Enhancement of H2-sensing Properties of F-doped SnO2 Sensorby Surface Modification with SiO2
Abstract
:Introduction
Materials and methods
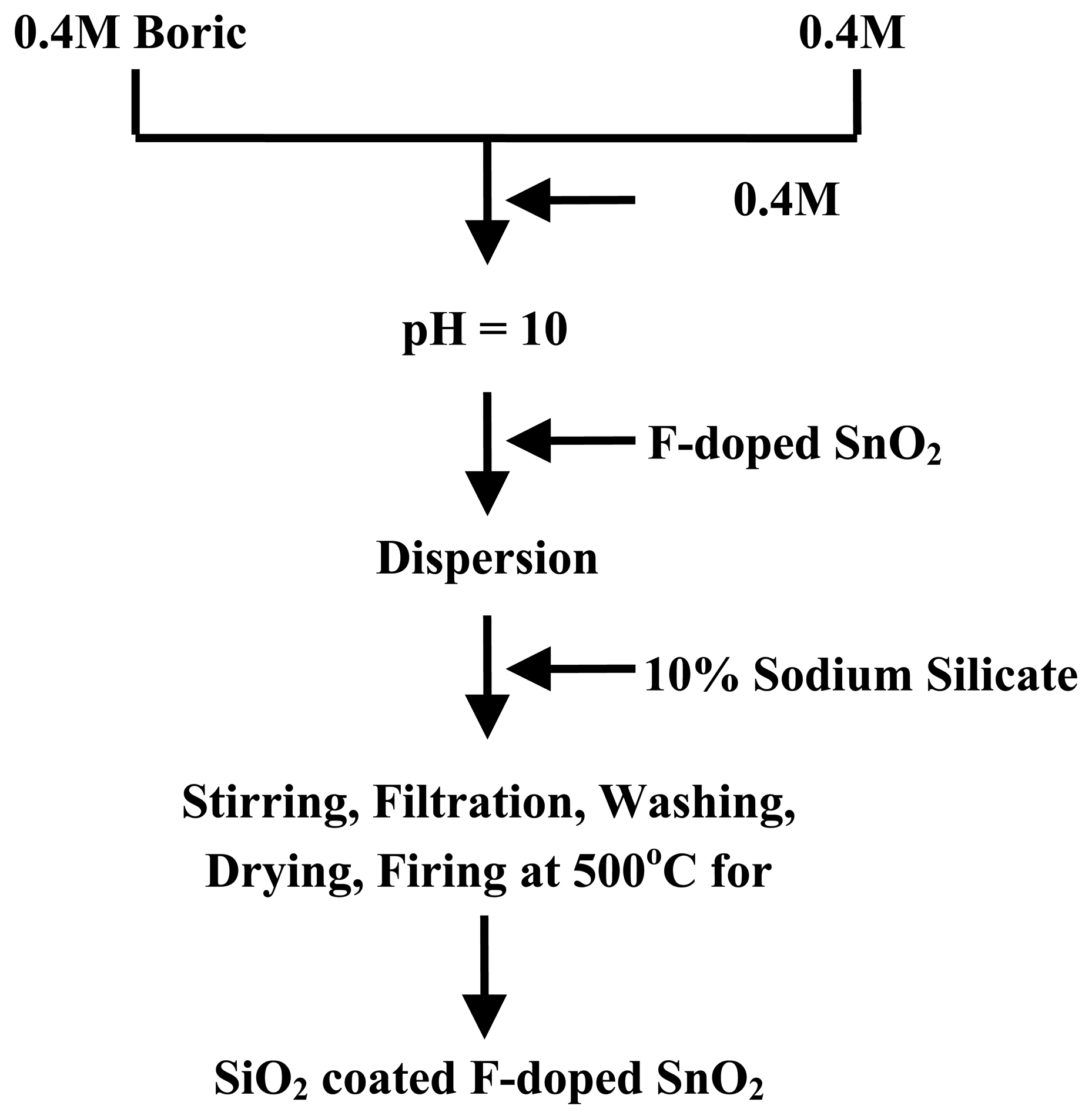
Preparation of sample
Characterization of the modified F-doped SnO2
Fabrication of Sensor
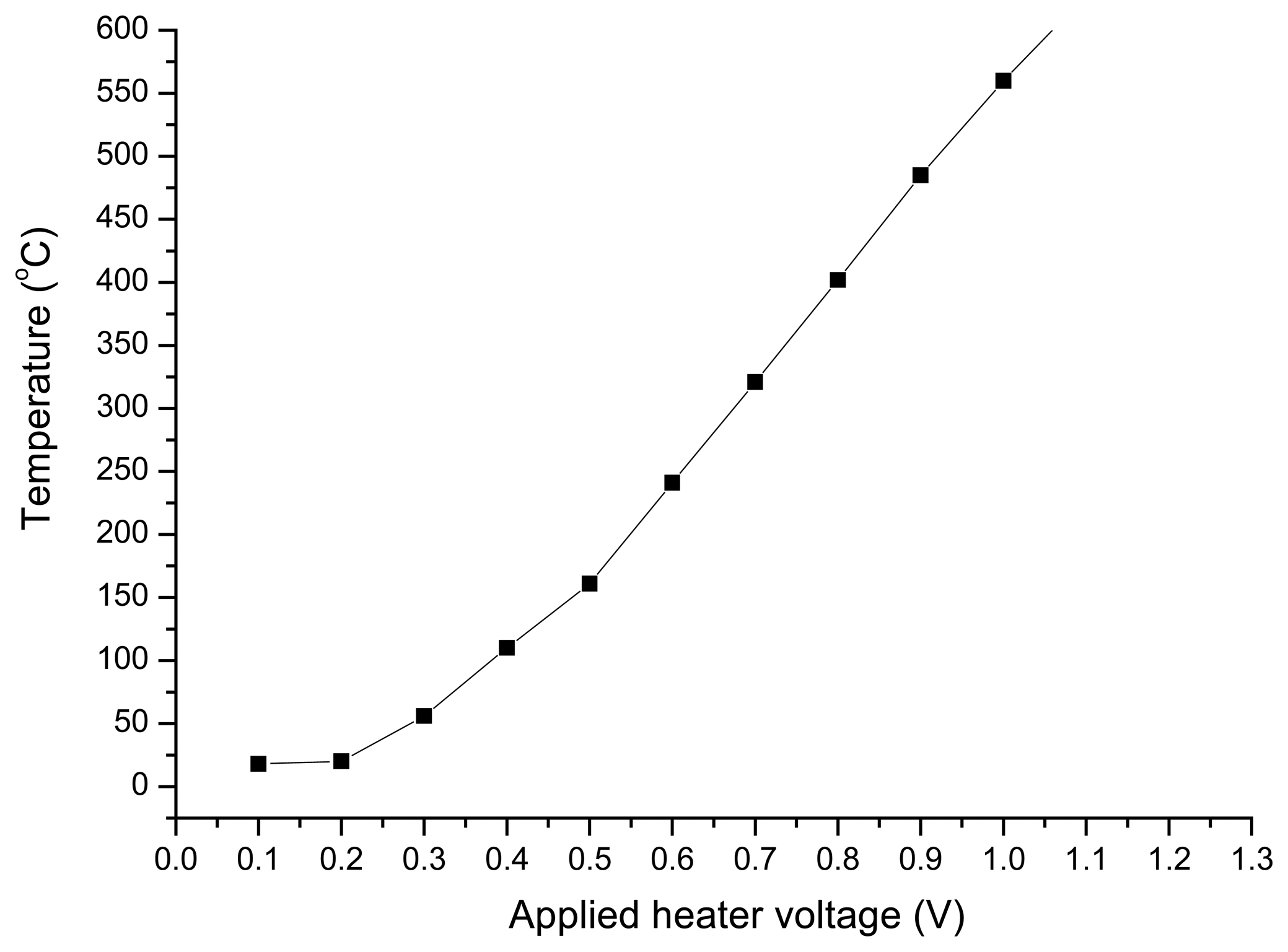
Temperature of sensor
Gas sensing test
Results and discussion
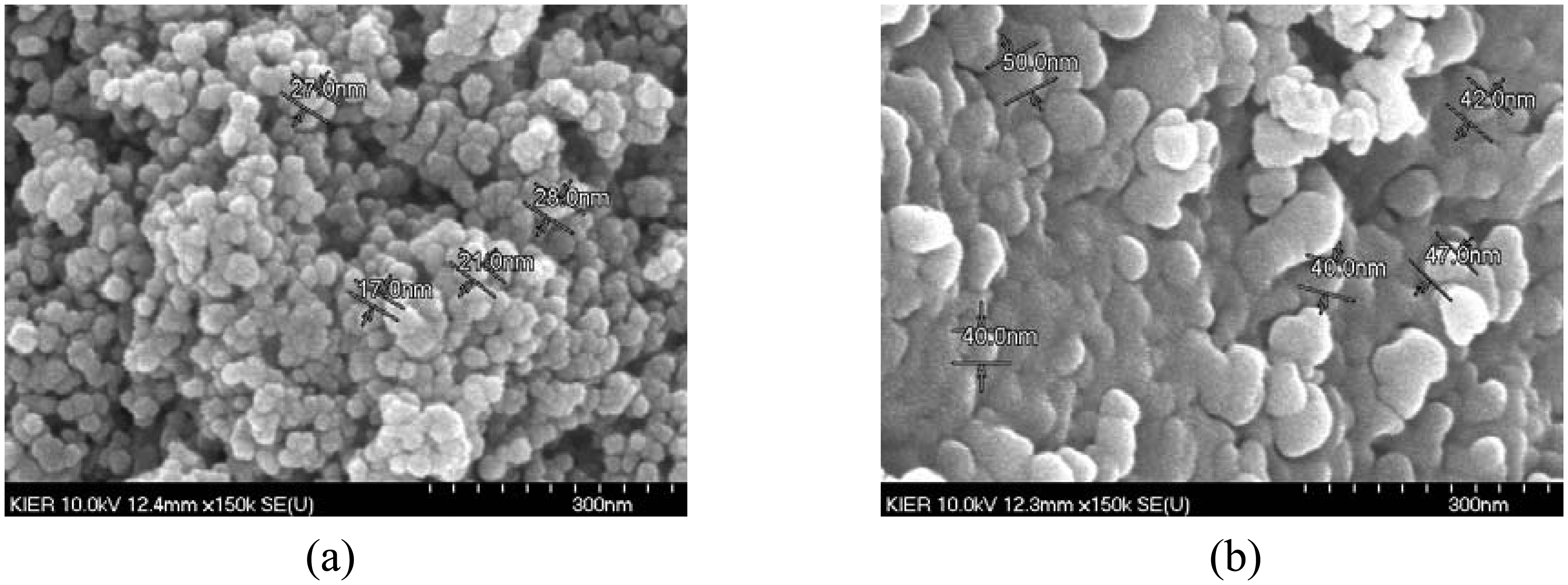
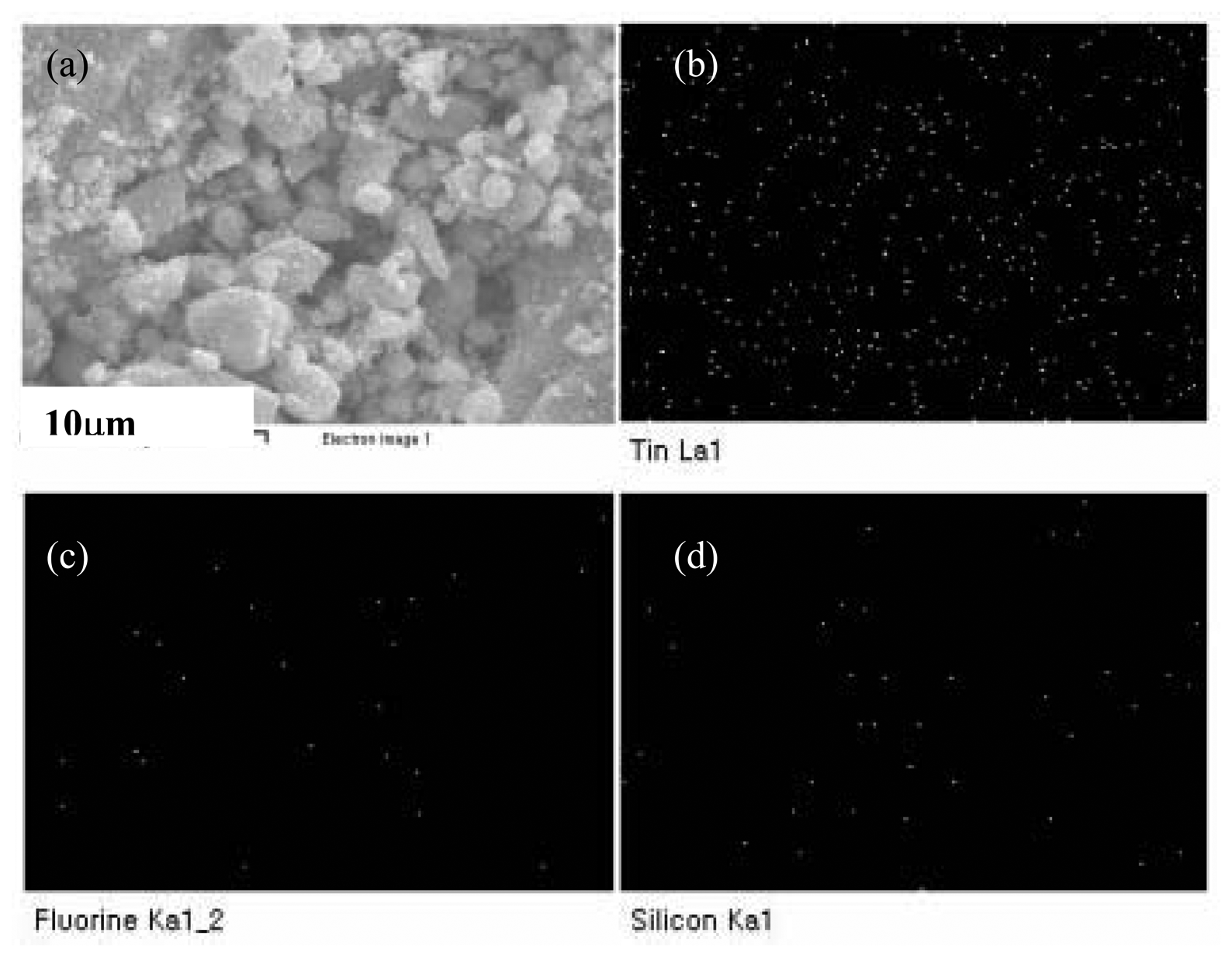
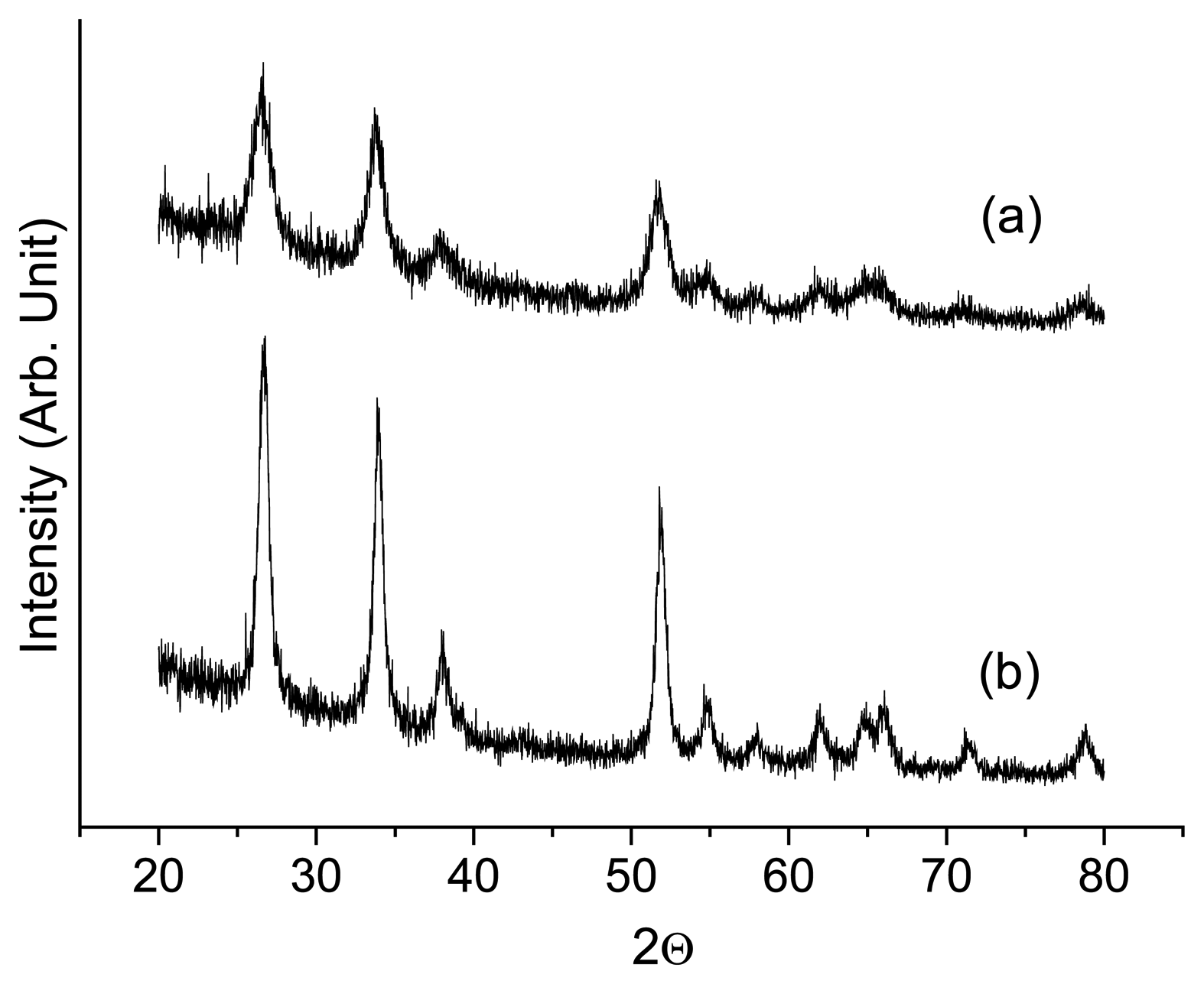
Microstructure of the sensor material
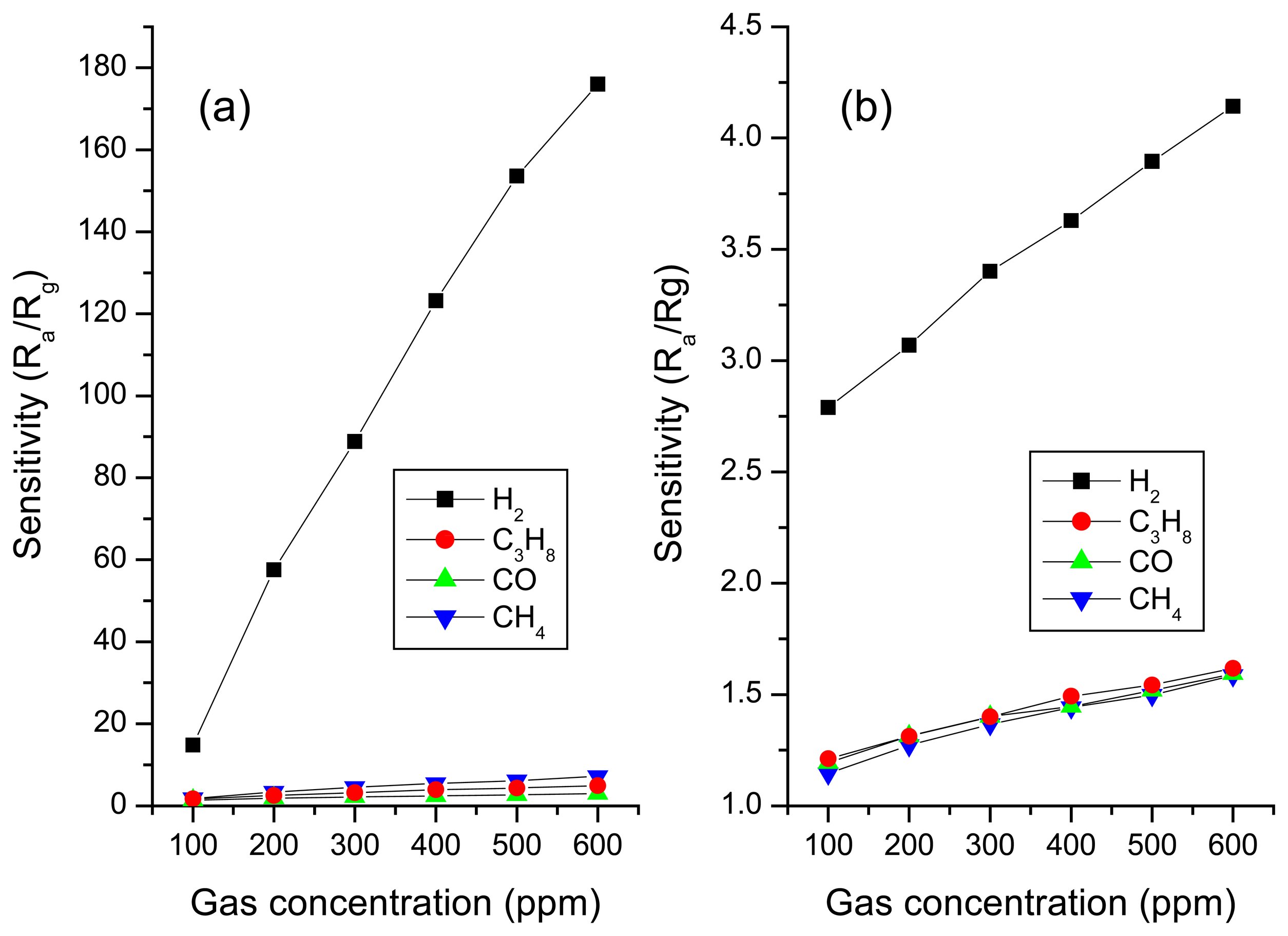
Gas sensing property
Comparison of the sensors made with surface modified F-doped SnO2 and unmodified F-doped SnO2
