Electrochemical Sensors for Detection of Acetylsalicylic Acid
Abstract
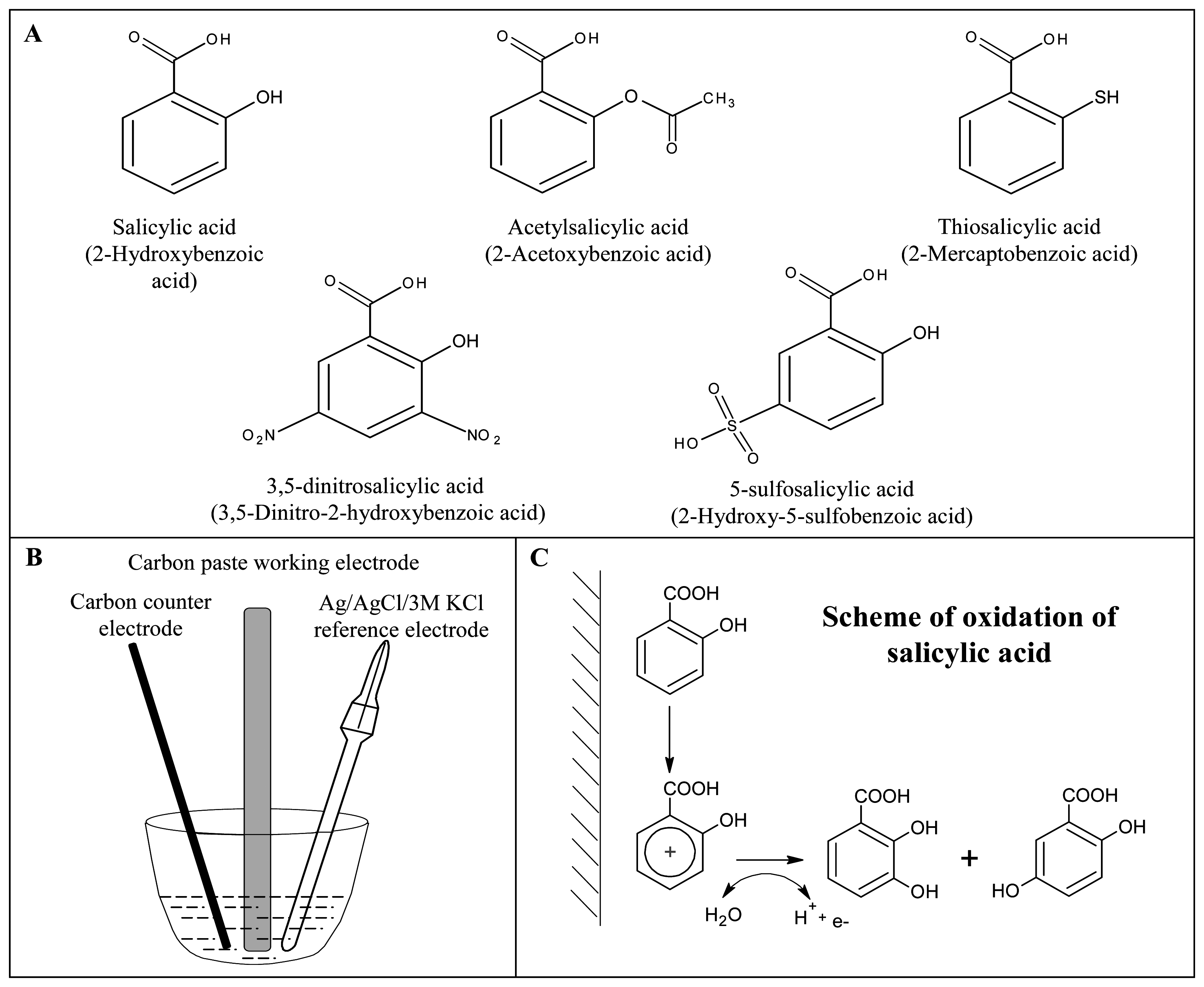
:1. Introduction
2. Material and methods
2.1. Chemicals
2.2. Preparation of pharmaceutical drug samples
2.3. Electrochemical measurement
2.3.1. Preparation of carbon paste electrode and carbon lead electrode
2.3.2 Electrochemical measurements
Cyclic voltammetry
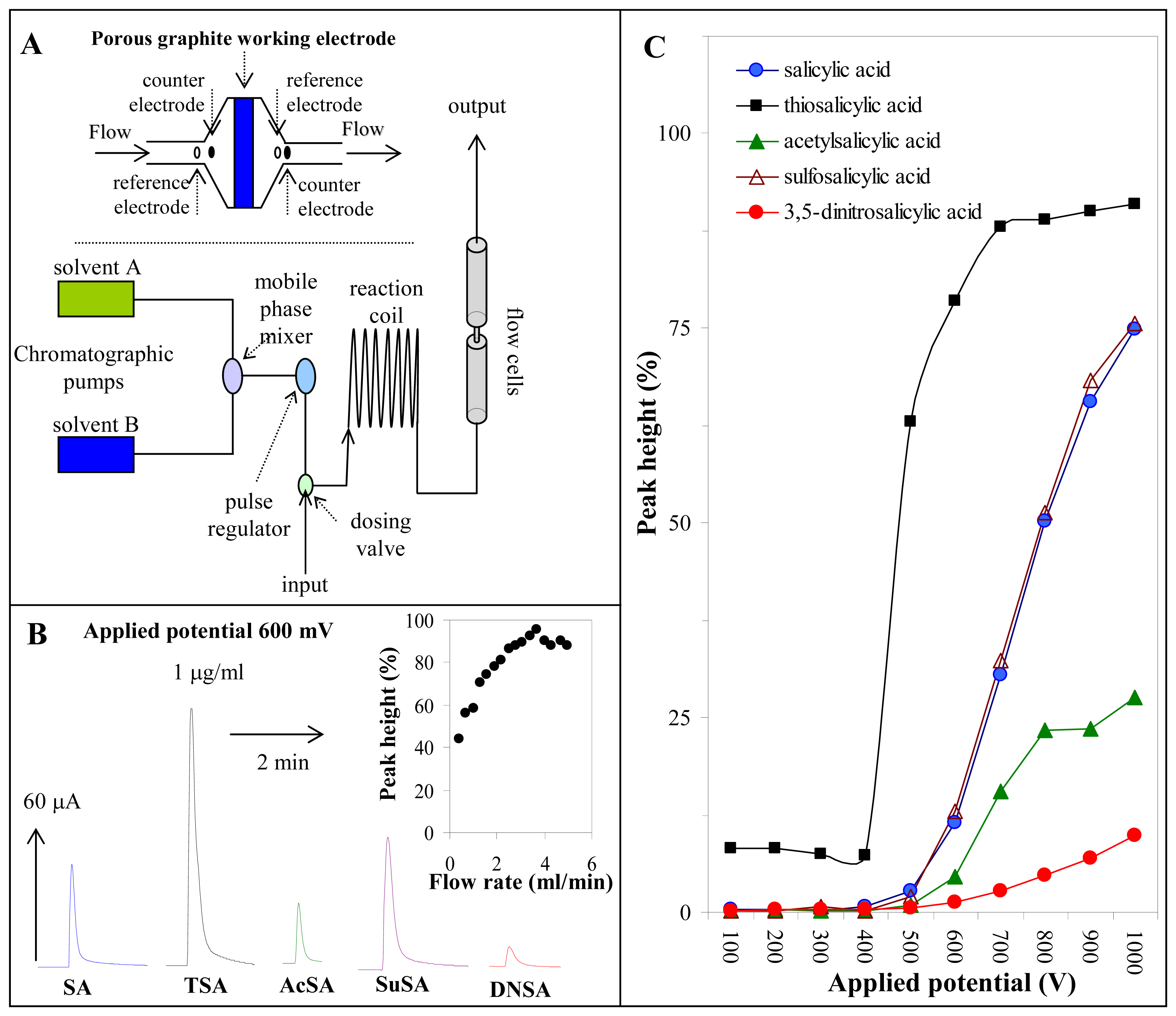
2.4. Flow injection analysis with electrochemical detection
2.5. Statistical analysis
3. Results and discussion
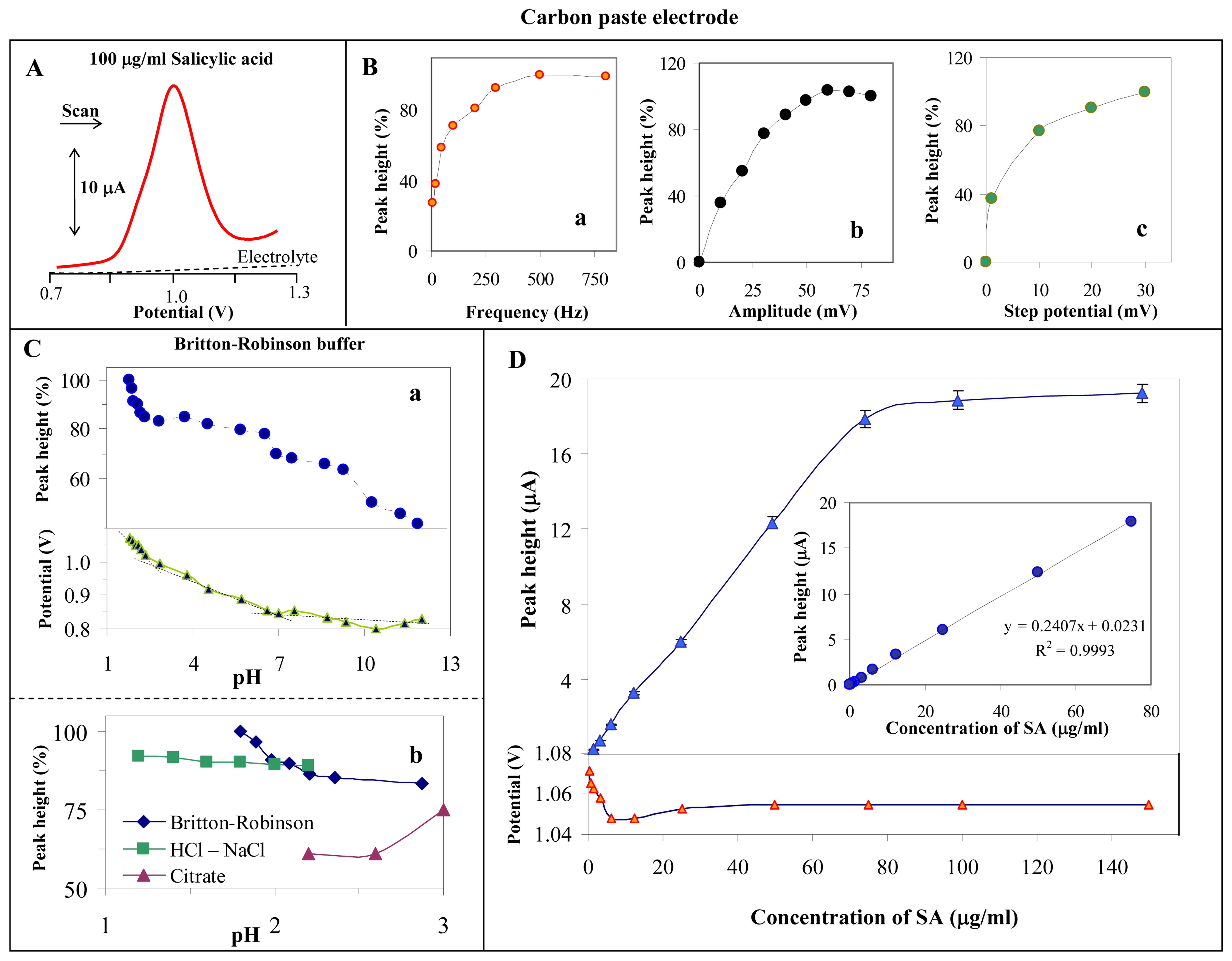
3.1. Study of electrochemical behaviour of salicylic acid on the surface of the carbon paste electrode
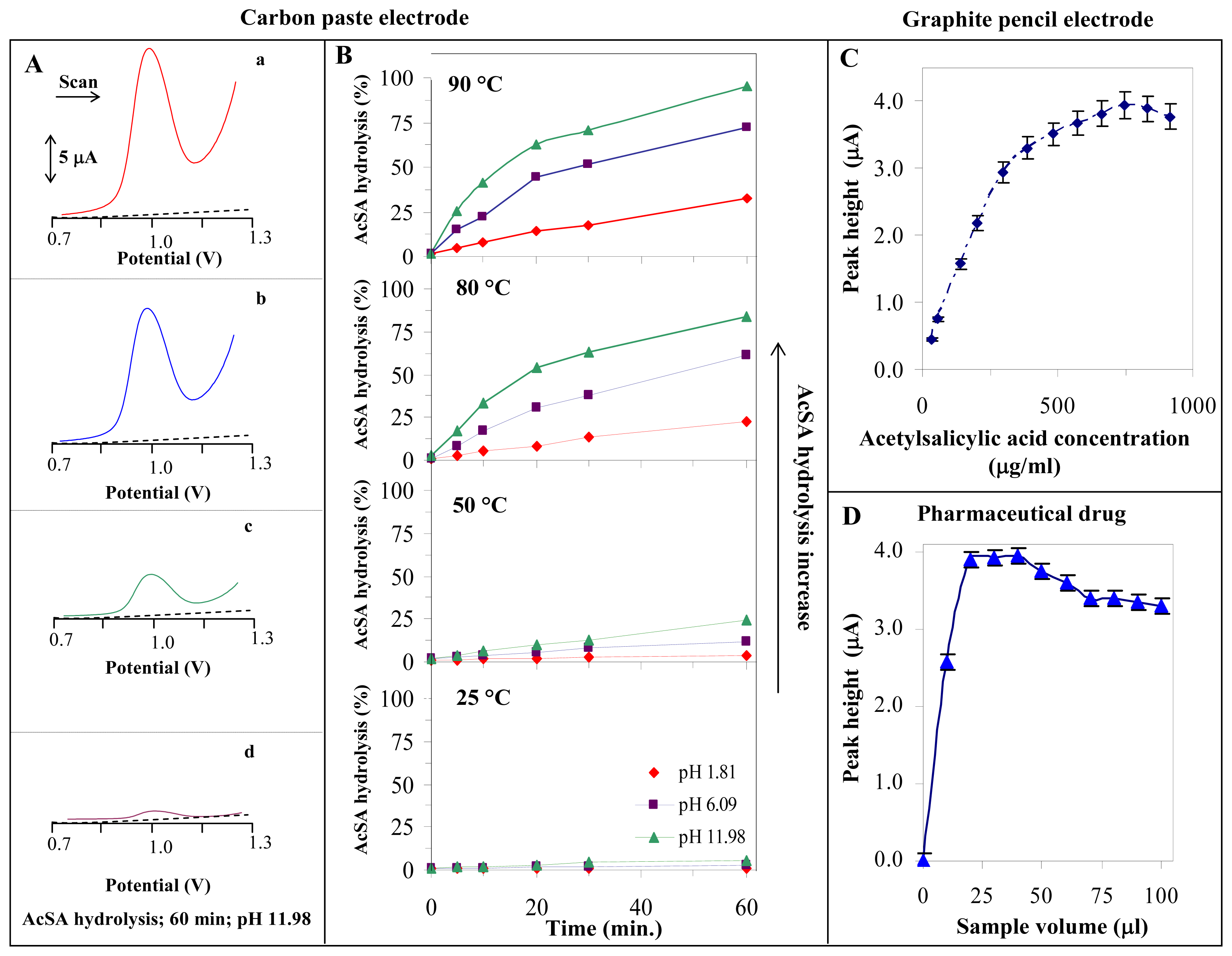
3.2. Indirect determination of acetylsalicylic acid
3.3. Indirect determination of acetylsalicylic acid in a pharmaceutical drug by SWV CPE
3.4. A determination of acetylsalicylic acid in the pharmaceutical drug using SWV GPE
3.5. Determination of salicylates by flow injection analysis with electrochemical detection
4. Conclusion
Acknowledgments
References
- Pirker, R.; Huck, C.W.; Popp, M.; Bonn, G.K. Simultaneous determination of gentisic, salicyluric and salicylic acid in human plasma using solid-phase extraction, liquid chromatography and electrospray ionization mass spectrometry. J. Chrom. B 2004, 809, 257–264. [Google Scholar]
- Stone, E. An account of the success of the bark of the willow tree in the cure of agues. Philos. Trans. R. Soc. Lond. 1763, 53, 195–200. [Google Scholar]
- Dreser, H. Pharmakologisches über aspirin (acetylsalicylsäure). Pfluger&aposs Arch. 1899, 76, 306–319. [Google Scholar]
- Vaneke, J.R.; Flower, R.J.; Botting, R.M. History of aspirin and its mechanism of action. Stroke 1990, 21 Suppl. S, 12–23. [Google Scholar]
- Thun, M.J.; Henley, S.J.; Patrono, C. Nonsteroidal anti-inflammatory drugs as anticancer agents: Mechanistic, pharmacologic, and clinical issues. J. Natl. Cancer Inst. 2002, 94, 252–266. [Google Scholar]
