Wet Chemical Synthesis and Screening of Thick Porous Oxide Films for Resistive Gas Sensing Applications
Abstract
:1. Introduction
2. Experimental
- SnO2: 2.40 ml acetylacetone in 4.00 ml THF are added to a solution of 3.03 g Sn(IV)isopropoxid (98%, Alfa Aesar) in 7.09 ml THF. 200 μl 1M HNO3 in 4.00 ml THF are added dropwise. After 5 min. 200 μl 1 M HNO3 are added. The resulting gel is dried at 100 °C and milled in an agate mortar. The resulting powder is calcined for 6 h at 400 °C (heating rate: 100 °C/h). The slurry is prepared from a mixture of 10 ml polyethylene glycol 600 (purum, Roth; 5 wt% in isopropanol), 116 mg SnO2 in 4 ml isopropanol, and, if necessary, 2 ml of the dopant solution in isopropanol.
- WO3: 584.1 μl HNO3 (65%), and, if necessary, 0.25 molar aqueous doping salt solutions are added to a solution of 8.18 g ammonium (meta)tungstate hydrate (purum, Fluka) in 30 ml water. The sol is dried at a temperature of 80 °C, milled and calcined for 6 h at 500 °C (heating rate 100 °C/h). The slurry is prepared from the powder and isopropanol with a ratio of 30 mg: 1 ml.
- ZrO2: 200 μl HNO3(65%) is added to a solution of 2.00 g zirconium(IV)-propoxid (70%, ABCR) in 2.00 ml THF and stirred for 90 min. The hydrolysis is carried out after adding 100 μl water in 6.5 ml THF. The sol is dried at 40 °C until it forms a transparent gel, which is milled and dried again, first for 2 h at 60 °C and then for 2 h at 80 °C. The calcination is carried out for 6 h at 500 °C (heating rate 100 °C/h). The slurry is prepared from 191 mg powder in 8 ml isopropanol, and, if necessary, 2 ml of the dopant solution in isopropanol. In order to improve the adhesion properties on the substrate a mediating sol, consisting of 2.88 g zirconium(IV)propoxid (70%), 0.45 ml ethyl acetoacetate, and 3.94 ml isopropanol is added.
- Bi2O3: 1.00 g HNO3 (65%), and 295 mg polyvinyl alcohol Mowiol 18-88 (Clariant, 10% in H2O) are added to a solution of 1.00 g Bi(NO3)3*5 H2O (>99%, Fluka). The sol is dried at 90 °C until a transparent glass is formed, which is milled and calcined for 6 h at 500 °C (heating rate 100 °C/h). The slurry is prepared from 168 mg powder in 5 ml 1-propanol, and, if necessary, 2 ml of doping solution in 1-propanol. The mediating sol is made by mixing 147 mg Bi(III)-2-ethyl-hexanoat (Alfa Aesar), 8.00 g 1-Propanol, and 23 mg acetylacetone and added to the slurry.
- TiO2: 15.2 g Ti(IV)isopropoxid (97%, Aldrich) is added dropwise to 24.3 g 1-propanol and 1.12 g HNO3 (65%). After stirring for 30 min 3.00 g water are added dropwise for hydrolysis. The gel is dried at 90 °C. Calcination is carried out for 6 h at 700 °C (heating rate of 100 °C/h). The slurry is prepared from 179 mg oxide in 10 ml isopropanol, and 0.62 g of the mediating sol in 9.38 ml 1-Propanol. The mediating sol consists of 1.90 g Ti(IV)isopropoxid, 1.34 g acetylacetone, 0.14 g HNO3 (65%), 0.188 g water, and 2.42 g 1-propanol.
- CeO2: 0.259 g HNO3 (65%), and 1.93 g polyvinyl alcohol Mowiol 18-88 (Clariant, 10 wt% in H2O) are added to 5.75 g cerium nitrate (99+%, Fluka) in 47.5 g water. The sol is dried at 90 °C. Calcination is carried out for 6 h at 700 °C (100 °C/h heating rate). The slurry is prepared from 298 mg oxide in 10 ml ethanol. The mediating sol consists of 0.58 g cerium nitrate, 144 mg polyethylene glycol 600 (10 wt% in ethanol), 26 μl HNO3 (65%), and 5.65 ml ethanol.
- In2O3: 2.00 g indium nitrate (99.99%, Aldrich) is mixed with 10 ml propionic acid and heated to 140 °C until the formation of nitrous oxides is completed (fill up again with propionic acid may become necessary). The transparent glass is milled and is calcined for 6 h at 500 °C (heating rate of 20 °C/h). The suspension consists of 150 mg oxide powder and 5 ml 1-propanol.
