Results and Discussion
Even trace amounts of organic solvent contamination in water impose a huge threat to the proper functionality of wastewater treatment plants. Therefore, they have to be detected and removed before reaching the biological clarifier. Although the detection directly in aqueous phase is in principle possible, it should be considered to use an apparatus that allows the application of gas phase sensing.
Figure 1.
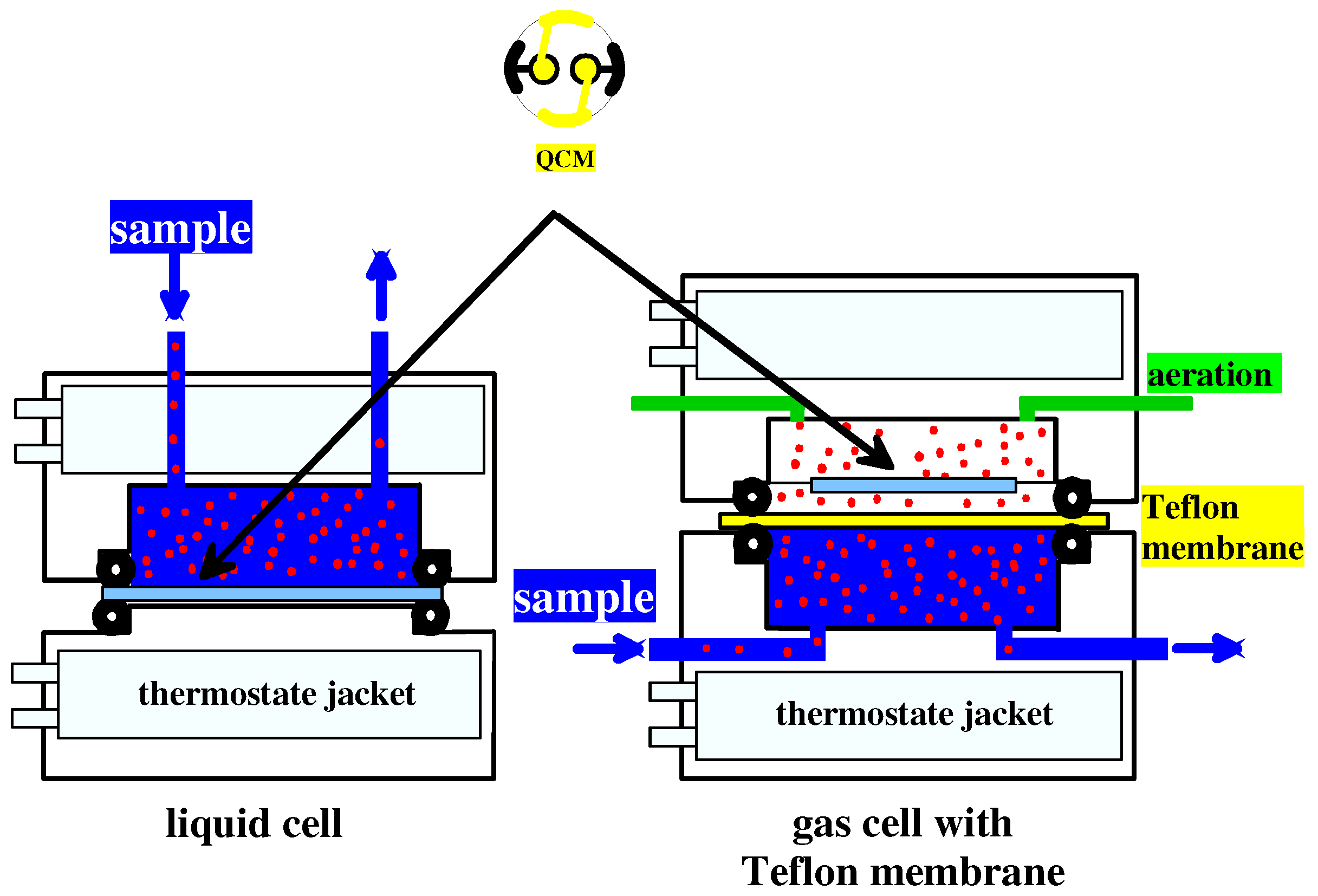
Liquid and gas cell for determining organic trace contaminants in wastewater
Figure 1.
