Studies on the Interaction Mechanism of 1,10-Phenanthroline Cobalt(II) Complex with DNA and Preparation of Electrochemical DNA Biosensor
Abstract
:1. Introduction
2. Experimental Section
2.1. Instrumentation
2.2 Reagents
2.3 Spectroscopic study on the interaction between [Co(phen)2(Cl)(H2O)]+ and DNA
2.4. Electrochemical study on the interaction between [Co(phen)2(Cl)(H2O)]+ and DNA
2.5. Preparation of the electrochemical DNA sensor
3. Results and Discussion
3.1. Fluorescence spectroscopic studies of the interaction between [Co(phen)2(Cl)(H2O)]+ and DNA
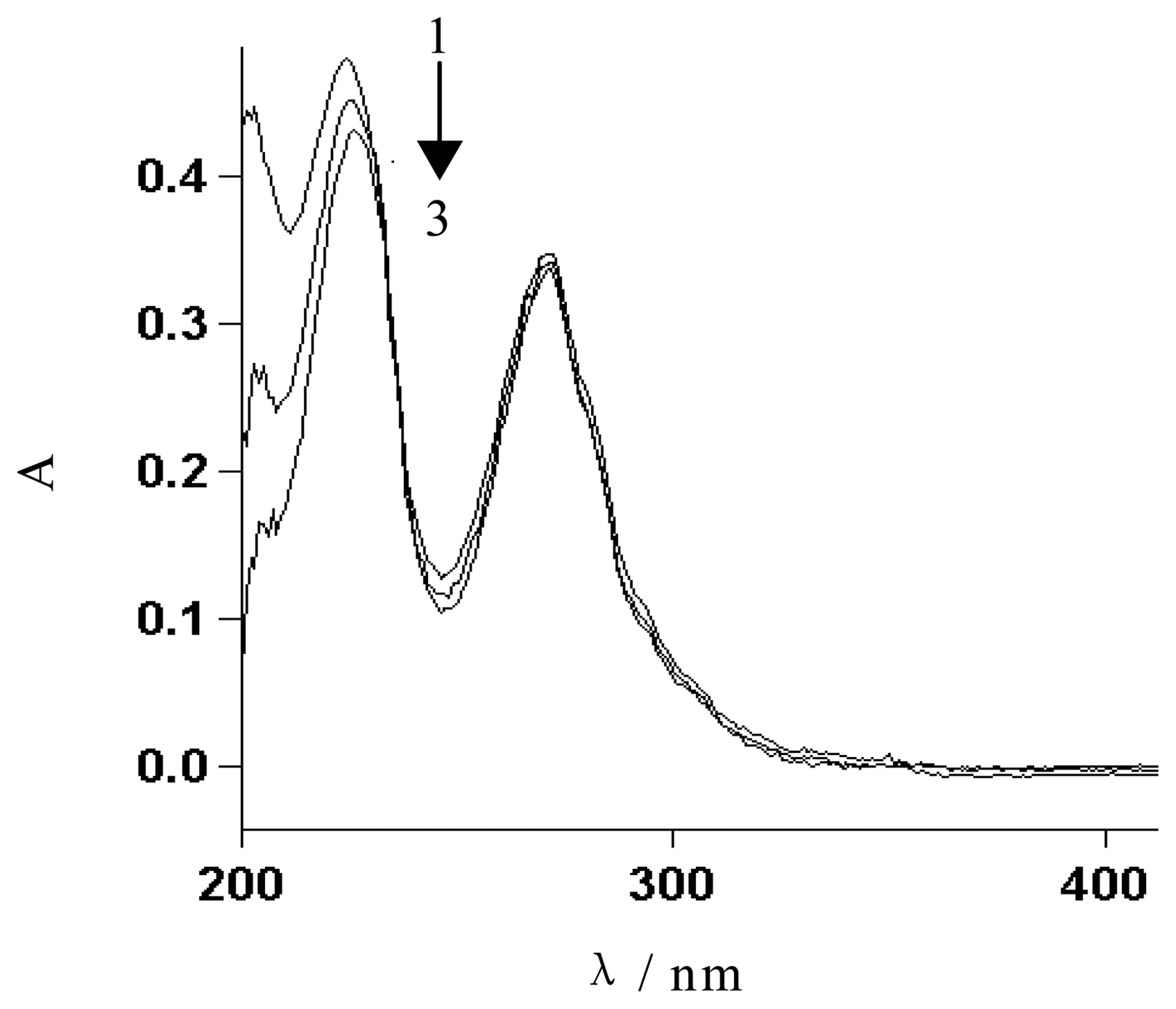
3.2. UV spectroscopic studies of the interaction between [Co(phen)2(Cl)(H2O)]+ and DNA
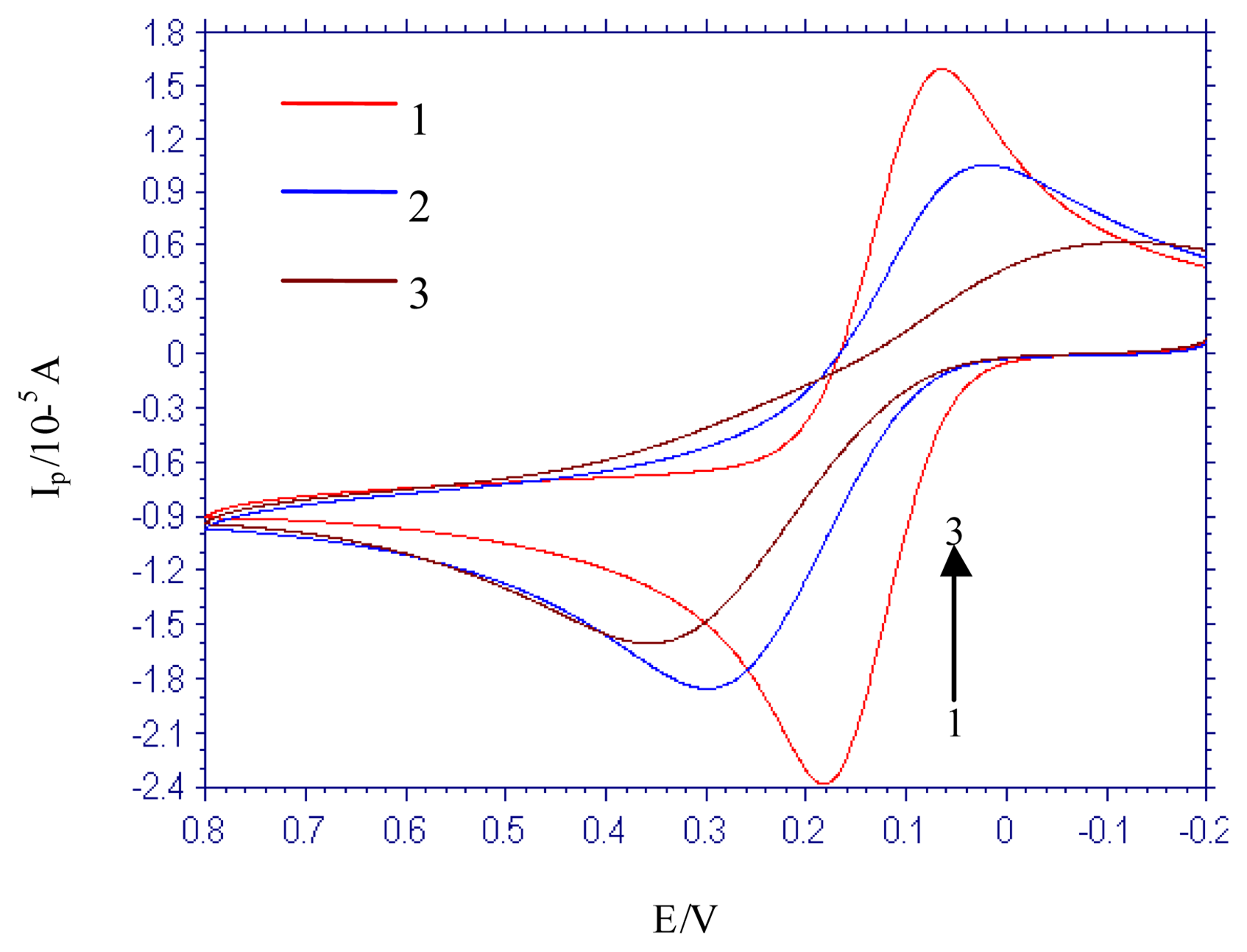
3.3. Electrochemical study on the interaction between [Co(phen)2(Cl)(H2O)]+ and DNA
3.4. The electrochemical characterization of modified GCE
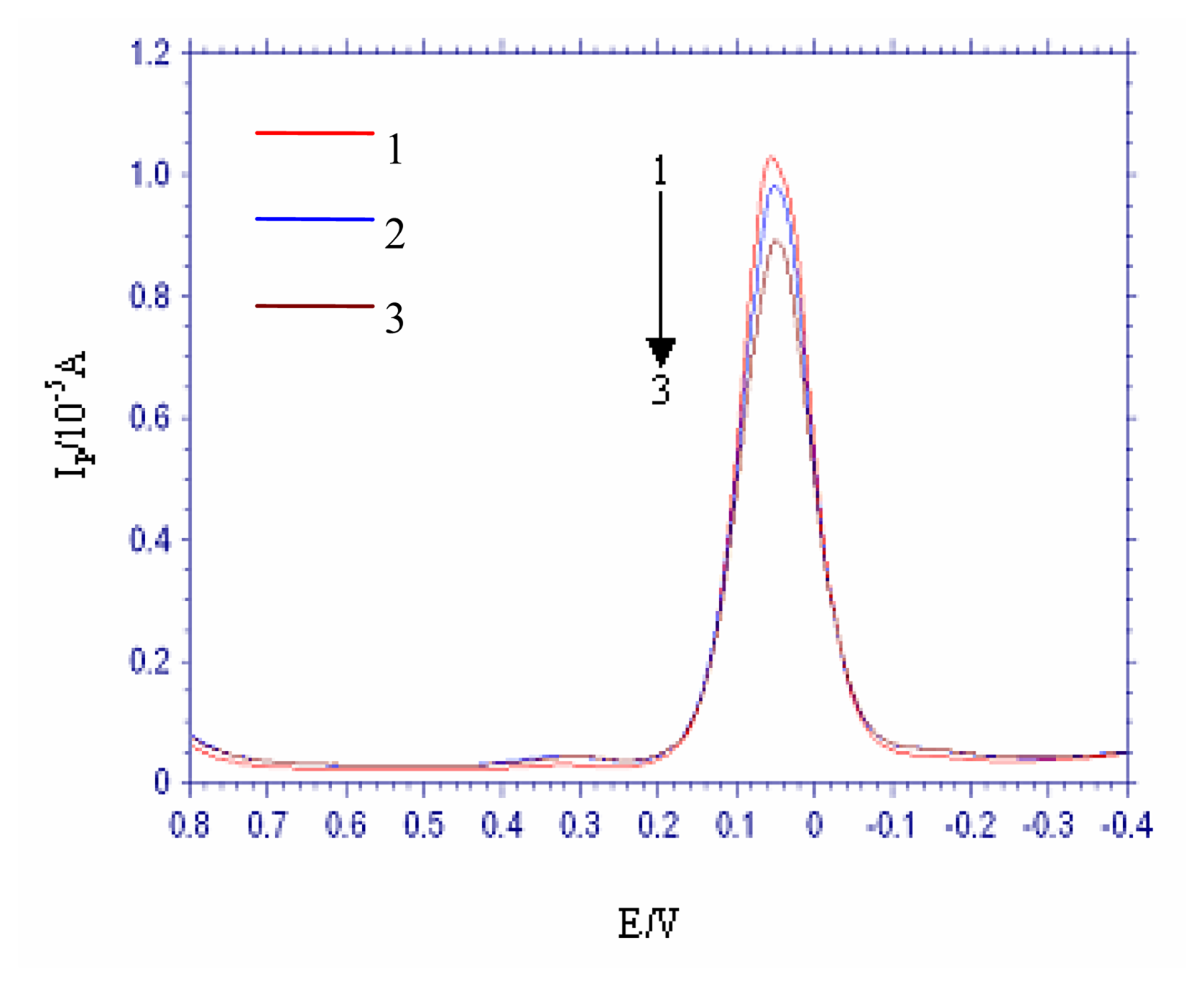
3.5. The selectivity of the DNA electrochemical biosensor
Acknowledgments
References and Notes
- Millan, K. M.; Saraullo, A.; Mikkelsen, S. R. Voltammetric DNA Biosensor for Cystic Fibrosis Based on a Modified Carbon Paste Electrode. Anal. Chem. 1994, 66, 943–2948. [Google Scholar]
- Wang, J.; Cai, X. H.; Rivas, G.; Shiraishi, H.; Farias, P. A. M.; Dontha, N. DNA Electrochemical Biosensor for the Detection of Short DNA Sequences Related to the Human Immunodeficiency Virus. Anal. Chem. 1996, 68, 2629–2934. [Google Scholar]