- In2O3/WO3 mixed oxide library: Adaption of the In2O3-recipe to the water-based WO3 oxide recipe. 0.367 g polyvinyl alcohol Mowiol 18-88 (Clariant, 10% in water), and 37 μl HNO3 (65%) are added to 1.74 g In(NO3)3*5 H2O in 6.77 ml water, and are stirred. An equimolar WO3 solution is made according to the above recipe. A composition spread is created using an increment of 10 at% under addition of a 0.02 molar solution of the bulk doping agent in water. 1.5 ml of the mixed oxide soles are pipetted into Eppendorf vials, shaken, dried at 70 °C and are calcined in GC vials for 6 h at 500 °C. The suspensions consist of 18 mg mixed-oxide powder in each case and 1.2 ml isopropyl alcohol.
3. Results
3.1. Sensormaterials based on single oxides
3.2. Sensormaterials based on mixed oxides
4. Summary and Conclusion
Acknowledgments
References and Notes
- Barsan, N. ; Weimar U. Conduction Model of Metal Oxide Gas Sensors. J. Electroceram. 2001, 7(3), 143–167. [Google Scholar]
- Mirsky, V.M.; Kulikov, V.; Hao, Q.; Wolfbeis, O.S. Multiparameter High Throughput Characterization of Combinatorial Chemical Microarrays of Chemosensitive Polymers. Macromol. Rapid. Commun. 2004, 25, 253–258. [Google Scholar]
- Apostolidis, A.; Klimant, I.; Andrzejewski, D.; Wolfbeis, O.S. A Combinatorial Approach for Development of Materials for Optical Sensing of Gases. J. Comb. Chem. 2004, (6), 325–331. [Google Scholar]
- Potyrailo, R.A. Polymeric Sensor Materials: Toward an Alliance of Combinatorial and Rational Design Tools? Angew. Chem. Int. Ed. 2006, 45, 702–723. [Google Scholar]
- Sullivan, M.G.; Utomo, H.; Fagan, P.J.; Ward, M.D. Automated Electrochemical Analysis with Combinatorial Electrode Arrays. Anal. Chem. 1999, 71, 4369–4375. [Google Scholar]
- Simon, U.; Sanders, D.; Jockel, J.; Heppel, C.; Brinz, T. Design Strategies for Multielectrode Arrays Applicable for High-Throughput Impedance Spectroscopy on Novel Gas Sensor Materials. J. Comb. Chem. 2002, 4, 511–515. [Google Scholar]
- Scheidtmann, J.; Frantzen, A.; Frenzer, G.; Maier, W. F. A combinatorial technique for the search of solid state gas sensor materials. Meas. Sci. Technol. 2005, 16, 119–127. [Google Scholar]
- Frantzen, A.; Scheidtmann, J.; Frenzer, G.; Maier, W.F.; Jockel, J.; Brinz, T.; Sanders, D.; Simon, U. High-Throughput Method for the Impedance Spectroscopic Characterization of Resistive Gas Sensors. Angew. Chem. Int. Ed. 2004, 43, 752–754. [Google Scholar]
- Koplin, T.J.; Siemons, M.; Ocen-Valentin, C.; Sanders, D.; Simon, U. Workflow for High Throughput Screening of Gas Sensing Materials. Sensors 2006, 6, 298–307. [Google Scholar]
- Yamada, Y.; Kobayashi, T. High-Throughput Analysis; Potyrailo, R. A., Amis, E. J., Eds.; Kluwer Academic / Plenum Publishers: New York, 2003; pp. 247–259. [Google Scholar]
- Barsoukov, E.; MacDonald, J.R. Impedance Spectroscopy. 2005.