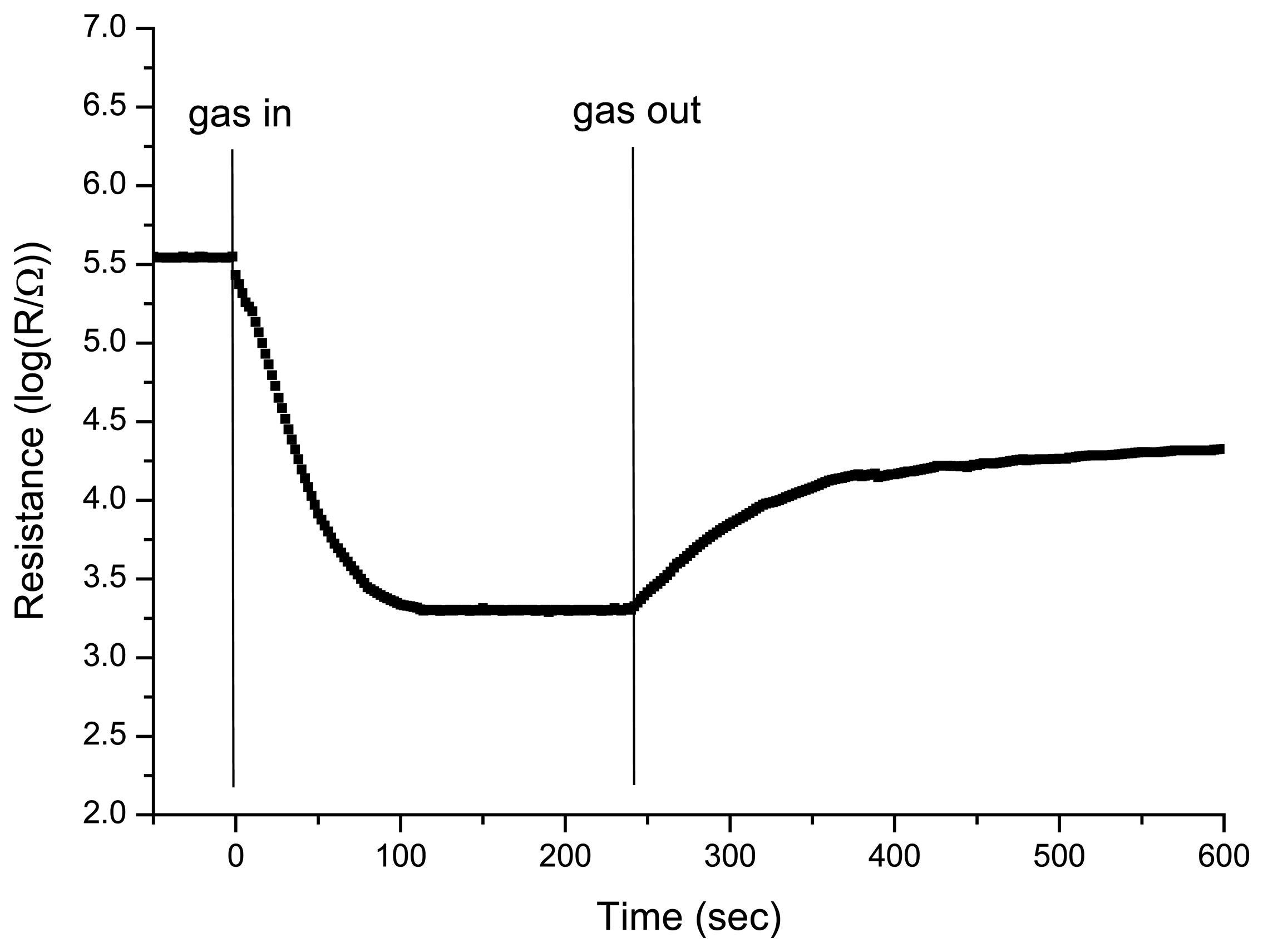
Response and recovery times of the sensor
Conclusions
Acknowledgments
References
- Grimes, C. A.; Ong, K. G.; Varghese, O.K.; Yang, X.; Mor, G.; Paulose, M.; Dickey, E.C.; Ruan, C.; Pishko, M.V.; Kendig, J.W.; Mason, A.J. A sentinel sensor network for hydrogen sensing. Sensors 2003, 3, 69–82. [Google Scholar]
- Katti, V.R.; Debnath, A.K.; Gadkari, S.C.; Gupta, S.K.; Sahni, V.C. Passivated thick film catalytic type H2 sensor operating room temperature. Sens. Actuators B 2002, 84, 219–225. [Google Scholar]