- Koji, H.; Keiko, I.; Yoshio, I. Novel DNA Sensor for Electrochemical Gene Detection. Anal. Chim. Acta 1994, 286, 219–224. [Google Scholar]
- Erdem, A.; Kosmider, B.; Osiecka, R.; Zyner, E.; Ochocki, J.; Ozsoz, M. Electrochemical genosensing of the Interaction Between the Potential Chemotherapeutic Agent, cis-bis(3-aminoflavone)dichloroplatinum(II) and DNA in Comparison with cis-DDP. J. Pharmaceut. Biomed. 2005, 38, 645–652. [Google Scholar]
- Rauf, S.; Gooding, J. J.; Akhtar, K.; Ghauri, M. A.; Rahman, M.; Anwar, M. A.; Khalid, A. M. Electrochemical Approach of Anticancer Drugs–DNA Interaction. J. Pharmaceut. Biomed. 2005, 37, 205–217. [Google Scholar]
- Wang, J.; Cai, X. H.; Rivas, G.; Shiraishi, H. Stripping Potentiometric Transduction of DNA Hybridization Processes. Anal. Chim. Acta 1996, 326, 141–147. [Google Scholar]
- Wang, J.; Rivas, G.; Cai, X. H.; Dontha, N.; Shiraishi, H.; Luo, D. B.; Valera, F. S. Sequence-specific Electrochemical Biosensing of M-tuberculosis DNA. Anal. Chim. Acta 1997, 337, 41–48. [Google Scholar]
- Ozkan, D.; Karadeniz, H.; Erdem, A.; Mascini, M.; Ozsoz, M. Electrochemical Genosensor for Mitomycin C–DNA Interaction Based on Guanine Signal. J. Pharmaceut. Biomed. 2004, 35, 905–912. [Google Scholar]
