Improvement of Amperometric Sensor Used for Determination of Nitrate with Polypyrrole Nanowires Modified Electrode
Abstract
:1. Introduction
2. Experimental
Reagents
Apparatus
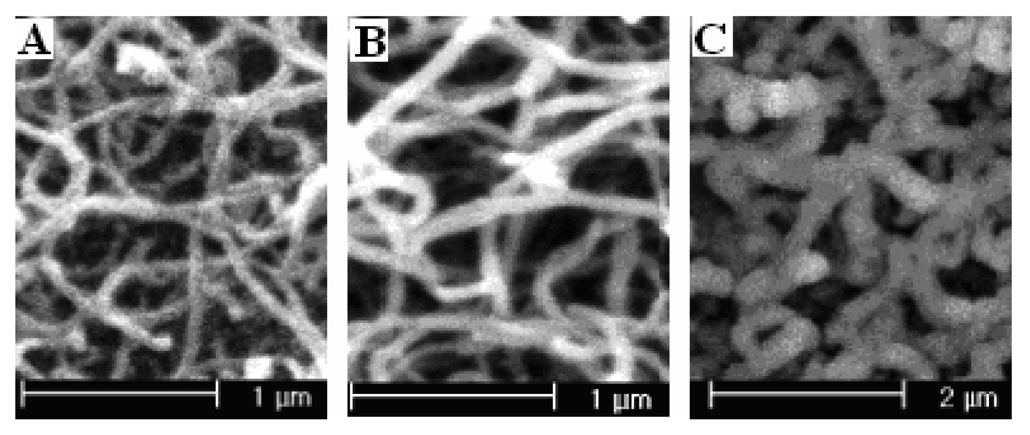
Preparation method of PPy nanowire modified electrodes
Measurement
3. Results and discussion
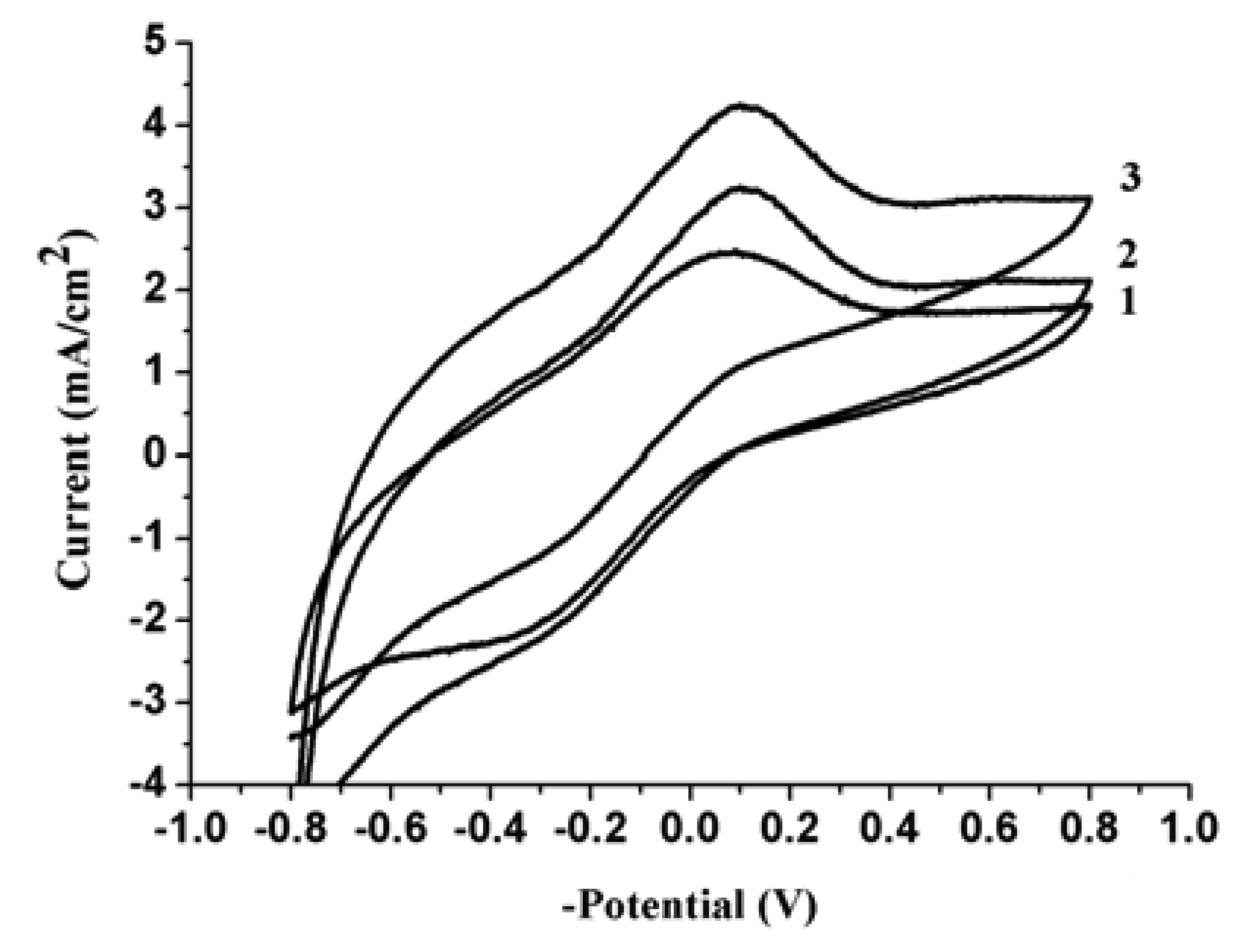
3.1. Cyclic voltammetry investigations of NO3- at PPy nanowires modified electrode
3.2. Effect of polymerization potential on the response characteristics
3.3. Effect of polymerization temperature on the response characteristics
3.4. Effect of polymerization time on the response characteristics
3.5. Effect of scan rate, upper anodic potential limits and number of cyclic voltammetric during polymerization of PPy on response characteristics of modified electrodes
3.6. Effect of test solution pH
3.7. Interference study
3.8. Reproducibility and stability of the modified electrode in Determination of NO3-
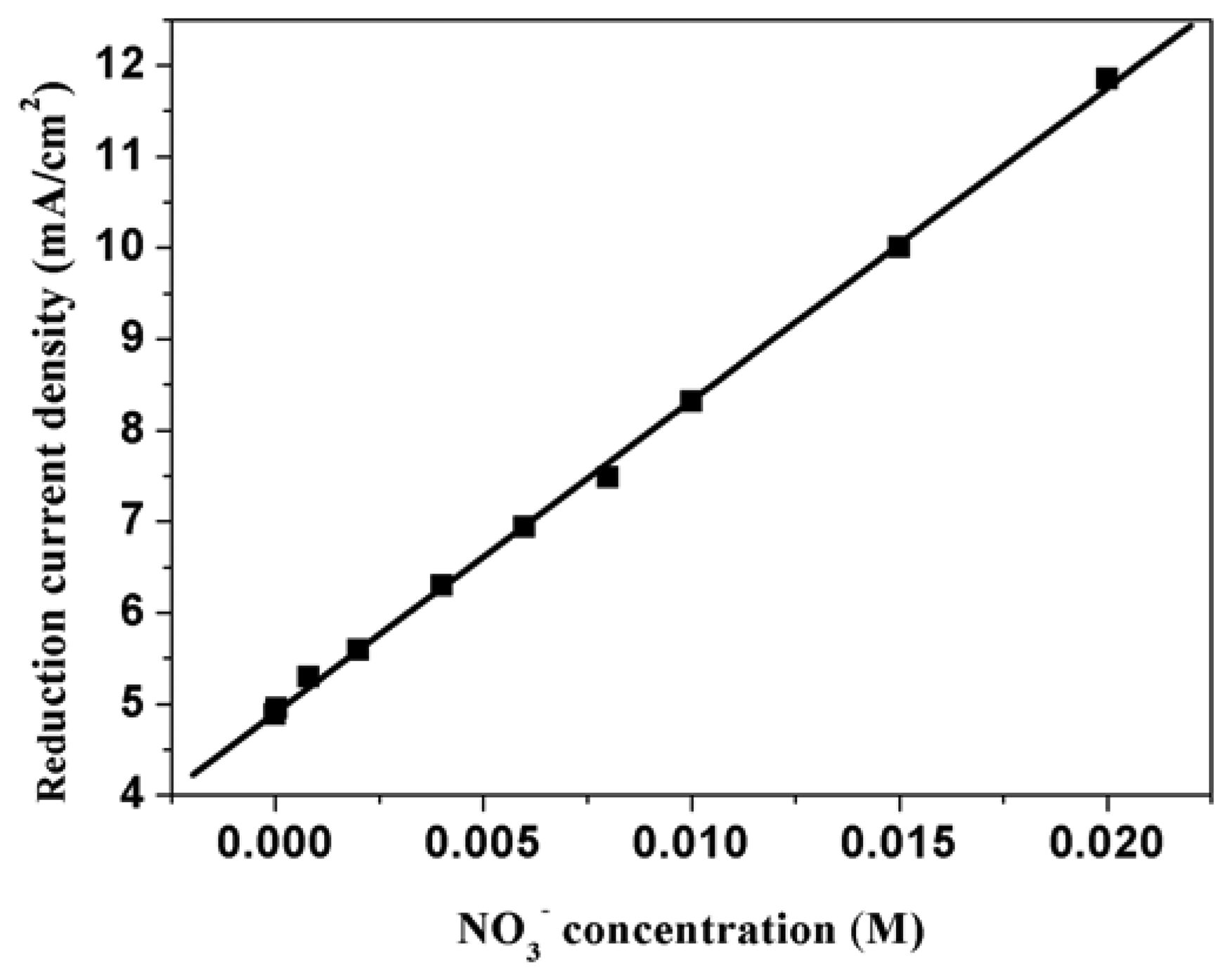
3.9. Applications
4. Conclusions
References
- Liu, H. Q.; Kameola, J.; Czaplewski, D. A.; Craighead, H. G. Polymeric nanowire chemical sensor. Nano. Lett. 2004, 4, 671–675. [Google Scholar]
- Alam, M. M.; Wang, J.; Guo, Y. Y.; Lee, S. P.; Tseng, H. R. Electrolyte-gated transistors based on conducting polymer nanowire junction arrays. J. Phys. Chem. B. 2005, 109, 12777–12784. [Google Scholar]
- Ramanathan, K.; Bangar, M. A.; Yun, M. H.; Chen, W.; Mulchandani, A.; Myung, N. V. Individually addressable conducting polymer nanowires array. Nano. Lett. 2004, 4, 1237–1239. [Google Scholar]
- Tsikas, D.; Gutzki, F. M.; Rossa, S.; Bauer, H.; Neumann, C.; Dockendorff, K.; Sandmann, J.; Frölich, J. C. Measurement of nitrite and nitrate in biological fluids by gas chromatography-mass spectrometry and by the Griess assay: problems with the Griess assay-solutions by gas chromatography-mass spectrometry. Anal. Biochem. 1997, 244, 208–220. [Google Scholar]