- Zakrzewska, K. Mixed oxides as gas sensors. Thin Solid Films 2001, 391(2), 229–238. [Google Scholar]
- Tanaka, S.; Esaka, T. Characterization of NOx sensor using doped In2O3. J. Mater. Res. 2001, 16(5), 1389–1395. [Google Scholar]
- Moos, R. A Brief Overview on Automotive Exhaust Gas Sensors Based on Electroceramics. Int. J. Appl. Ceram. Technol. 2005, 2(5), 345–428. [Google Scholar]
- Kunimoto, A.; Abe, N.; Uchida, H.; Katsube, T. Highly sensitive semiconductor NOx gas sensor operating at room temperature. Sens. Actuators, B 2000, 65, 122–124. [Google Scholar]
- Kumar, M.M.; Post, M. L. Effect of grain boundaries on hydrocarbon sensing in Fe-doped p-type semiconducting perovskite SrTiO3 films. J. Appl. Phys. 2005, 97(11), 114916/114911–114916/114918. [Google Scholar]
- Schmid, W.; Barsan, N.; Weimar, U. Sensing of hydrocarbons with tin oxide sensors: possible reaction path as revealed by consumption measurements. Sens. Actuators, B 2003, 89(3), 232–236. [Google Scholar]
- Jain, K.; Pant, R.P.; Lakshmikumar, S.T. Effect of Ni doping on thick film SnO2 gas sensor. Sens. Actuators, B 2006, 113(2), 823–829. [Google Scholar]
- Kwon, C.H.; Yun, D.H.; Hong, H.-K.; Kim, S.-R.; Lee, K.; Lim, H.Y.; Yoon, K.H. Multi-layered thick-film gas sensor array for selective sensing by catalytic filtering technology. Sens. Actuators, B 2000, 65, 327–330. [Google Scholar]
- Williams, D.E.; Moseley, P.T. Dopant effects on the response of gas-sensitive resistors utilising semiconducting oxides. J. Mater. Chem. 1999, 1(5), 809–814. [Google Scholar]
- Dabney, W.S.; Antolino, N.E.; Luisi, B.S.; Richard, A.P.; Edwards, D.D. Sol–gel deposition and characterization of In6WO12 thin films. Thin Solid Films 2002, 411(2), 192–197. [Google Scholar]
- Solis, J.L.; Lantto, V. Gas-sensing properties of SnxWO3+x mixed oxide thick films. Sens. Actuators, B 1998, 48(1-3), 322–327. [Google Scholar]
- Kaciulis, S.; Pandolfi, L.; Viticoli, S.; Sberveglieri, G.; Zampiceni, E.; Wlodarski, W.; Galatsis, K.; Li, Y.X. Investigation of thin films of mixed oxides for gas-sensing applications. Surf. Interface Anal. 2002, 34(1), 672–676. [Google Scholar]
- Minami, H.; Itaka, K.; Kawaji, H.; Wang, Q.J.; Koinuma, H.; Lippmaa, M. Rapid synthesis and characterization of (Ca1-xBax)3Co4O9thin films using combinatorial methods. Appl. Surf. Sci. 2002, 197-198, 442–447. [Google Scholar]
- Koinuma, H.; Kawasaki, M.; Itoh, T.; Ohtomo, A.; Murakami, M.; Jin, Z.; Matsumoto, Y. Concept and development of combinatorial laser MBE for oxide electronics. Physica C 2000, 335, 245–250. [Google Scholar]
- Reichenbach, H.M.; McGinn, P.J. Combinatorial synthesis of oxide powders. J. Mater. Res. 2001, 16(4), 967–974. [Google Scholar]