- Heilig, A.; Barsan, N.; Weimer, U.; Schweizer-Berberich, M.; Gardner, J.W.; Gopel, W. Gas idenfication by modulating temperatures of SnO2-based thick film sensors. Sens. Actuators B 1997, 43, 45–51. [Google Scholar]
- Becker, Th.; Ahlers, S.; Bosch-Braunmihl, Chr.; Muller, G.; Kiesewetter, O. Gas sensing properties of thin and thick film tin-oxide materials. Sens. Actuators B 2001, 77, 55–61. [Google Scholar]
- Shukla, S.; Patil, S.; Kuiry, S.C.; Rahman, Z.; Du, T.; Ludwig, L.; Parish, C.; Seal, S. Synthesis and characterization of sol-gel derived nano-crystalline tin oxide thin film as hydrogen sensor. Sens. Actuators B 2003, 96, 343–353. [Google Scholar]
- Starke, T.K.H.; Coles, G.S.V. Laser-ablated nano-crystalline SnO2 materials for low-level CO detection. Sens. Actuators B 2003, 88, 227–233. [Google Scholar]
- Wang, Y.; Wie, X.; Li, Y.; Zhou, Z. Meso-structured SnO2 as sensing materials for gas sensors. Solid State Elect. 2004, 48, 627–632. [Google Scholar]
- Morrison, S.R. Semiconductor gas sensor. Sens. Actuators 1982, 2, 329–341. [Google Scholar]
- Kohl, D. Surface process in the detection of reducing gases with SnO2-based devices. Sens. Actuators 1989, 18, 71–113. [Google Scholar]
- Shukla, S.; Zhang, P.; Cho, H.J.; Rahman, Z.; Drake, C.; Seal, S.; Craciun, V.; Ludwig, L. Hydrogen-discriminating nanocrystalline doped-tin-oxide room-temperature micro sensor. J. Appl. Phys. 2005, 98, 104306 1–15. [Google Scholar]
- Shukla, S.; Agarwal, R.; Cho, H.J.; Seal, S.; Ludwig, L.; Parish, C. Effect of ultraviolet radiation exposure on room-temperature hydrogen sensitivity of nanocrystalline doped tin oxide sensor incorporated into microelectromechanical system device. J. Appl. Phys. 2005, 97, 054307 1–13. [Google Scholar]
- Shukla, S.; Ludwig, L.; Parish, C.; Seal, S. Inverse-catalyst-effect observed for nanocrystalline-doped tin oxide sensor at lower operating temperature. Sens. Actuators B 2005, 104, 223–231. [Google Scholar]
- Wie, B.; Hsu, M.; Su, P.; Lin, H.; Wu, P.; Lai, H. A novel SnO2 gas sensor doped with carbon nanotubes operating at room temperature. Sens. Actuators B 2004, 101, 81–89. [Google Scholar]
- Han, C.-H.; Han, S.-D.; Singh, I.; Toupance, T. Micro-bead of nano-crystalline F-doped SnO2 as a sensitive hydrogen gas sensoe. Sens. Actuators B 2005, 109, 264–269. [Google Scholar]
- Yamazoe, N.; Muta, Y.; Seiyama, T. Tin oxide gas sensor insensitive to ethanol gas. J. Surf. Sci. Soc. Jpn. 1984, 5, 241–247. [Google Scholar]
- Feng, C.-D.; Shimizu, Y.; Egashira, M. Effect of gas diffusion process on sensing properties of SnO2thin film sensor in a SiO2/SnO2layer-built structure fabricated by sol-gel process. J. Electrochem. Soc. 1994, 141, 220–225. [Google Scholar]