- Karadeniz, H.; Gulmez, B.; Sahinci, F.; Erdem, A.; Kaya, G. I.; Unver, N.; Kivcak, B.; Ozsoz, M. Disposable Electrochemical Biosensor for the Detection of the Interaction Between DNA and Lycorine based on Guanine and Adenine Signals. J. Pharmaceut. Biomed. 2003, 33, 295–302. [Google Scholar]
- Millan, K. M.; Mikkelsen, S. R. Sequence-selective Biosensor for DNA Based on Electroactive Hybridization Indicators. Anal. Chem. 1993, 65, 2317–2323. [Google Scholar]
- Pang, D. W.; Abruna, H. D. Interactions of Benzyl Viologen with Surface-Bound Single- and Double-Stranded DNA. Anal. Chem. 2000, 72, 4700–4706. [Google Scholar]
- Ju, H. X.; Ye, Y. K.; Zhao, J. H.; Zhu, Y. L. Hybridization Biosensor Using di(2,2′-bipyridine)osmium (III) as Electrochemical Indicator for Detection of Polymerase Chain Reaction Product of Hepatitis B Virus DNA. Anal. Biochem. 2003, 313, 255–261. [Google Scholar]
- Girousi, S. T.; Gherghi, I. C.; Karava, M. K. DNA-modified Carbon Paste Electrode Applied to the Study of Interaction Between Rifampicin (RIF) and DNA in Solution and at the Electrode Surface. J. Pharmaceut. Biomed. 2004, 36, 851–858. [Google Scholar]
- Fin, L.; Yang, P. Synthesis and DNA Binding Studies of Cobalt (III) Mixed-polypyridyl Complex. J. Inorg. Biochem. 1997, 68, 79–83. [Google Scholar]