- Scheidtmann, J.; Klär, D.; Saalfrank, J.W.; Schmidt, T.; Maier, W.F. Quantitative Composition Activity Relationships (QCAR) of Co-Ni-Mn-Mixed Oxide and M1-M2-Mixed Oxide Catalysts. QSAR & Comb. Sci. 2005, 24(2), 203–210. [Google Scholar]
- Henderson, S.J.; Armstrong, J.A.; Hector, A.L.; Weller, M.T. High-throughput methods to optically functional oxide and oxide–nitride materials. J. Mater. Chem. 2005, 15(15), 1528–1536. [Google Scholar]
- Savage, N.; Chwieroth, B.; Ginwalla, A.; Patton, B.R.; Akbar, S.A.; Dutta, P.K. Composite n–p semiconducting titanium oxides as gas sensors. Sens. Actuators, B 2001, 79(1), 17–27. [Google Scholar]
- Sterzel, H.-J.; Kuehling, K. Dosage of suspensions in microscale for the production of material samples in combination with material research and testing. In Ger. Offen.; 2002; BASF AG: Germany, De. p. 8 PP. [Google Scholar]
- Chen, L.; Bao, J.; Gao, C.; Huang, S.; Liu, C.; Liu, W. Combinatorial Synthesis of Insoluble Oxide Library from Ultrafine/Nano Particle Suspension Using a Drop-on-Demand Inkjet Delivery System. J. Comb. Chem. 2004, 6(5), 699–702. [Google Scholar]
- Bene, R.; Perczel, I.V.; Réti, F.; Meyer, F.A.; Fleisher, M.; Meixner, H. Chemical reactions in the detection of acetone and NO by a CeO2 thin film. Sens. Actuators, B 2000, 71(1-2), 36–41. [Google Scholar]
- Tanaka, S.; Esaka, T. Characterization of NOx sensor using doped In2O3. J. Mater. Res. 2001, 16(5), 1389–1395. [Google Scholar]
- Armelao, L.; Colombo, P.; Fabrizio, M. Synthesis of Bi2O3 and Bi4(SiO4)3 Thin Films by the Sol-Gel Method. J. Sol-Gel Sci. Technol. 1998, 13(1-3), 213–217. [Google Scholar]
- Simon, U.; Sanders, D.; Jockel, J.; Brinz, T. Setup for High-Throughput Impedance Screening of Gas-Sensing Materials. J. Comb. Chem. 2005, 7(5), 682–687. [Google Scholar]
- Frantzen, A.; Sanders, D.; Jockel, J.; Scheidtmann, J.; Simon, U.; Maier, W.F. A flexible Database for Combinatorial and High-Throughput Materials Science. QSAR & Comb. Sci. 2004, 24, 22–27. [Google Scholar]
- Köhler, J.; Imanaka, N.; Adachi, G. -Y. Indiumwolframat, In2(WO4)3 - ein In3+ leitender Festkörperelektrolyt. Z. Anorg. Allg. Chem. 1999, 625(11), 1890–1896. [Google Scholar]
- Scheidtmann, J.; Frantzen, A.; Frenzer, G.; Maier, W.F. A combinatorial technique for the search of solid state gas sensor materials. Meas. Sci. Technol. 2005, 16(1), 119–127. [Google Scholar]



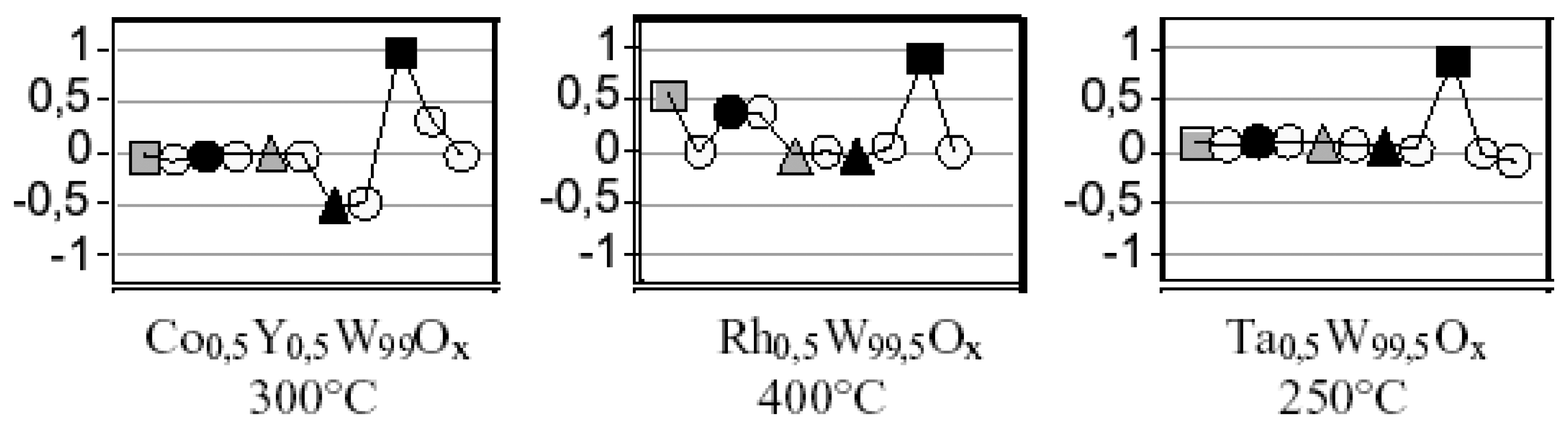
 H2, ● CO,
H2, ● CO,
 NO, ▲ NO2,
NO, ▲ NO2,
 propene, synthetic air.
propene, synthetic air.
 H2, ● CO,
H2, ● CO,
 NO, ▲ NO2,
NO, ▲ NO2,
 propene, synthetic air.