- Weiss, H.J.; Aledort, L.M. Impaired platelet-connective-tissue reaction in man after aspirin ingestion. Lancet 1967, 2, 495–497. [Google Scholar]
- Awtry, E.H.; Loscalzo, J. Aspirin. Circulation 2000, 101, 1206–1218. [Google Scholar]
- Wong, S.; Appleberg, M.; Ward, C.M.; Lewis, D.R. Aspirin resistance in cardiovascular disease: A review. Eur. J. Vasc. Endovasc. 2004, 27, 456–465. [Google Scholar]
- Patrono, C. Aspirin as an antiplatelet drug. New Engl. J. Med. 1994, 330, 1287–1294. [Google Scholar]
- Fuster, V.; Dyken, M.L.; Vokonas, P.S.; Hennekens, C. Aspirin as a therapeutic agent in cardiovascula disease. Circulation 1993, 87, 659–675. [Google Scholar]
- Hennekens, C.H. Aspirin in the treatment and prevention of cardiovascular disease. Ann. Rev. Pub. Health 1997, 18, 37–49. [Google Scholar]
- Manson, J.E.; Stampfer, M.J.; Colditz, G.A.; Willet, W.C.; Rosner, B.; Speizer, F.E.; Hennekens, C.H. A prospective-study of aspirin use and primary prevention of cardiovascular-disease in women. JAMA - J. Am. Med. Assoc. 1991, 266, 521–527. [Google Scholar]
- Reilly, I.A.G.; Fitzgerald, G.A. Aspirin in cardiovascular-disease. Drugs 1988, 35, 154–176. [Google Scholar]
- Shen, S.C.; Ko, C.H.; Hsu, K.C.; Chen, Y.C. 3-OH flavone inhibition of epidermal growth factor-induced proliferation through blocking prostaglandin E-2 production. Int. J. Cancer 2004, 108, 502–510. [Google Scholar]
- Kelloff, G.J.; Schilsky, R.L.; Alberts, D.S.; Day, R.W.; Guyton, K.Z.; Pearce, H.L.; Peck, J.C.; Phillips, R.; Sigman, C.C. Colorectal adenomas: A prototype for the use of surrogate end points in the development of cancer prevention drugs. Clin. Cancer Res. 2004, 10, 3908–3918. [Google Scholar]
- Hardwick, J.C.H.; van Santen, M.; van den Brink, G.R.; van Deventer, S.J.H.; Peppelenbosch, M.P. DNA array analysis of the effects of aspirin on colon cancer cells: involvement of Rac1. Carcinogenesis 2004, 25, 1293–1298. [Google Scholar]