- Stratford, M. R. L. Measurement of nitrite and nitrate by high-performance ion chromatography. Method. Enzymol. 1999, 301, 259–269. [Google Scholar]
- Di Matteo, V.; Pierucci, M.; Esposito, E. Methods for the determination of nitrite by high-performance liquid chromatography with electrochemical detection. J. Chromatogr. A. 1997, 789, 213–219. [Google Scholar]
- Hussein, W. R.; Guilbault, G. G. Nitrate and ammonium ion selective electrodes as sensors. I. In bacterial growth curves for isolation of nitrate and nitrite reductases from Escherichia coli. Anal. Chim. Acta. 1974, 72, 381–390. [Google Scholar]
- Davenport, R. J.; Johnson, D. C. Voltammetric determination of nitrate and nitrite ions using a rotating cadmium disk electrode. Anal. Chem. 1973, 45, 1979–1980. [Google Scholar]
- Casella, I. G.; Gatta, M. Electrochemical reduction of NO3- and NO2- on a composite copper thallium electrode in alkaline solutions. J. Electroanal. Chem. 2004, 568, 183–188. [Google Scholar]
- Da Silva, S.; Shan, D.; Cosnier, S. Improvement of biosensor performances for nitrate determination using a new hydrophilic poly(pyrrole-viologen) film. Sensor. Actuat. B-Chem. 2004, 103, 397–402. [Google Scholar]
- Jain, A. K.; Gupta, V. K.; Singh, L. P.; Raisoni, J. R. Chelating ionophore based membrane sensors for copper(II) ions. Talanta. 2005, 66, 1355–1361. [Google Scholar]
- Han, G. Y.; Shi, G. Q. Conducting polymer electrochemical actuator made of high-strength three-layered composite films of polythiophene and polypyrrole. Sensor. Actuat. B-Chem. 2004, 99, 525–531. [Google Scholar]
- Lei, J. P.; Ju, H. X.; Ikeda, O. A novel supramolecular assembly film of porphyrin bound DNA: Characterization and catalytic behaviors towards nitric oxide. Sensor. 2005, 5, 171–184. [Google Scholar]