propene, synthetic air.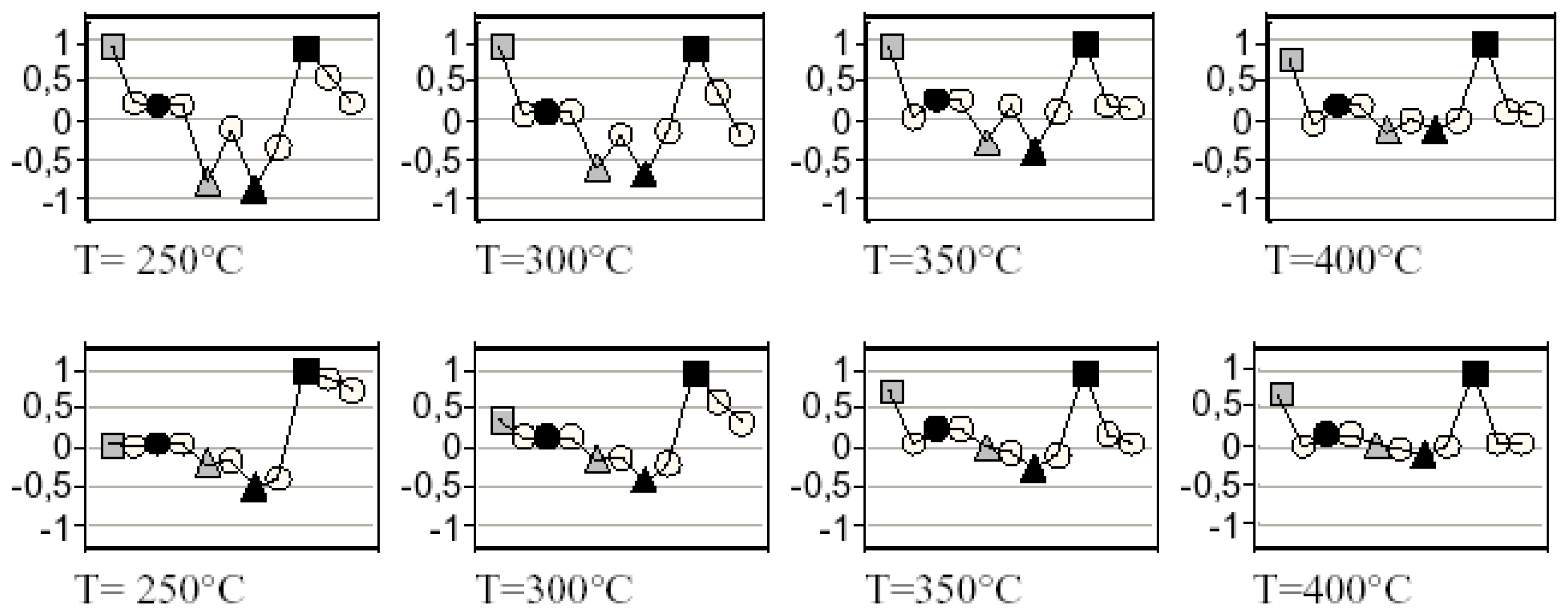
 H2, ● CO,
H2, ● CO,
 NO, ▲ NO2, propene,
NO, ▲ NO2, propene,
 synthetic air. Framed sensor material: Mg0,5Ta 0,2Y1W98,3Ox
synthetic air. Framed sensor material: Mg0,5Ta 0,2Y1W98,3Ox
 H2, ● CO,
H2, ● CO,
 NO, ▲ NO2, propene,
NO, ▲ NO2, propene,
 synthetic air. Framed sensor material: Mg0,5Ta 0,2Y1W98,3Ox
synthetic air. Framed sensor material: Mg0,5Ta 0,2Y1W98,3Ox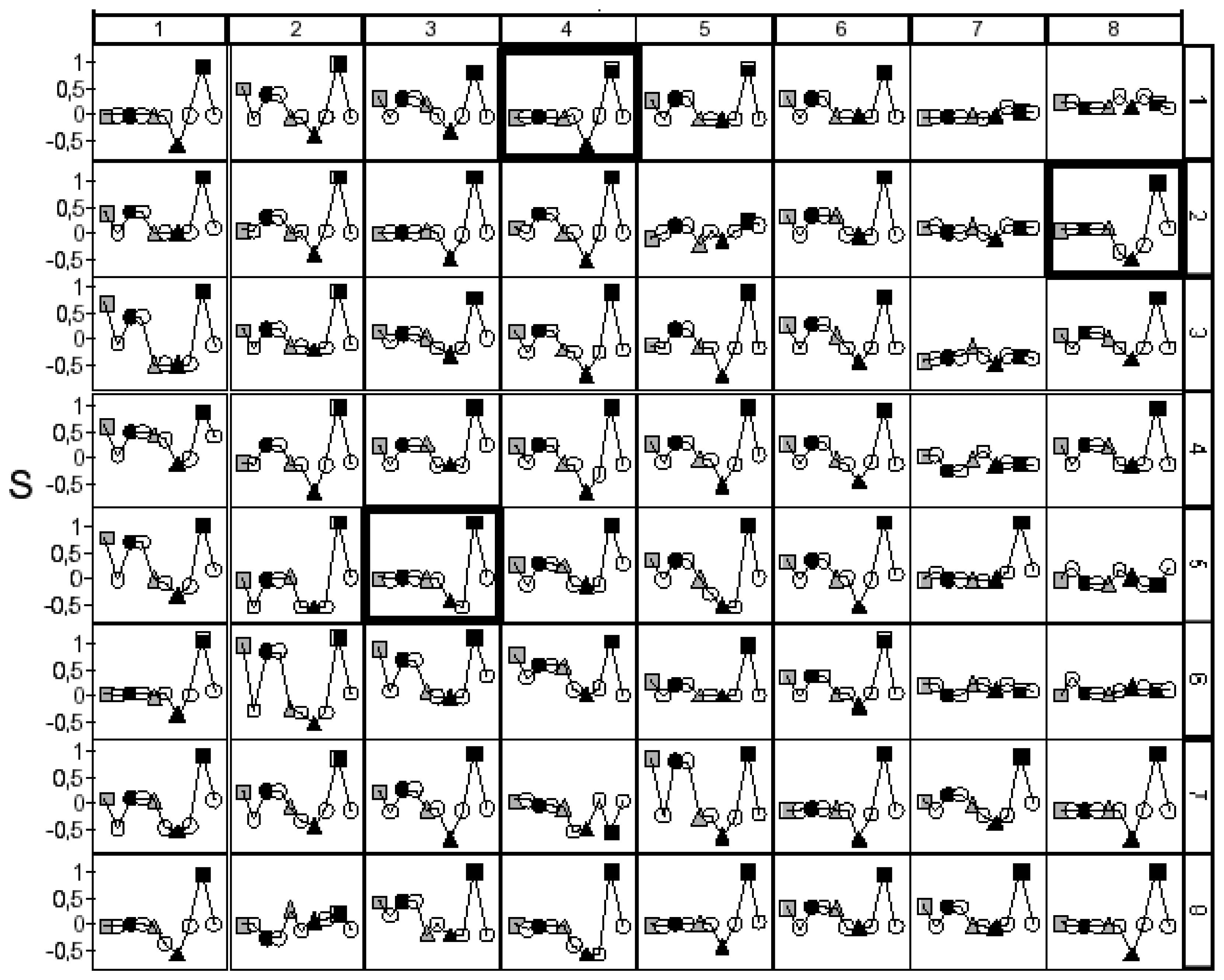
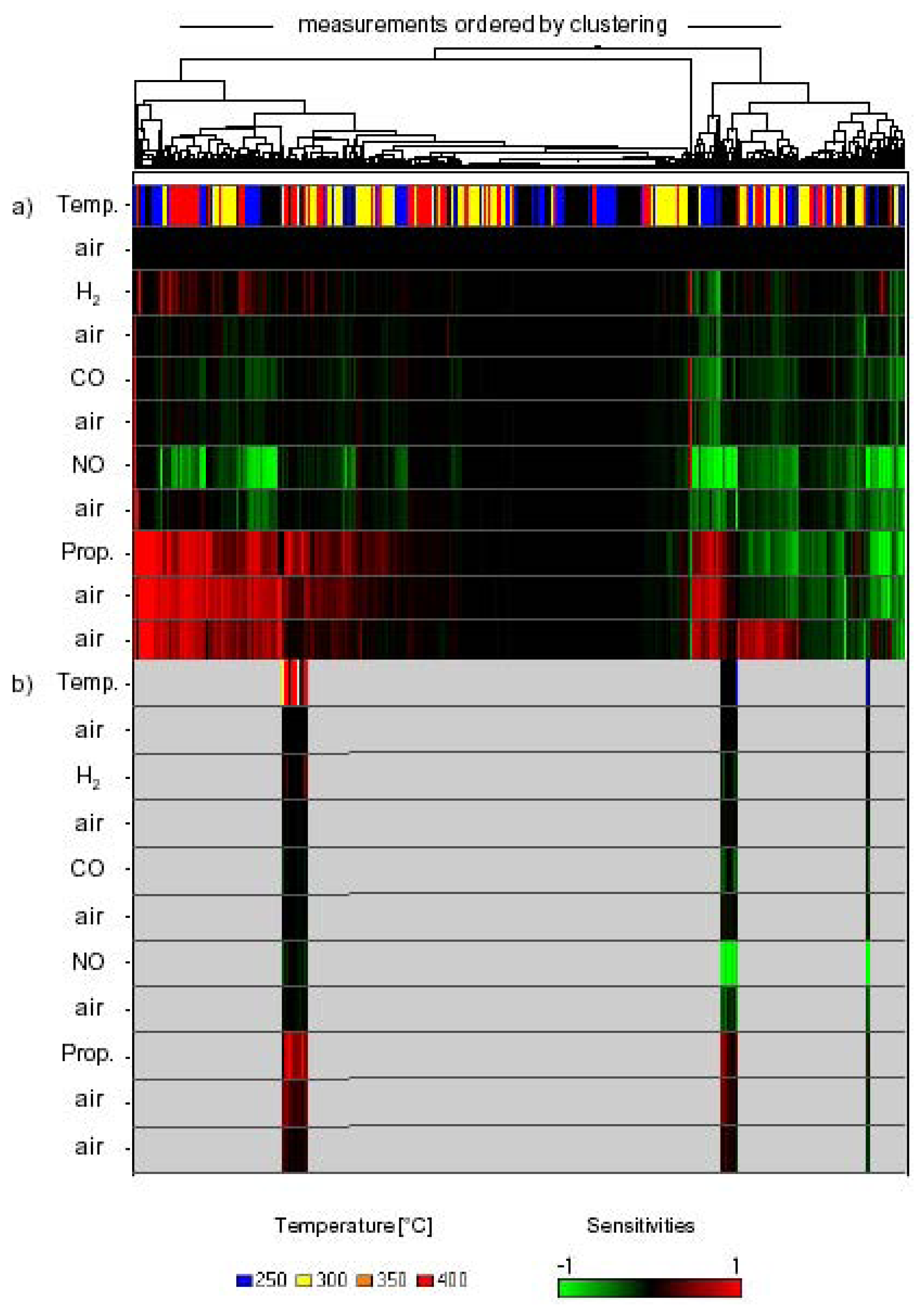
| materials used | test gas | response temperature | c(gas) [ppm]/LOD [1/ppm] | cross sensitivity against | ref. |
|---|---|---|---|---|---|
| 0.5 at% Ba/In2O3 | NO, NO2 | 300°C | 10/∼1 3/∼4 | octane | 13 |
| Pt/NaZSM5 | C3H8 | 500/∼6·10-3 | - | 14 | |