- Martnett, J. Aspirin and the potential role of prostaglandins in colon cancer. Cancer Res. 1992, 52, 5575–5589. [Google Scholar]
- Wang, W.H.; Wong, W.M.; Dailidiene, D.; Berg, D.E.; Gu, Q.; Lai, K.C.; Lam, S.K.; Wong, B.C.Y. Aspirin inhibits the growth of Helicobacter pylori and enhances its susceptibility to antimicrobial agents. Gut 2003, 52, 490–495. [Google Scholar]
- Kupferwasser, L.I.; Yeaman, M.R.; Nast, C.C.; Kupferwasser, D.; Xiong, Y.Q.; Palma, M.; Cheung, A.L.; Bayer, A.S. Salicylic acid attenuates virulence in endovascular infections by targeting global regulatory pathways in Staphylococcus aureus. J. Clin. Invest. 2003, 112, 222–233. [Google Scholar]
- Matias, F.A.A.; Vila, M.M.D.C.; Tubino, M. Quantitative reflectance spot test for the determination of acetylsalicylic acid in pharmaceutical preparations. J. Braz. Chem. Soc. 2004, 15, 327–330. [Google Scholar]
- Criado, A.; Cardenas, S.; Gallego, M.; Valcarcel, M. Continuous flow spectrophotometric determination of paracetamol in pharmaceuticals following continuous microwave assisted alkaline hydrolysis. Talanta 2000, 53, 417–423. [Google Scholar]
- Arancibia, J.A.; Olivieri, A.C.; Escandar, G.M. First- and second-order multivariate calibration applied to biological samples: determination of anti-inflammatories in serum and urine. Anal. Bioanal. Chem. 2002, 374, 451–459. [Google Scholar]
- Moreira, A.B.; Dias, I.L.T.; Neto, G.O.; Zagatto, E.A.G.; Kubota, L.T. Solid-phase fluorescence spectroscopy for the determination of acetylsalicylic acid in powdered pharmaceutical samples. Anal. Chim. Acta 2004, 523, 49–52. [Google Scholar]
- Quintino, M.S.M.; Angnes, L. Bia-amperometric quantification of salbutamol in pharmaceutical products. Talanta 2004, 62, 231–236. [Google Scholar]
- Rover, L.; Garcia, C.A.B.; Neto, C.D.; Kubota, L.T.; Galembeck, F. Acetylsalicylic acid determination in pharmaceutical samples by FIA-potentiometry using a salicylate-sensitive tubular electrode with an ethylene-vinyl acetate membrane. Anal. Chim. Acta 1998, 366, 103–109. [Google Scholar]
