Studies of the Cataluminescence of Benzene Homologues onNanosized γ–Al2O3/Eu2O3 and the Development of a Gas Sensorfor Benzene Homologue Vapors
Abstract
:1. Introduction
2. Experimental Section
2.1. Apparatus
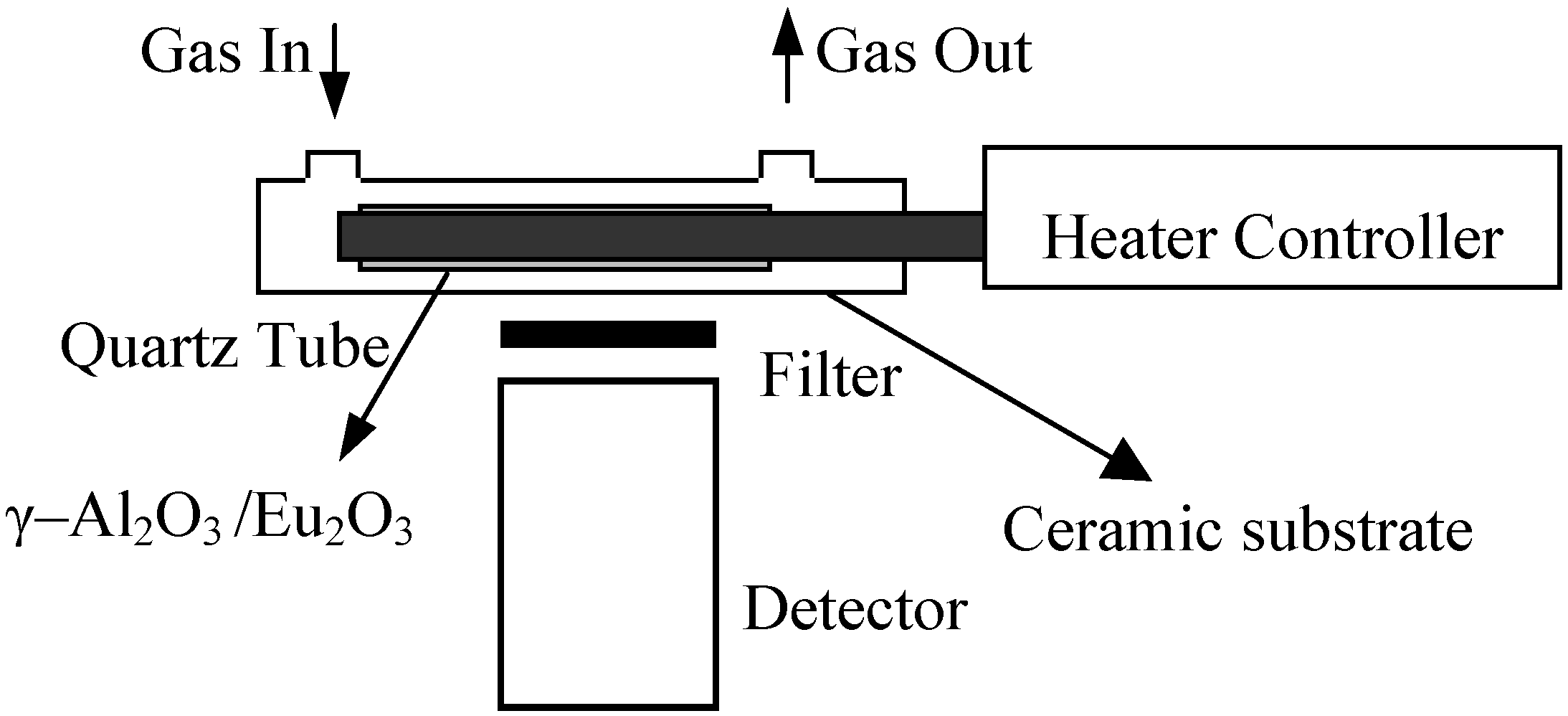
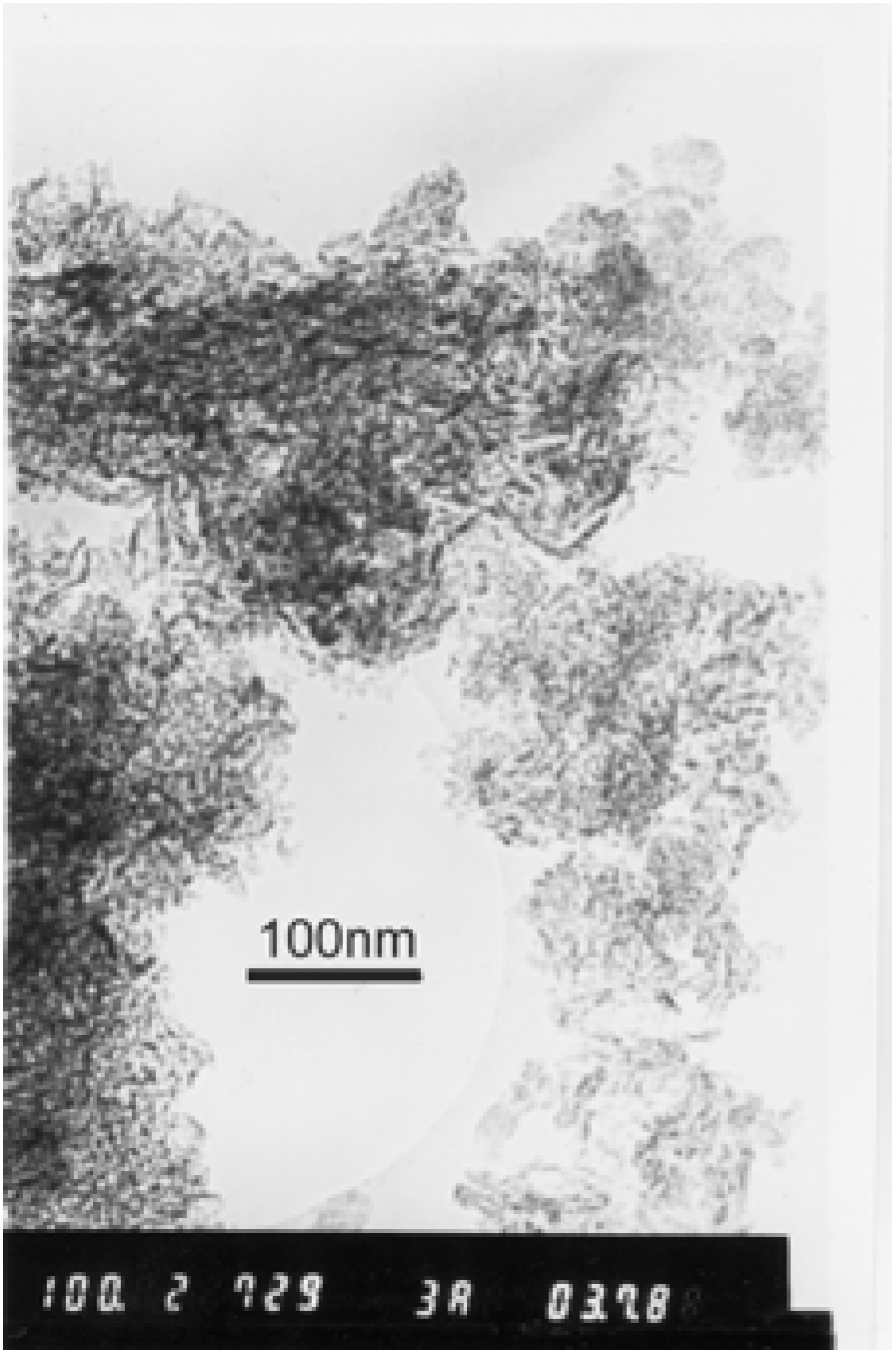
2.2. Preparation of the nanosized materials
3. Results and Discussion
3.1. Estimation of the nanosized materials
| Material | CTL intensity (S/N) | Optimum temperature and wavelength |
|---|---|---|
| γ–Al2O3 | 4.23 | 395 ºC, 425nm |
| γ–Al2O3/Eu2O3 (98 mol %/2 mol %) | 7.50 | 432 ºC, 425nm |
| γ–Al2O3/Eu2O3 (98.5 mol %/1.5 mol %) | 6.59 | 383 ºC, 425nm |
| ZrO2 | 5.45 | 373 ºC, 425nm |
| ZrO2/Eu2O3 (98 mol %/2 mol %) | 4.86 | 247 ºC, 620nm |
3.2. The CTL response profile of benzene on the surface of nanosized γ–Al2O3 /Eu2O3
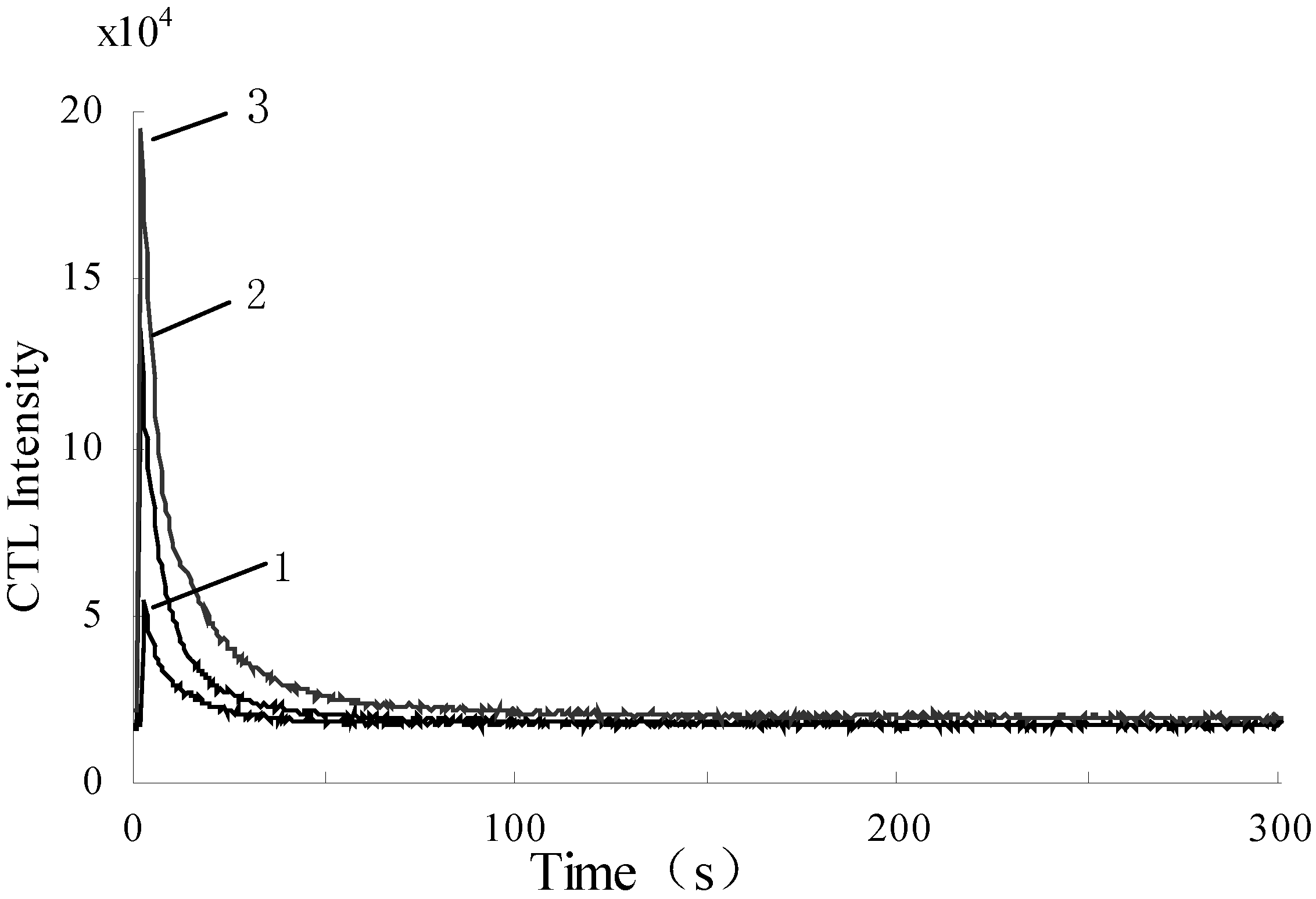
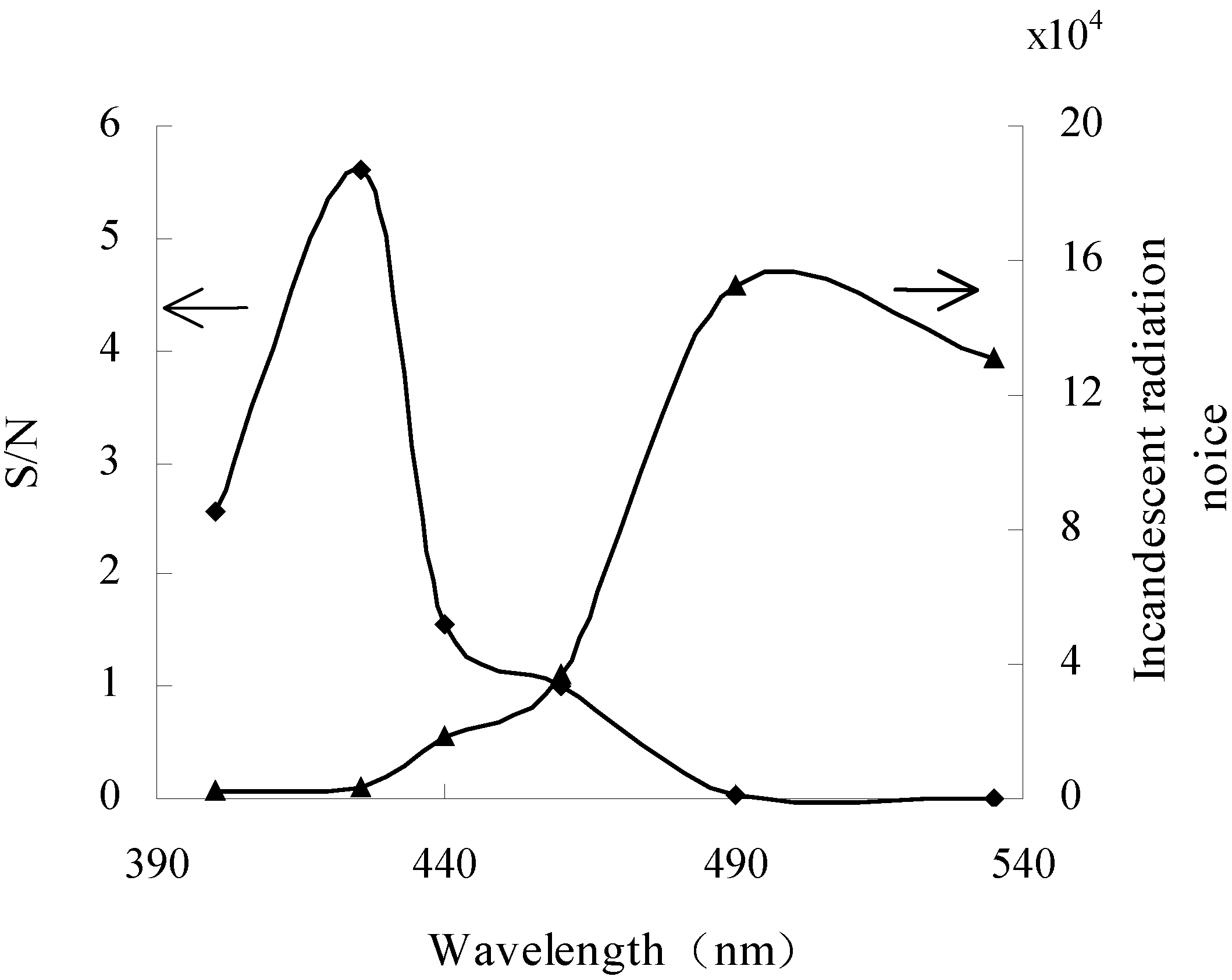