- Jin, B. K.; Ji, X. P.; Nakamura, T. Voltammetric Study of Interaction of Co(phen)33+ with DNA at Gold Nanoparticle Self-assembly Electrode. Electrochimica Acta 2004, 50, 1049–1055. [Google Scholar]
- Srinivasan, S.; Annaraj, J.; Athappan, P. R. Spectral and Redox Studies on Mixed Ligand Complexes of Cobalt(III) Phenanthroline/bipyridyl and Benzoylhydrazones, Their DNA Binding and Antimicrobial Activity. J. Inorg Biochem. 2005, 99, 876–882. [Google Scholar]
- Sastri, C. V.; Eswaramoorthy, D.; Giribabu, L.; Maiya, B. G. DNA Interactions of New Mixed-ligand Complexes of Cobalt(III) and Nickel(II) that Incorporate Modified Phenanthroline Ligands. J. Inorg Biochem. 2003, 94, 128–145. [Google Scholar]
- Zhao, P. S.; Lu, L. D.; Jian, F. F. Structure of Chloro bis(1,10-phenanthroline)cobalt(II) Complex, [Co(phen)2(Cl)(H2O)]Cl·2H2O. J. Korean Chem. Soc. 2003, 47, 334–336. [Google Scholar]
- Zhang, S. S.; Niu, S. Y.; Qu, B.; Jie, G. F.; Xu, H.; Ding, C. F. Studies on the Interaction Mechanism Between Hexakis(imidazole) Manganese(II) Terephthalate and DNA and Preparation of DNA Electrochemical Sensor. J. Inorg. Biochem. 2005, 99, 2340–2347. [Google Scholar]
- Song, Y. M.; Yang, P. J.; Wang, L. F.; Yang, M. L.; Kang, J. W. Study on the Interactions Between Sm(RA)2·Ac·4H2O and DNA. Acta Chim. Sinica 2003, 61, 1266–1270. [Google Scholar]
- Long, E. C.; Barton, J. K. On Demonstrating DNA Intercalation. Accounts Chem. Res. 1990, 23, 271–273. [Google Scholar]
- Carter, M. T.; Rodriguez, M.; Bard, A. J. Voltammetric Studies of the Interaction of Metal Chelates with DNA. 2. Tris-chelated Complexes of Cobalt(III) and Iron(II) with 1,10-phenanthroline and 2,2′-bipyridine. J. Am. Chem. Soc. 1989, 111, 8901–8911. [Google Scholar]




© 2006 by MDPI ( http://www.mdpi.org) Reproduction is permitted for noncommercial purposes.
Share and Cite
Niu, S.; Li, F.; Zhang, S.; Wang, L.; Li, X.; Wang, S. Studies on the Interaction Mechanism of 1,10-Phenanthroline Cobalt(II) Complex with DNA and Preparation of Electrochemical DNA Biosensor. Sensors 2006, 6, 1234-1244. https://doi.org/10.3390/s6101234
Niu S, Li F, Zhang S, Wang L, Li X, Wang S. Studies on the Interaction Mechanism of 1,10-Phenanthroline Cobalt(II) Complex with DNA and Preparation of Electrochemical DNA Biosensor. Sensors. 2006; 6(10):1234-1244. https://doi.org/10.3390/s6101234
Chicago/Turabian StyleNiu, Shuyan, Feng Li, Shusheng Zhang, Long Wang, Xuemei Li, and Shiying Wang. 2006. "Studies on the Interaction Mechanism of 1,10-Phenanthroline Cobalt(II) Complex with DNA and Preparation of Electrochemical DNA Biosensor" Sensors 6, no. 10: 1234-1244. https://doi.org/10.3390/s6101234