- Garrido, J.M.P.J.; Lima, J.L.F.C.; Matos, C.D. Flow injection determination of acetylsalicylic acid in pharmaceutical preparations with an amperometric detector. Collect. Czech. Chem. Commun. 2000, 65, 954–962. [Google Scholar]
- Quintino, M.D.M.; Corbo, D.; Bertotti, M.; Angnes, L. Amperometric determination of acetylsalicylic acid in drugs by batch injection analysis at a copper electrode in alkaline solutions. Talanta 2002, 58, 943–949. [Google Scholar]
- Catarino, R.I.L.; Conceicao, A.C.L.; Garcia, M.B.Q.; Goncalves, M.L.S.; Lima, J.L.F.C.; dos Santos, M.M.C. Flow amperometric determination of pharmaceuticals with on-line electrode surface renewal. J. Pharmaceut. Biomed. 2003, 33, 571–580. [Google Scholar]
- Satinsky, D.; Neto, I.; Solich, P.; Sklenarova, H.; Conceicao, M.; Montenegro, B.S.M.; Araujo, A.N. Sequential injection chromatographic determination of paracetamol, caffeine, and acetylsalicylic acid in pharmaceutical tablets. J. Sep. Sci. 2004, 27, 529–536. [Google Scholar]
- Stolker, A.A.M.; Niesing, W.; Hogendoorn, E.A.; Versteegh, J.F.M.; Fuchs, R.; Brinkman, U.A.T. Liquid chromatography with triple-quadrupole or quadrupole-time of flight mass spectrometry for screening and confirmation of residues of pharmaceuticals in water. Anal. Bioanal. Chem. 2004, 378, 955–963. [Google Scholar]
- Franeta, J.T.; Agbaba, D.; Eric, S.; Pavkov, S.; Aleksic, M.; Vladimirov, S. HPLC assay of acetylsalicylic acid, paracetamol, caffeine and phenobarbital in tablets. Farmaco 2002, 57, 709–713. [Google Scholar]
- Prasek, J.; Adamek, M.; Hubalek, J.; Adam, V.; Trnkova, L.; Kizek, R. New Hydrodynamic Electrochemical Arrangement for Heavy Metals Detection Using Thick-Film Chemical Sensor Electrodes. Sensors 2006, 11, 1498–1512. [Google Scholar]