| Pt/SnO2 | NO, NO2 | room temperature | 20/∼0.83 30/∼0.24 | - | 15 |
| SrTi0.6Fe0.4O3 | C3H8 | 400°C | 3000/∼9.2·10-4 | - | 16 |
| SnO2 | C3H8 | 250°C | 400/∼0.14 | CH4, toluene | 17 |
| Al, Ni/SnO2 | LPG | 300°C | 600/∼0.16 | - | 18 |
| Pd, Sb, In/SnO2 | C3H8 | 500°C | 500/∼0.04 | C2H5OH | 19 |
| BaTiO3 | C2H4 | 500°C | 700/∼2.3·10-3 | O2, NH3 | 20 |
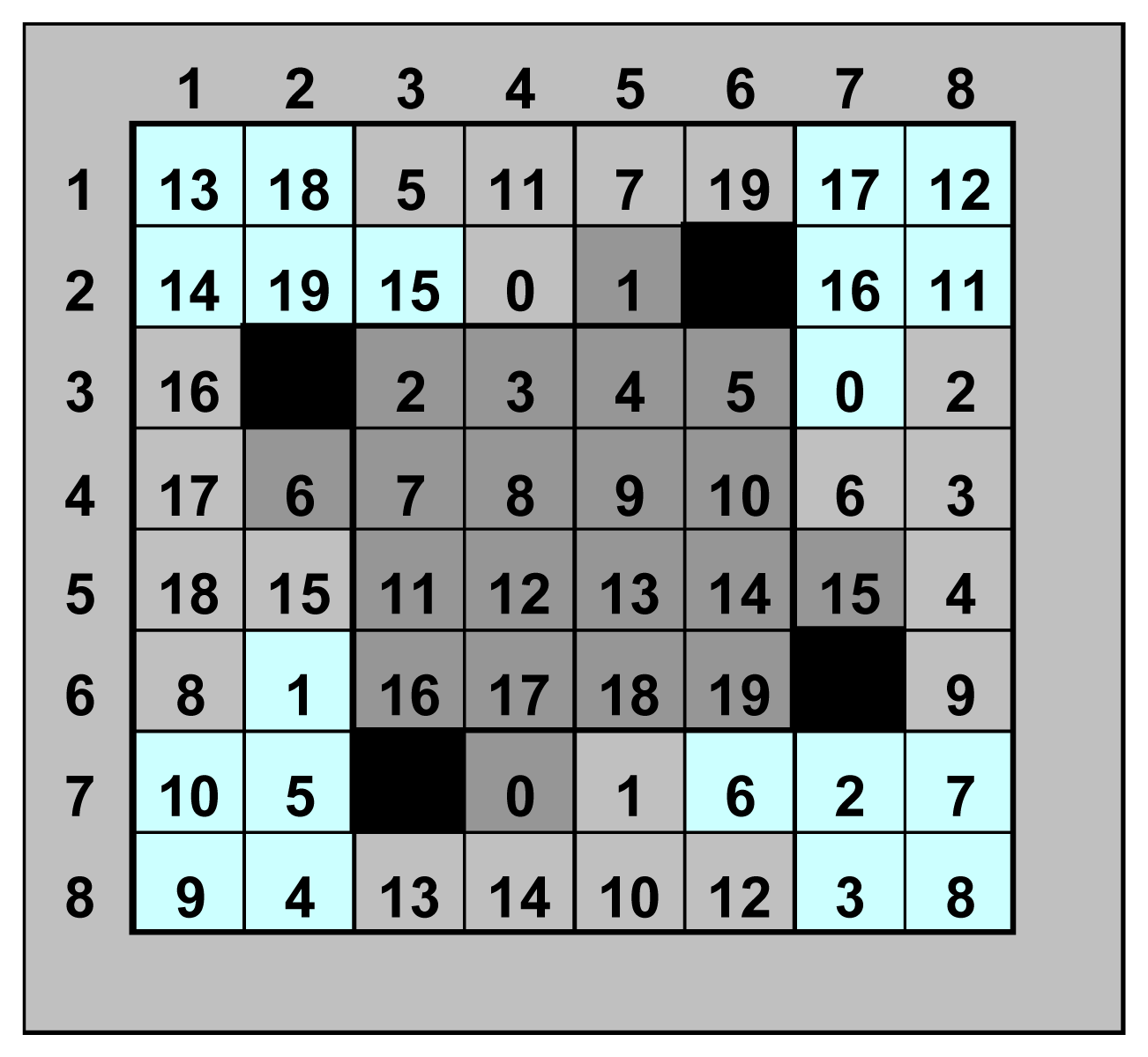
| bulk oxide | libraries | surface-dopants | content [At%] | bulk-dopants | content [At%] | primer |
|---|---|---|---|---|---|---|
| WO3 | 3 | 40 | 0.5 | 20 | 0.5 | 10% W-sol |
| SnO2 | 3 | 40 | 0.5 | 20 | 0.5 | PEG 600 |
| ZrO2 | 1 | 20 | 0.5 | - | - | 10% Zr-sol |
| TiO2 | 1 | 20 | 0.5 | - | - | 10% Ti-sol |
| CeO2 | 2 | 20 | 0.5 | 20 | 0.5 | 10% Ce-sol |
| Bi2O3 | 1 | 20 | 0.5 | - | - | 10% Bi-sol |