3.3. Optimization of wavelengths
3.4. Optimization of working temperature
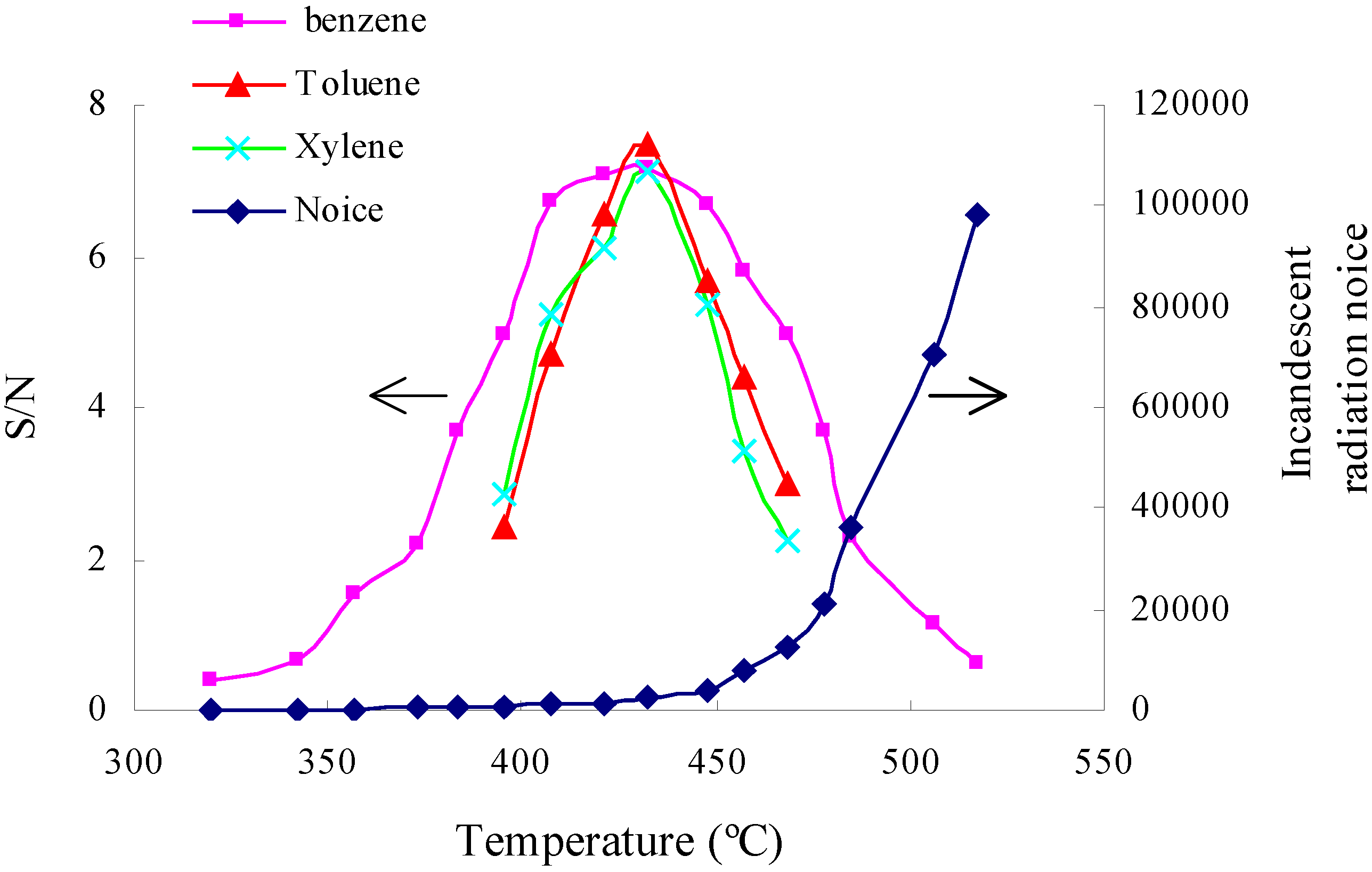
3.5. Optimization of flow rate of carrier gas
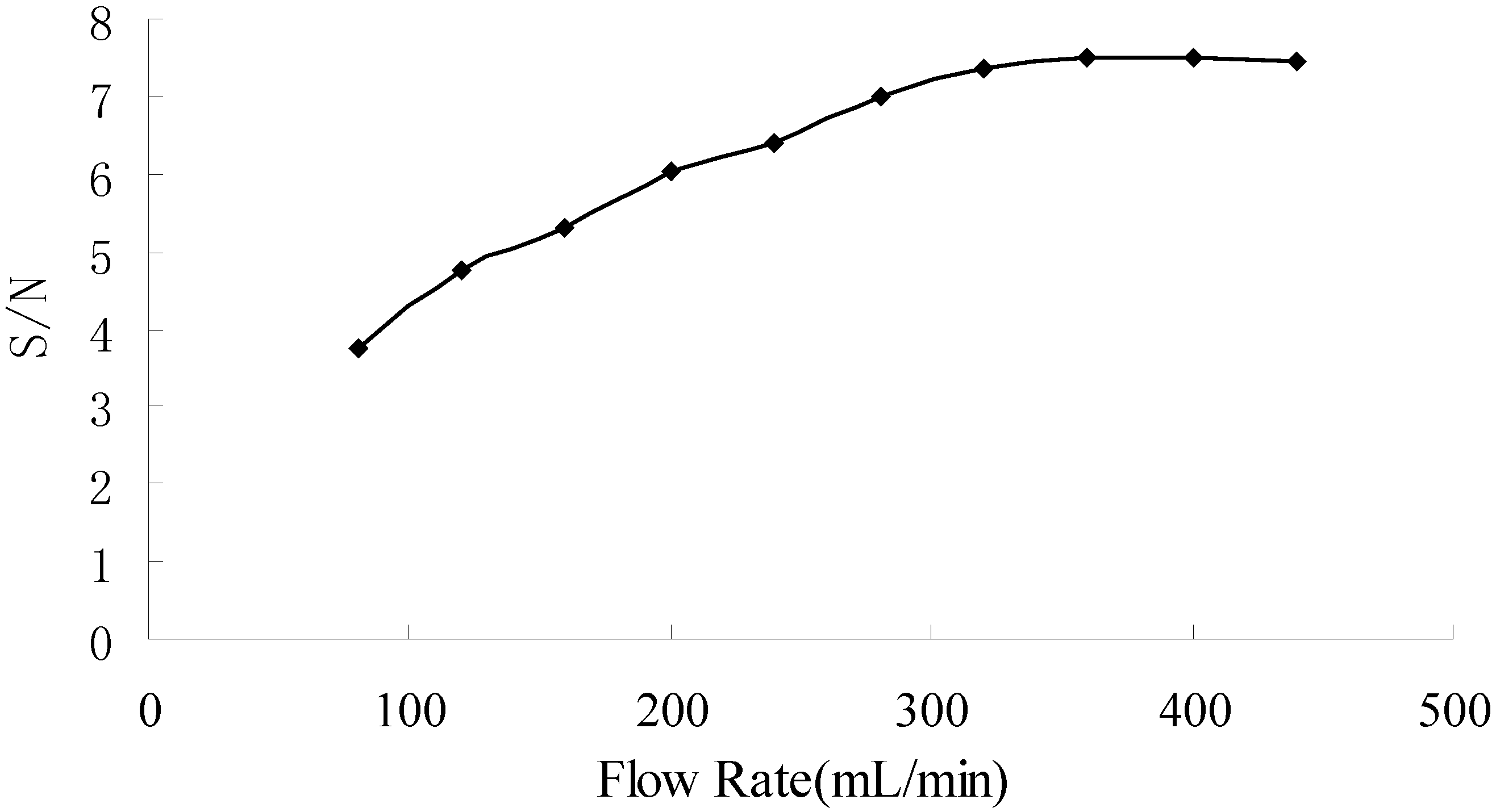
3.6. Analytical Characteristics
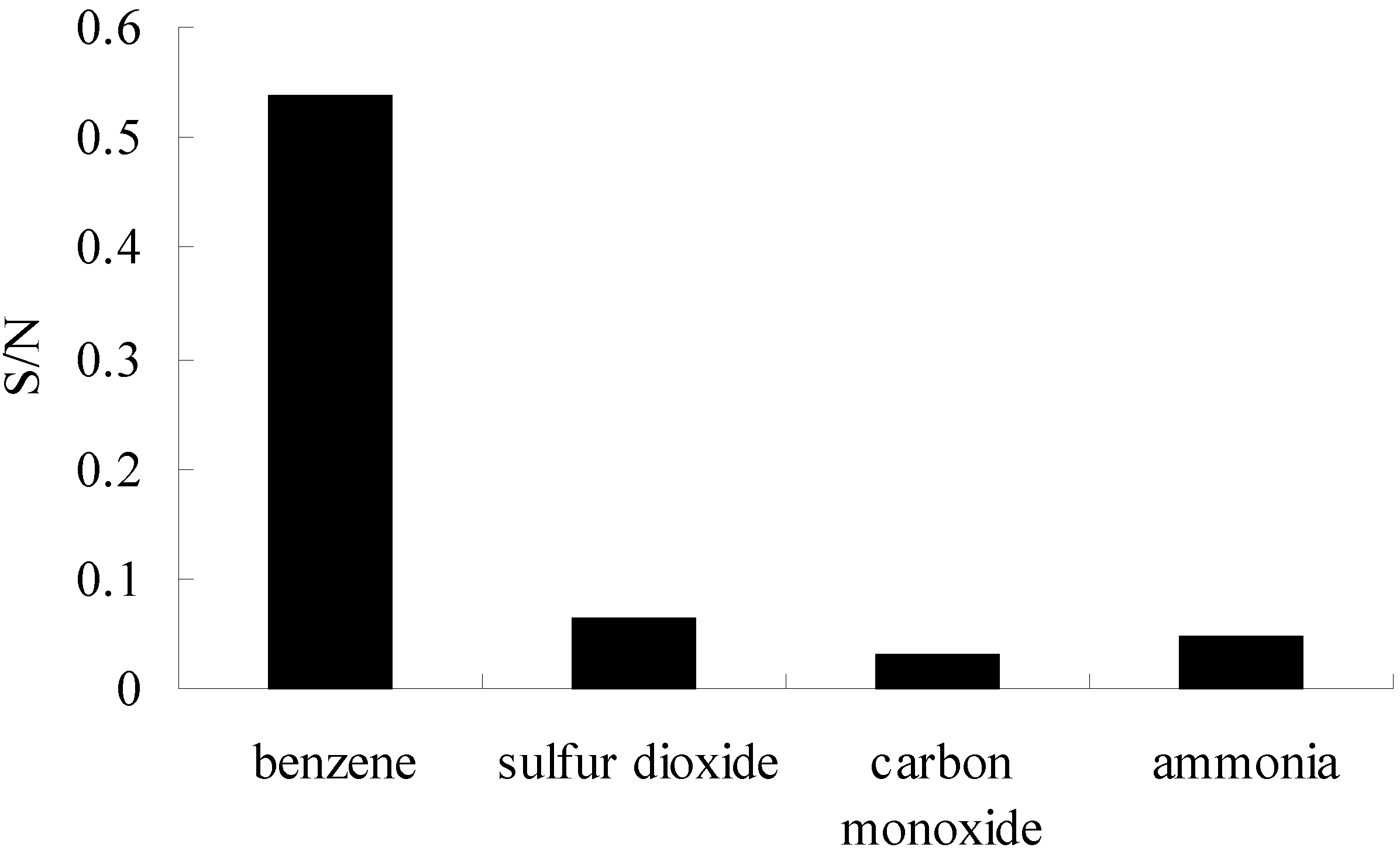
3.7. Selectivity
| Measured Gas | Linear Range (mL/m3) | Regression Equation | Correlation Coefficient | Limit of Detection (mL/m3) |
|---|---|---|---|---|
| Benzene | 2.4~100 | I=16.31c+119 | 0.9991 | 1.8 |
| 100~5000 | I=2.560c+393 | 0.9994 | ||
| Toluene | 4.0~100 | I=30.89c+78.9 | 0.9997 | 3 |
| 100~5000 | I=3.616c+351 | 0.9921 | ||
| Xylene | 6.8~100 | I=29.79c+18.6 | 0.9958 | 3.4 |
| 100~5000 | I=3.970c+142 | 0.9974 |
3.8. Lifetime of the gas sensor
3.9. Determination of benzene or benzene homologues in the synthetic samples