- Babula, P.; Huska, D.; Hanustiak, P.; Baloun, J.; Krizkova, S.; Adam, V.; Hubalek, J.; Havel, L.; Zemlicka, M.; Horna, A.; Beklova, M.; Kizek, R. Flow Injection Analysis Coupled with Carbon Electrodes as the Tool for Investigation of Affecting and Content of Naphthoquinones in Biological Samples. Sensors 2006, 11, 1466–1482. [Google Scholar]
- Vidal, A.D.; Barrales, P.O.; Diaz, A.M. Simultaneous determination of paracetamol, caffeine and propyphenazone in pharmaceuticals by means of a single flow-through UV multiparameter sensor. Microchim. Acta 2003, 141, 157–163. [Google Scholar]
- Loh, H.C.; Ahmad, M.; Taib, M.N. A novel salicylic acid optical fibre probe fabrication. Sens. Actuator B-Chem. 2005, 107, 59–63. [Google Scholar]
- Vidal, A.D.; Reyes, J.F.G.; Barrales, P.O.; Diaz, A.M. UV spectrophotometric flow-through multiparameter sensor for the simultaneous determination of acetaminophen, acetylsalicylic acid, and caffeine. Anal. Lett. 2002, 35, 2433–2447. [Google Scholar]
- Ruiz-Medina, A.; Fernandez-de Cordova, M.L.; Ortega-Barrales, P.; Molina-Diaz, A. Flow-through UV spectrophotometric sensor for determination of (acetyl)salicylic acid in pharmaceutical preparations. Int. J. Pharm. 2001, 216, 95–104. [Google Scholar]
- Pasekova, H.; Sales, M.G.; Montenegro, M.C.; Araujo, A.N.; Polasek, M. Potentiometric determination of acetylsalicylic acid by sequential injection analysis (SIA) using a tubular salicylate-selective electrode. J. Pharm. Biomed. Anal. 2001, 24, 1027–1036. [Google Scholar]
- Kubota, L.T.; Fernandes, J.C.B.; Rover, L.; Neto, G.D. Determination of acetylsalicylic acid by FIA-potentiometric system in drugs after on-line hydrolysis. Talanta 1999, 50, 661–667. [Google Scholar]
- Ai, S.Y.; Wang, Q.J.; Li, H.; Jin, L.T. Study on production of free hydroxyl radical and its reaction with salicylic acid at lead dioxide electrode. J. Electroanal. Chem. 2005, 578, 223–229. [Google Scholar]