| In2O3 | 2 | 20 | 0.5 | 20 | 0.5 | - |
| bulk doped oxides | test gas | c(gas) [ppm]/LOD [1/ppm] | selectivity to | temperature [°C] |
|---|---|---|---|---|
| In99,5Co0.5Ox | NO/NO2 | 5/0.18; 0.26 | H2, CO | 250 |
| W99Co0.5Y0.5Oxa | NO2 | 5/0.40 | NO, H2, CO | 300 |
| W98.3Ta0.2Y1Mg0.5Oxb | NO2 | 5/0.17 | NO, H2, CO | 300 |
| W99.5Ta0.5Ox | Propene | 50/2.1·10-3 | all gases | 250 |
| W99.5Rh0.5Ox | CO | 50/5.2·10-3 | NO, NO2 | 400 |
| bulk oxide | test gas | temperature [°C] | bulk dopants [0.5 At%] | surface dopants [0.5 At%] |
|---|---|---|---|---|
| Sn | NO | 250-300 | - | - |
| Bi | NO | 250-300 | - | pure; Er, Sm, Sc, Y |
| pure; Au, Co, Cr, Mg, Sc, | ||||
| W | propene | 350 | Y | Eu |
| propene | 300-400 | Ta | pure, Cu | |
| In | propene | 350 | Ce | - |
© 2006 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Frenzer, G.; Frantzen, A.; Sanders, D.; Simon, U.; Maier, W.F. Wet Chemical Synthesis and Screening of Thick Porous Oxide Films for Resistive Gas Sensing Applications. Sensors 2006, 6, 1568-1586. https://doi.org/10.3390/s6111568
Frenzer G, Frantzen A, Sanders D, Simon U, Maier WF. Wet Chemical Synthesis and Screening of Thick Porous Oxide Films for Resistive Gas Sensing Applications. Sensors. 2006; 6(11):1568-1586. https://doi.org/10.3390/s6111568
Chicago/Turabian StyleFrenzer, Gerald, Andreas Frantzen, Daniel Sanders, Ulrich Simon, and Wilhelm F. Maier. 2006. "Wet Chemical Synthesis and Screening of Thick Porous Oxide Films for Resistive Gas Sensing Applications" Sensors 6, no. 11: 1568-1586. https://doi.org/10.3390/s6111568