- Da Rocha, J. R. C.; Angnes, L.; Bertotti, M.; Araki, K.; Toma, H. E. Amperometric detection of nitrite and nitrate at tetraruthenated porphyrin-modified electrodes in a continuous-flow assembly. Anal. Chim. Acta. 2002, 452, 23–28. [Google Scholar]
- Asghari, A.; Amini, M. K.; Mansour, H. R.; Salavati-Niasari, M.; Rajabi, M. Nitrate-selective membrane electrode based on bis(2-hydroxyanil)acetylacetone lead(II) neutral carrier. Anal. Sci. 2003, 19, 1121–1125. [Google Scholar]
- Badea, M.; Amine, A.; Palleschi, G.; Moscone, D.; Volpe, G.; Curulli, A. New electrochemical sensors for detection of nitrites and nitrates. J. Electroanal. Chem. 2001, 509, 66–72. [Google Scholar]
- Davis, J.; Moorcroft, M. J.; Wilkins, S. J.; Compton, R. G.; Cardosi, M. Electrochemical detection of nitrate at a copper modified electrode under the influence of ultrasound. Electroanal. 2000, 12, 1363–1367. [Google Scholar]
- Ugo, P.; Moretto, L. M.; Mazzocchin, G. A.; Guerriero, P.; Martin, C. R. Electrochemical preparation and characterization of an anion-permselective composite membrane for sensor technology. Electroanal. 1998, 10, 1168–1173. [Google Scholar]
- Cosnier, S.; Galland, B.; Innocent, C. New electropolymerizable amphiphilic viologens for the immobilization and electrical wiring of a nitrate reductase. J. Electroanal. Chem. 1997, 433, 113–119. [Google Scholar]
- Sun, B.T.; Fitch, P. G. Nitrate ion-selective sensor based on electrochemically prepared conducting polypyrrole films. Electroanal. 1997, 6, 494–497. [Google Scholar]
- Hutchins, R. S.; Bachas, L. G. Nitrate-selevtive electrode developed by electrochemically mediated imprinting/doping of polypyrrole. Anal. Chem. 1995, 67, 1654–1660. [Google Scholar]
- Cosnier, S.; Innocent, C.; Jouanneau, Y. Amperometric detection of nitrate via a nitrate reductase immobilized and electrically wired at the electrode surface. Anal. Chem. 1994, 66, 3198–3201. [Google Scholar]