- Stradins, J.; Hasanli, B. Anodic Voltammetry of Phenol and Benzenethiol Derivatives.1. Influence of Ph on Electrooxidation Potentials of Substituted Phenols and Evaluation of Pk(a) from Anodic Voltammetry Data. J. Electroanal. Chem. 1993, 353, 57–69. [Google Scholar]
- Kizek, R.; Masarik, M.; Kramer, K.J.; Potesil, D.; Bailey, M.; Howard, J.A.; Klejdus, B.; Mikelova, R.; Adam, V.; Trnkova, L.; Jelen, F. An analysis of avidin, biotin and their interaction at attomole levels by voltammetric and chromatographic techniques. Anal. Bioanal. Chem. 2005, 381, 1167–1178. [Google Scholar]
- Masarik, M.; Kizek, R.; Kramer, K.J.; Billova, S.; Brazdova, M.; Vacek, J.; Bailey, M.; Jelen, F.; Howard, J.A. Application of avidin-biotin technology and adsorptive transfer stripping square-wave voltammetry for detection of DNA hybridization and avidin in transgenic avidin maize. Anal. Chem. 2003, 75, 2663–2669. [Google Scholar]
- Wang, J.; Kawde, A.N.; Sahlin, E. Renewable pencil electrodes for highly sensitive stripping potentiometric measurements of DNA and RNA. Analyst 2000, 125, 5–7. [Google Scholar]
- Ly, S.Y.; Jung, Y.S.; Kim, M.H.; Han, I.K.; Jung, W.W.; Kim, H.S. Determination of caffeine using a simple graphite pencil electrode with square-wave anodic stripping voltammetry. Microchim. Acta 2004, 146, 207–213. [Google Scholar]
- Bond, A.M.; Mahon, P.J.; Schiewe, J.; VicenteBeckett, V. An inexpensive and renewable pencil electrode for use in field-based stripping voltammetry. Anal. Chim. Acta 1997, 345, 67–74. [Google Scholar]
- Perdicakis, M.; Aubriet, H.; Walcarius, A. Use of a commercially available wood-free resin pencil as convenient electrode for the ‘voltammetry of microparticles’ technique. Electroanalysis 2004, 16, 2042–2050. [Google Scholar]
- Ozsoz, M.; Erdem, A.; Kara, P.; Kerman, K.; Ozkan, D. Electrochemical biosensor for the detection of interaction between arsenic trioxide and DNA based on guanine signal. Electroanalysis 2003, 15, 613–619. [Google Scholar]