| Sample No. | Composition | Standard Values (mL/m3) | Measured Values (mL/m3, n=6) | Benzene(or Benzene Homologues) Recovery (%) |
|---|---|---|---|---|
| 1 | Benzene | 807 | 887±64 | 110 |
| Sulfur Dioxide | 100 | |||
| 2 | Benzene | 807 | 814±95 | 101 |
| Ammonia | 100 | |||
| Benzene | 897 | |||
| 3 | Toluene | 354 | 1416±64 | 102 |
| Xylene | 132 |
4. Conclusions
Acknowledgements
References and Notes
- U.S. Environmental Protection Agency. National air toxics assessment. http://www.epa.gov/ttn/atw/nata.
- Qin, J. P.; Yu, H. M.; Tang, J. H. Analysis of benzene, toluene and xylenes by capillary gas chromatography. J. Nanjing Univ. 2005, 27, 58–60. [Google Scholar]
- Cao, J.; Xu, Y.; Wang, Y. M.; Luo, G. A. Determination of macro-porous resin residue in herba hyperici perforat extraction by flow gas capture method. Chinese J. Anal. Chem. 2004, 32, 309–312. [Google Scholar]
- Angelucci, R.; Poggi, A.; Dori, L.; Cardinali, G. C.; Parisini, A.; Tagliani, A.; Mariasaldi, M.; Cavani, F. Permeated porous silicon for hydrocarbon sensor fabrication. Sens. Actuators B 1999, 74, 95–99. [Google Scholar] [CrossRef]
- Mabrook, M.; Hawkins, P. Benzene Sensing Using Thin Films of Titanium Dioxide Operating at Room Temperature. Sensors 2002, 2, 374–382. [Google Scholar] [CrossRef]
- Yang, J.W.; Cho, H.J.; Lee, S.H.; Lee, J.Y. Characterization of SnO2 ceramic gas sensor for exhaust gas monitoring of SVE process. Environ. Monit. Assess. 2004, 92, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Breysse, M.; Claudel, B.; Faure, L.; Guenin, M; Williams, R. J. J.; Wolkenstein, T. J. Chemiluminescence during the catalysis of carbon monoxide oxidation on a thoria surface. J. Catal. 1976, 45, 137–144. [Google Scholar] [CrossRef]
- Nakagawa, M.; Kawabata, S.; Nishiyama, K.; Utsunomiya, K.; Yamamoto, I.; Wada, T.; Yamashita, Y.; Yamashita, N. Analytical detection system of mixed odor vapors using chemiluminescence-based gas sensor. Sens. Actuators B 1996, 34, 334–338. [Google Scholar] [CrossRef]
- Utsunomiya, K.; Nakagawa, M.; Tomiyama, T.; Yamamoto, I.; Matsuura, Y.; Chikamori, S.; Wada, T.; Yamashita, N.; Yamashita, Y. An adsorption-luminescent Al2O3 sheet for determining vapor of odor substances in air. Sens. Actuators B 1993, 13-14, 627–628. [Google Scholar] [CrossRef]
- Nakagawa, M. A new chemiluminescence based sensor for discriminating and determining constituents in mixed gases. Sens. Actuators B 1995, 29, 94–100. [Google Scholar] [CrossRef]
- Nakagawa, M.; Okabayashi, T.; Fujimoto, T.; Utsunomiya, K.; Yamamoto, I.; Wada, T.; Yamashita, Y.; Yamashita, N. A new method for recognizing organic vapor by spectroscopic image on cataluminescence based gas sensor. Sens. Actuators B 1998, 51, 159–162. [Google Scholar] [CrossRef]
- Okabayashi, T; Matsuo, N; Yamamoto, I; Utsunomiya, K.; Yamashita, N.; Nakagawa, M. Temperature-programmed sensing for gas identification using the cataluminescence-based sensors. Sens. Actuators B 2005, 108, 515–520. [Google Scholar] [CrossRef]
- Okabayashi, T.; Fujimoto, T.; Yamamoto, I.; Utsunomiya, K.; Wada, T.; Yamashita, Y.; Yamashita, N.; Nakagawa, M. High sensitive hydrocarbon gas sensor utilizing cataluminescence of gamma- Al2O3 activated with Dy3+. Sens. Actuators B 2000, 64, 54–58. [Google Scholar] [CrossRef]
- Zhu, Y. F.; Shi, J. J.; Zhang, Z. Y.; Zhang, C.; Zhang, X. R. Development of a gas sensor utilizing chemiluminescence on nanosized titanium dioxide. Anal. Chem. 2002, 74, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Shi, J. J.; Li, J. J.; Zhu, Y. F.; Wei, F.; Zhang, X. R. Nanosized SrCO3-based chemiluminescence sensor for ethanol. Anal. Chim. Acta 2002, 66, 69–78. [Google Scholar] [CrossRef]
- Zhang, Z. Y.; Zhang, C.; Zhang, X. R. Development of a chemiluminescence ethanol sensor based on nanosized ZrO2. Analyst 2002, 127, 792–796. [Google Scholar] [CrossRef] [PubMed]
- Cao, X. A.; Zhang, Z. Y.; Zhang, X. R. A study of gaseous acetaldehyde sensor utilizing cataluminescence on nanosized SrCO3. Chinese J. Anal. Chem. 2004, 32, 1567–1570. [Google Scholar]
- Cao, X. A.; Zhang, Z. Y.; Zhang, X. R. A novel gaseous acetaldehyde sensor utilizing cataluminescence on nanosized-BaCO3. Sens. Actuators B 2004, 99, 30–35. [Google Scholar] [CrossRef]
- Cao, X. A.; Feng, G. M.; Gao, H. H.; Luo, X. Q.; Lu, H. L. Nanosized γ-Al2O3 + Nd2O3 –based cataluminescence sensor for ethylene dichloride. Luminescence 2005, 20, 104–108. [Google Scholar] [CrossRef] [PubMed]
© 2006 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Lu, J.; Cao, X.; Pan, C.; Yang, L.; Lai, G.; Chen, J.; Wu, C. Studies of the Cataluminescence of Benzene Homologues onNanosized γ–Al2O3/Eu2O3 and the Development of a Gas Sensorfor Benzene Homologue Vapors. Sensors 2006, 6, 1827-1836. https://doi.org/10.3390/s6121827
Lu J, Cao X, Pan C, Yang L, Lai G, Chen J, Wu C. Studies of the Cataluminescence of Benzene Homologues onNanosized γ–Al2O3/Eu2O3 and the Development of a Gas Sensorfor Benzene Homologue Vapors. Sensors. 2006; 6(12):1827-1836. https://doi.org/10.3390/s6121827
Chicago/Turabian StyleLu, Jieshan, Xiaoan Cao, Canying Pan, Lianfeng Yang, Guangbo Lai, Jianling Chen, and Cuiqin Wu. 2006. "Studies of the Cataluminescence of Benzene Homologues onNanosized γ–Al2O3/Eu2O3 and the Development of a Gas Sensorfor Benzene Homologue Vapors" Sensors 6, no. 12: 1827-1836. https://doi.org/10.3390/s6121827