Liquid and gas cell for determining organic trace contaminants in wastewater
This is favorable due to the substantially lower noise level occurring in the oscillators driving the respective mass-sensitive device, which results from decreased electronic loss of the sensor caused by viscous interactions between the sensor and its environment [
5].
Figure 1 shows both the experimental setups for liquid as well as for gaseous phase used for these measurements. Whereas the first consists of a flow-cell, where the sensitive area is directly exposed to the samples, in the second case the sensor chamber is separated from the water stream by the means of a thin Teflon membrane with pores sizing 200nm. Preliminary experiments showed that this is impermeable for water molecules, whereas organic solvents pass through readily.
Figure 2.
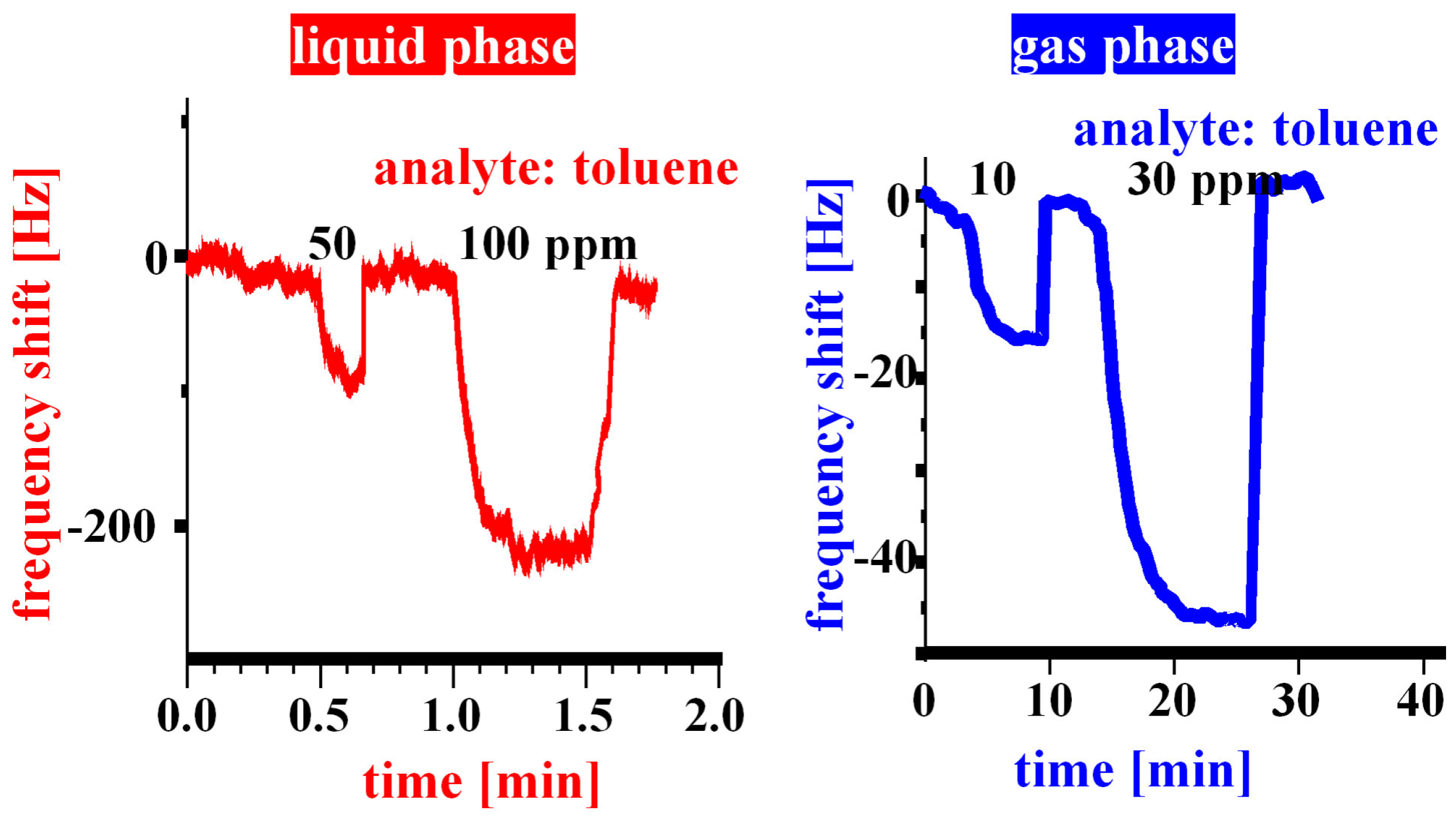
Sensor responses against toluene obtained in liquid and in gas phase.
Figure 2.
Sensor responses against toluene obtained in liquid and in gas phase.
The results of this procedure are given in
Figure 2, where typical sensor responses for either type of measurement are given. In both cases toluene was used for templating. Comparing the two datasets with each other, one can clearly see that especially the response time is highly affected by the measuring environments. The sensor immersed into the liquid reacts much faster than the one with the Teflon membrane separating the measurement chamber and the flow chamber from each other. The resulting sensor effects are in the same order of magnitude, therefore the thin polymer material obviously is fully permeable for the toluene molecules. The system with the gas sensing chamber reacts sluggishly, however, it shows the much lower noise level (around 1 Hz compared with about 10 Hz for the fluid cell) thus leading to lower detection limits, namely less than one ppm, whereas in the liquid it is about two orders of magnitude higher. This is a result of the higher electronic damping that QCMs undergo in contact with a fluid, because here a part of the oscillation energy is dissipated into the adjacent liquid layers due to viscous coupling with the resonating device. Thus, by suitable alterations of the sensor housing, the resulting system can be tuned to either fast reaction times or lower detection limits. Of course, on first sight it is not logical why the gas measurements proceed more slowly, because usually sensors exposed to gas streams have substantially lower response times than in liquids samples. Therefore, the sensor response in this case is determined by the coupled kinetics of analyte evaporation from the water through the porous membrane and the sensor-analyte interaction.
Of course, chemical sensors are not restricted to determine distinct analytes but also more complex phenomena can be evaulated. Although one could apply modern separation techniques in this case, sensors still offer the advantage of providing direct on-line measurements. However, a single sensor will usually fail in this case, as it is only capable of yielding a sum signal of all analyte changes. Therefore, applying an array of devices [
6] with up to six channels or more showing different selectivities followed by multivariate data analysis leads to reliable sensor results. We demonstrated this principle by mounting a QCM containing six electrode pairs in the headspace of a small composter and monitoring the concentrations of four predefined key-analytes, namely small aliphatic alcohols, ethyl acetate, limonene and humidity. Each of these compounds can be used as a lead analyte characterizing a different stage of plant degradation.
Figure 3.
Composter and sensor array used
Figure 3.
Composter and sensor array used
Figure 4.
Trends for three key analytes obtained by an artificial neural network.
Figure 4.
Trends for three key analytes obtained by an artificial neural network.
In special cases, however, even processes in complex mixtures can be observed by the means of one single sensor element, e.g. when monitoring engine oil degradation [
7], which are similar for different brands and types of oil. Therefore, it should be possible to design a sensor system that is capable of addressing all these oxidative processes and thus yielding a total cumulative signal. Organic polymers have been imprinted with the entire oil matrix of a fresh and a used material, the resulting sensors showed powerful selectivities. In this paper we want to concentrate on inorganic polymers, such as sol-gel materials. They have proven excellent properties concerning ruggedness, chemical tolerance and thermostability. When using Ti-alkoxides as precursors, thick layers with very low electronic loss are generated. This allows for the construction of a sensor system where the QCM is operated as the frequency-determining device in an oscillator circuit. Consequently, active measuring by applying a frequency counter can be used instead of (expensive) network analyzer equipment.
Figure 5.
Commercial oil level probe with mounted sensor and frequency response of the system on the change from fresh oil to waste oil.
Figure 5.
Commercial oil level probe with mounted sensor and frequency response of the system on the change from fresh oil to waste oil.
An example of a degradation sensor mounted on a commercial oil level probe can be found in
figure 5. The system is equipped with an electronic mixer device so that the chemical effects are directly read out, i.e. they are already corrected by the viscosity effects. Additionally,
figure 5 contains a sensor response curve at the change from fresh oil to waste oil. The resulting mass effect of 1.2 kHz can be measured with high statistical significance, especially when regarding the noise level in the range of 10 Hz. Thus, by the means of suitable imprinted polymers, the complex procedures occurring during the oxidative degradation of engine oils can be translated into a single frequency shift that contains the entirety of chemical information. This gives a very straightforward measurand for the remaining lubricant usability based on chemical information (rather than a physical one).
Usually, in molecular imprinting one single template is used to achieve ideal selectivity. However, there are analytical tasks where it has turned out to be advantageous to apply more than one template. Polycyclic aromatic hydrocarbons e.g. are members of a hazardous group of compounds that combine high toxicity and cancerogenous potential, so and thorough efforts are made for their analysis [
8]. Earlier measurements with single-imprinted chemical sensors showed good selectivities and excellent sensitivities [
9]. The sensitivity patterns obtained for different templates lead to the conclusion that the best re-inclusion can be observed for molecules being slightly larger than the template. To further investigate this phenomenon, mixtures of templates were tested [
10].
Figure 6 shows a resulting set of data, where both the ratio between the two template molecules as well as the polymerization temperature is varied.
Figure 6.
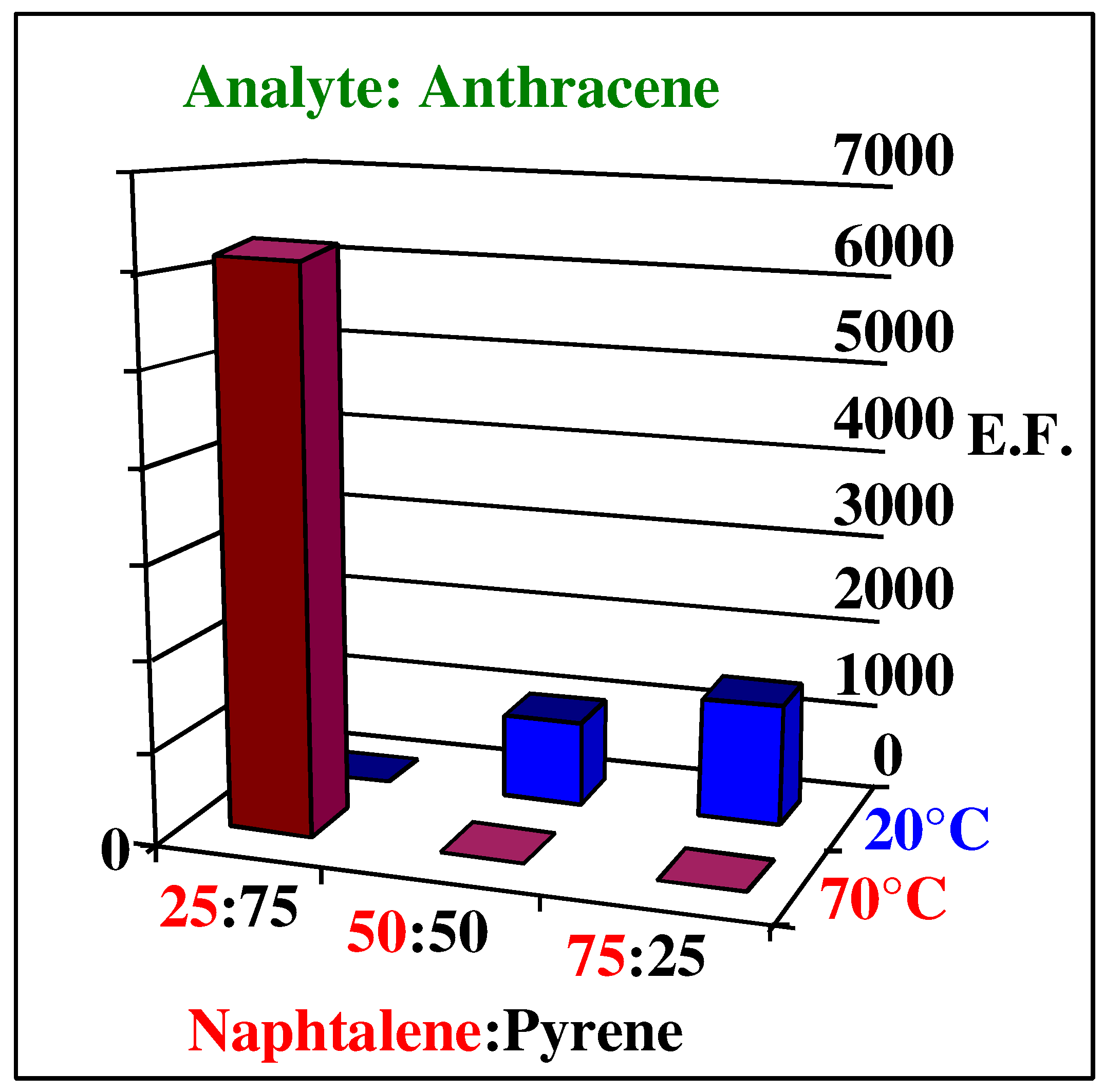
Enrichment factors of polyurethane layers imprinted with different template mixtures at 20°C and 70°C
Figure 6.
Enrichment factors of polyurethane layers imprinted with different template mixtures at 20°C and 70°C
As with the single molecular templates, the temperature again has a high influence on the geometrical fit. At 20°C anthracene is obviously incorporated into the cavities generated by naphthalene, whereas the ones caused by pyrene are already too large to allow optimized interactions. This is the same observation as for single imprinted layers, where we could show that at low temperatures the cavities produced are larger than the templates. A second evidence for this phenomenon is the fact that the enrichment of the analyte monotonously depends on the amount of naphthalene in the template mixture. At higher temperatures, where the geometrical arrangement between the forming polymer and the template is much better, the larger pyrene molecule determines the re-inclusion properties. The smaller template molecule here acts as a porogen and opens otherwise inaccessible sites in the material. This is underpinned by the fact that the signals achieved at 70°C are much higher than for the other layers thus suggesting optimized uptake of the analyte.
Imprinting strategies are not restricted to analytes of molecular scale, but also much larger particles can be used, such as enzymes or entire microorganisms. The synthetic procedures, however, have to be slightly modified, as imprinting into bulk materials is not possible in this case. First, for microorganisms with several µm in diameter the resulting layers would be much too thick to be useful for mass-sensitive detection. Second, as the analytes in this case are very bulky, they would diffuse through a three-dimensional polymer only very slowly (not speaking about the limitations imposed on template removal during synthesis) thus greatly enhancing the sensor response time and making it useless for sensing applications. For this purpose surface imprinting procedures have shown to be a suitable tool for sensor layer development. This principle has been successfully applied to yeast, that served as the microorganism used for the fundamental developments on the way to these sensors [
11].
Figure 7.
AFM-image of a yeast-imprinted polyurethane and sensor effect of that material towards a suspension of the template microorganism in buffer.
Figure 7.
AFM-image of a yeast-imprinted polyurethane and sensor effect of that material towards a suspension of the template microorganism in buffer.
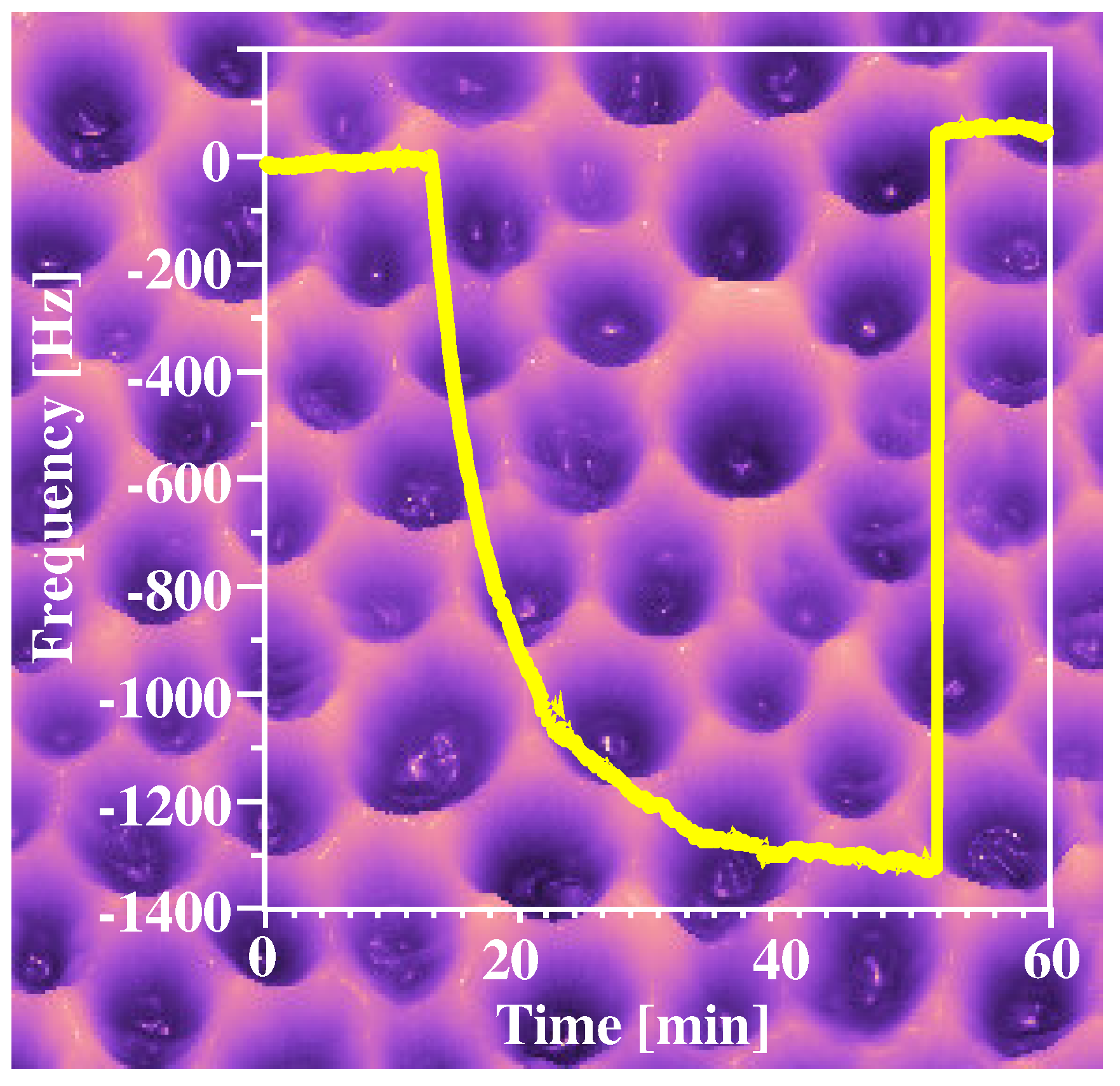
Figure 7 shows an AFM-image of one of these layers prepared by a surface stamping procedure as well as the respective sensor response obtained with a 10 MHz QCM towards yeast. The microorganisms on the stamp obtain a hexagonal packing and the pits left behind represent the typical size distribution of yeast cultures. These pits are optimally adapted to re-include cells from suspensions, which can also be seen in the sensor responses. The QCM frequency shift is perfectly reversible, the 1200 Hz obtained is the expected value for a monolayer of yeasts on the surface. Thus, the imprinted materials achieve antibody-like interaction properties towards the microorganisms used. The main difference to natural antibodies, however, is the ability to remove the cells from the material surface. This exactly meets the requirements of chemical sensors, as in this case high selectivity and reversibility is desired, which is often difficult to achieve.
Viruses represent a group of microorganisms, for which it would be highly interesting to develop selective sensors systems, especially because up to now no easy on-line method for their detection exists. This is a result of their size (below 1 µm) which makes them literally invisible for methods operating in the visible range of the electromagnetic spectrum (i.e. microscopy and scattering methods). The sensor layers are produced by a stamping method similarly to the yeast imprints, the procedure is shown in
figure 8.
Thus, the stamp and the polymer coating are prepared separately and the final material again is obtained by mechanically pressing the two substrates together. The polymerization takes place in the submicrometre-area around the templates that ensure an ideal adaptation of the cavities generated by forming an optimized interaction network between the reacting oligomeric chains and functional groups on the virus surface. An AFM-image of such a functionalized polymer is given in
figure 9.
The resulting pits once again can clearly be seen, however, the self organization of the yeasts is more pronounced. Nonetheless, the virus-temlated materials show large frequency shifts on mass-sensitive transducers, as can also be seen in
figure 10, where the sensor characteristic for such a TMV-imprinted material is given.
Figure 8.
Stamping technique for viruses
Figure 8.
Stamping technique for viruses
Figure 9.
TMV-imprinted polyurethane layer.
Figure 9.
TMV-imprinted polyurethane layer.
Figure 10.
Sensor characteristic for a TMV-imprinted material
Figure 10.
Sensor characteristic for a TMV-imprinted material
It shows several advantageous features: the dynamic range is comparably high (three orders of magnitude), in the lower concentration range of the suspension the sensor system shows very favourable sensitivity dynamic behaviour, as in this case the differential signal is much more pronounced than at higher concentrations. Another main advantage of the system shall be once more emphasized: these sensors represent a fast, on-line detection method for viruses and thus overcome the limitations imposed on optical methods in the visual range. Compared to sensors for biological analytes already existing [
12,
13,
14], these imprint layers represent artificial antibodies and thus combine biological selectivity with the stability of a man-made material.
Experimental
General
10 MHz-QCMs were prepared by screen-printing the desired electrode structure onto an AT-cut quartz blank with 10mm or 15.5 mm diameter and burning the organic residues afterwards. Mass-sensitive measurements were carried out with a home-made oscillator circuit connected to commercially available frequency counters (HP 53131A and Keithley 775A), the resulting sensor data is read out into a computer by a self-written software.
Layer synthesis
Imprinted polystyrene layers for the detection of organic solvent traces in water are synthesized by mixing 210 µl of divinylbenzene (DVB), 90 µl of styrene, 3 mg of azo-isobutyronitrile (AIBN) and 4.4 mg biphenyl (working as additional porogen) with 100µl of the imprinting template, i.e. benzene or toluene. The resulting solution was spin-coated onto the QCM and polymerized over night at 40°C in a saturated solvent athmosphere. Afterwards the sensors are washed with o-xylene and dried at 150°C to remove the template as well as the biphenyl.
The six sensitive materials used as array coatings in composting were prepared as follows:
PVA(H2O): for preparation10 mg of polyvinylalcohol with a molar mass of 15000 g mol-1 is dissolved in 25 ml of deionized water. 60 mg of acrylic acid is added to this mixture together with a small amount of AIBN and pre-polimerized for 2 hours at 70°C.
PS(Lim): A mixture of 30 µl of styrene, 70 µl of divinylbenzene (DVB), 1 mg of AIBN and 300 µl of limonene is pre-polymerized for 15 min at 45°C. For spin-coating the resulting solution was diluted 1+4 with limonene.
PS (EtAc): The polymer was synthesized by mixing 40 µl of styrene and 60 µl of divinylbenzene together with 1 mg of AIBN. After pre-polymerizing for 10 min at 70°C 200 µl of ethyl acetate were added.
PU(PrOH) and PU(BuOH): For preparing the polyurethane 1 g of DPDI was mixed with 1.97 g of bisphenol A (BPA), 0.22 g of phloroglucinol and 2 ml of tetrahydrofurane (THF). After dissolution 970 µl of the respective alcohol (1-propanol or 1-butanol) were added to 30 µl of the pre-reacted solution, for spin-coating both mixtures were diluted 1+30 with the template solvent.
PU(EtAc): Solutions of 2.5 mmol of DPDI, BPA, phloroglucinol and triethanolamine (TEA) in 2 ml of ethyl acetate were prepared, respectively. These were mixed in the following ratio: 500 µl ethyl acetate, 148 µl BPA, 90 µl ploroglucinol, 10 µl TEA and 231 µl BPA. For preparing the layers the solution was diluted 1+9 with ethyl acetate.
For the synthesis of PAH-imprinted polyurethanes 4,4´-diisocyanato-diphenylmethane (DPDI) and polyethyleneglycol (molar mass 200; PEG200) and the template (mixtures) were dissolved in water-free pyridine, respectively. The resulting solutions contained 100 mg/ml of the monomer and were mixed in the following ratio: 545µl DPDI, 455 µl PEG200 and 50µl template yielding to a total of 5% imprint molecule in the matrix. After 15 minutes of pre-polymerisation the mixture was spin-coated onto the device and the template removed by heating at 90°C for 45 minutes.
Polyurethane was imprintied with microorganisms (yeasts). Bisphenol A, 4,4'-diphenylmethanediisocyanate and phloroglucinol were pre-polymerized in tetrahydrofurane (THF) at 70°C for around one hour until the gel point was reached. The ratio of isocyanate:hydroxy functionalities was 1:2. The diluted pre-polymer is drop or spin coated (~ 1000 rpm).
Polymers from divinylbenzene, (meth-)acrylic acid, 4-vinylpyridine and styrene were again prepolymerized at 70°C in THF using azoisbutyronitrile (AIBN) as radical starter. The polymer solutions are spin coated with up to 5000 rpm to generate flat surfaces. After stamping the polymer is cured with UV light (substrate and QCM are made of quartz)