- Kang, S. C.; Lee, K. S.; Kim, J. D.; Kim, K. J. Polypyrrole modified electrode as a nitrate sensor. Bull. Korean Chem. Soc. 1990, 11, 124–126. [Google Scholar]
- Rau, J. R.; Chen, S. C.; Sun, H. W. Characterization of a polypyrrole microsensor for nitrate and nitrite ions. Electrochim. Acta. 1994, 39, 2773–2779. [Google Scholar]
- Wu, J. C.; Mullett, W. M.; Pawliszyn, J. Electrochemically controlled solid-phase microextraction based on conductive polypyrrole films. Anal. Chem. 2002, 74, 4855–4859. [Google Scholar]
- Bruheim, I.; Liu, X. C.; Pawliszyn, J. Thin-film microextraction. Anal. Chem. 2003, 75, 1002–1010. [Google Scholar]











| J(mA/cm2) | Standard deviation (SD) | Mean | RSD(%) |
|---|---|---|---|
| 4.947 | |||
| 4.932 | |||
| 4.915 | 0.09258 | 4.8822 | 1.896 |
| 4.897 | |||
| 4.720 |
| Samples | (A) Proposed method (10-4 mol 1-1) | (B) Ion chromatography (10-4 mol 1-1) | (B-A)/B (%) |
|---|---|---|---|
| water | 1.163 | 1.221 | 4.75 |
| River | 4.742 | 4.839 | 2.01 |
© 2005 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Zhang, X.-l.; Wang, J.-x.; Wang, Z.; Wang, S.-c. Improvement of Amperometric Sensor Used for Determination of Nitrate with Polypyrrole Nanowires Modified Electrode. Sensors 2005, 5, 580-593. https://doi.org/10.3390/s5120580
Zhang X-l, Wang J-x, Wang Z, Wang S-c. Improvement of Amperometric Sensor Used for Determination of Nitrate with Polypyrrole Nanowires Modified Electrode. Sensors. 2005; 5(12):580-593. https://doi.org/10.3390/s5120580
Chicago/Turabian StyleZhang, Xiu-ling, Ji-xiao Wang, Zhi Wang, and Shi-chang Wang. 2005. "Improvement of Amperometric Sensor Used for Determination of Nitrate with Polypyrrole Nanowires Modified Electrode" Sensors 5, no. 12: 580-593. https://doi.org/10.3390/s5120580