- Wada, K.; Egashira, M. Improvement of gas-sensing properties of Pd/SnO2 sensor by SiO2 coating films formed by dipping method. J. Ceram. Soc. Jpn. 1998, 106, 84–88. [Google Scholar]
- Wada, K.; Egashira, M. Gas-sensing propertied of Pd/SnO2 sensors dipped in a diethoxy silane sol solution. J. Ceram. Soc. Jpn. 1998, 106, 621–626. [Google Scholar]
- Wada, K.; Egashira, M. Hydrogen sensing properties of SnO2 subjected to surface chemical modification with ethoxysilanes. Sens. Actuators B 2000, 62, 211–219. [Google Scholar]
- Garmard, A.; Babot, G.; Rascle, M.C.; Jousseaume, B.; Toupance, T.; Campet, G. Conductive F-doped tin dioxide sol-gel materials from fluorinated β-diketonate tin(IV) complexes characterization and thermolytic behavior. Chem. Mater. 2000, 12, 3419–3426. [Google Scholar]
- Carr, J. J. Sensors and Circuits; PTR Prentice Hall: Englewool Cliffs, New Jersey 07632, 1993; p. p. 75. [Google Scholar]
- Weast, R.C. Handbook of Chemistry and Physics; CRC Press: Cleveland, 1976; p. p. D-107. [Google Scholar]
- Seal, S.; Shukla, S. Nano crystalline SnO2 gas sensors in view of surface reactions and modifications. JOM 2002, 54, 35–38. [Google Scholar]
- Gong, J.; Chen, Q.; Fei, W.; Seal, S. Micro-machined nano-crystalline SnO2 chemical gas sensors for electronic nose. