W. Anal. Chem. 1981, 53, 2267–2269. [PubMed]
- Schlue, W. R.; Thomas, R. C. J. Physiol. 1985, 364, 327–338. [PubMed]
- Moss, S. D.; Janata, J.; Johnson, C. C. Anal. Chem. 1975, 47, 2238–2243.
- Mcbride, P.T.; Jánata, J.; Comte, P.A.; Moss, S.D.; Johnson, C.C. Anal. Chim. Acta 1978, 101, 239–245.
- Oesch, U.; Caras, S.; Janata, J. Anal. Chem. 1981, 53, 1983–1986.
- Janata, J.; Bezegh, A. Anal. Chem. 1988, 60, 62R–74R. [PubMed]
- Ye, Q.S.; Keresztes, Z.; Horvai, G. Electroanalysis 1999, 11, 729–734.
- Oesch, U.; Simon, W. Anal. Chem. 1980, 52, 692–700.
- Dinten, O.; Spichiger, U.; Chaniotakis, E.N.; Gehrig, P.; Rusterholz, B.; Morf, W.E.; Simon, W. Anal. Chem. 1991, 63, 596–603. [PubMed]
- Harrison, D.J.; Cunningham, L.L.; Li, X.; Teclemariam, A.; Permann, D. J. Electrochem. Soc. 1988, 135, 2473–2478.
- Moody, G. J.; Thomas, J.D.R.; Slater, M. J. M. Analyst 1988, 113, 1703–1707.
- Blackburn, G.; Janata, J. J. Electrochem. Soc. 1982, 129, 2580–2584.
- Cattrall, R.; Walles, P.J.; Hamilton, L.C. Anal. Chim. Acta 1985, 169, 403–406. [PubMed]
- Cardwell, T.J.; Cattrall, R.; Walles, P. J.; Hamilton, L.C. Anal. Chim. Acta 1985, 177, 239–242.
- Lindner, E.; Cosofret, V.V.; Kusy, R.P.; Buck, R.P.; Rosatzin, T.; Schaller, U.; Simon, W.; Jeney, J.; Tóth, K.; Pungor, E. Talanta 1993, 40, 957–967. [PubMed]
- Puig-Lleixà, C.; Jiménez, C.; Fàbregas, E.; Bartrolí, J. Sensors and Actuators B 1998, 49, 211–217.
- Malinowska, E.; Oklejas, V.; Hower, R.W.; Brown, R.B.; Meyerhoff, M.E. Sensors and Actuators B 1996, 33, 161–167.
- Moody, G.J.; Thomas, J.D.R. Analyst 1988, 113, 1703–1707.
- Ma, S.C.; Chaniotakis, N.A.; Meyerhoff, M.E. Anal. Chem. 1988, 60, 2293–2299. [PubMed]
- van der Wal, P.D.; Sudhölter, E.J.R.; Boukamp, B.A.; Bouwmeester, H.J.M.; Reinhoudt, D.N. J. Electroanal. Chem. 1991, 317, 153–168.
- Lindner, E.; Niegreisz, Z.; Toth, K.; Pongor, E.; Berube, T.R.; Buck, R.P. J. Electroanal. Chem. 1989, 259, 67–80.
- Punger, E.; Toth, K. Anal. Proc. 1985, 22, 352–353.
- Oh, B.K.; Kim, C.Y.; Lee, H.J.; Rho, K.L.; Cha, G.S.; Nam, H.H. Anal. Chem. 1996, 68, 503–508. [PubMed]
- Buck, R.; Lindner, P.E. Pure & Appl. Chem. 1994, 66, 2527–2536.
- Jeong, E.D.; Won, M.S.; Shim, Y.B. J. Power Sources 1998, 70, 70–77.
- Sample Availability: Available from the authors.
© 2003 by MDPI (http://www.mdpi.net). Reproduction is permitted for noncommercial purposes.
Share and Cite
Piao, M.-H.; Yoon, J.-H.; Jeon, G.; Shim, Y.-B. Characterization of All Solid State Hydrogen Ion Selective Electrode Based on PVC-SR Hybrid Membranes. Sensors 2003, 3, 192-201. https://doi.org/10.3390/s30600192
Piao M-H, Yoon J-H, Jeon G, Shim Y-B. Characterization of All Solid State Hydrogen Ion Selective Electrode Based on PVC-SR Hybrid Membranes. Sensors. 2003; 3(6):192-201. https://doi.org/10.3390/s30600192
Chicago/Turabian StylePiao, Ming-Hua, Jang-Hee Yoon, Gerok Jeon, and Yoon-Bo Shim. 2003. "Characterization of All Solid State Hydrogen Ion Selective Electrode Based on PVC-SR Hybrid Membranes" Sensors 3, no. 6: 192-201. https://doi.org/10.3390/s30600192