- Fojta, M.; Havran, L.; Billova, S.; Kostecka, P.; Masarik, M.; Kizek, R. Two-surface strategy in electrochemical DNA hybridization assays: Detection of osmium-labeled target DNA at carbon electrodes. Electroanalysis 2003, 15, 431–440. [Google Scholar]
- Bromba, M.U.A.; Ziegler, H. Application hints for Savitzky-Golay digital smoothing filtres. Anal.Chem. 1981, 53, 1583–1586. [Google Scholar]
- Kizek, R.; Vacek, J.; Trnkova, L.; Klejdus, B.; Havel, L. Application of catalytic reactions on a mercury electrode for electrochemical detection of metallothioneins. Chem. Listy 2004, 98, 166–173. [Google Scholar]
- Petrlova, J.; Potesil, D.; Mikelova, R.; Blastik, O.; Adam, V.; Trnkova, L.; Jelen, F.; Prusa, R.; Kukacka, J.; Kizek, R. Attomole voltammetric determination of metallothionein. Electrochim. Acta 2006, 51, 5112–5119. [Google Scholar]
- Petrek, J.; Havel, L.; Petrlova, J.; Adam, V.; Potesil, D.; Babula, P.; Kizek, R. Analysis of salicylic acid in willow barks and branches by an electrochemical method. Russ. J. Plant. Physiol. 2006, in press. [Google Scholar]
- Torriero, A.A.J.; Luco, J.M.; Sereno, L.; Raba, J. Voltammetric determination of salicylic acid in pharmaceuticals formulations of acetylsalicylic acid. Talanta 2004, 62, 247–254. [Google Scholar]
- Vacek, J.; Andrysik, Z.; Trnkova, L.; Kizek, R. Determination of azidothymidine - an antiproliferative and virostatic drug by square-wave voltammetry. Electroanalysis 2004, 16, 224–230. [Google Scholar]
- Gattrell, M.; Kirk, D.W. A study of the oxidation of phenol at platinum and preoxidized platinum surfaces. J. Electrochem. Soc. 1993, 140, 1534–1540. [Google Scholar]
- Gattrell, M.; Kirk, D.W. A study od electrode passivation during aqueous phenol electrolysis. J. Electrochem. Soc. 1993, 140, 903–911. [Google Scholar]
- Lopez-Cueto, G.; Ostra, M.; Ubide, C. The acetylsalicylic acid-bromine system for multicomponent kinetic determinations. Anal. Bioanal. Chem. 2002, 374, 915–922. [Google Scholar]
- Connors, K.A.; Amidon, G.L.; Kennon, L. Chemical stability of pharmaceuticals; Wiley, 1979. [Google Scholar]
- Achuo, E.A.; Audenaert, K.; Meziane, H.; Hofte, M. The salicylic acid-dependent defence pathway is effective against different pathogens in tomato and tobacco. Plant Pathol. 2004, 53, 65–72. [Google Scholar]
- Potesil, D.; Zelena, J.; Petrlova, J.; Adam, V.; Vacek, J.; Klejdus, B.; Zehnalek, J.; Trnkova, L.; Havel, L.; Kizek, R. Simultaneous femtomole determination of cysteine, reduced and oxidized glutathione, and phytochelatin in maize (Zea mays L.) kernels using highe-performance liquid chromatography with electrochemical detection. J. Chromatogr. A 2005, 1084, 134–144. [Google Scholar]
| A compound of interest | Regression equation | aR2 | bLOD (nM) | cLOD (fmol) |
|---|---|---|---|---|
| SA | y = 17.811x – 1.9008 | 0.9980 | 0.195 | 0.977 |
| AcSA | y = 3.5721x + 0.3521 | 0.9987 | 0.527 | 2.63 |
| TSA | y = 7.0741x – 1.7902 | 0.9964 | 0.529 | 2.64 |
| SuSA | y = 4.0618x + 0.8322 | 0.9985 | 0.427 | 2.13 |
| DNSA | y = 0.0382x – 0.058 | 0.9988 | 50.6 | 253 |
© 2006 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Supalkova, V.; Petrek, J.; Havel, L.; Krizkova, S.; Petrlova, J.; Adam, V.; Potesil, D.; Babula, P.; Beklova, M.; Horna, A.; et al. Electrochemical Sensors for Detection of Acetylsalicylic Acid. Sensors 2006, 6, 1483-1497. https://doi.org/10.3390/s6111483
Supalkova V, Petrek J, Havel L, Krizkova S, Petrlova J, Adam V, Potesil D, Babula P, Beklova M, Horna A, et al. Electrochemical Sensors for Detection of Acetylsalicylic Acid. Sensors. 2006; 6(11):1483-1497. https://doi.org/10.3390/s6111483
Chicago/Turabian StyleSupalkova, Veronika, Jiri Petrek, Ladislav Havel, Sona Krizkova, Jitka Petrlova, Vojtech Adam, David Potesil, Petr Babula, Miroslava Beklova, Ales Horna, and et al. 2006. "Electrochemical Sensors for Detection of Acetylsalicylic Acid" Sensors 6, no. 11: 1483-1497. https://doi.org/10.3390/s6111483




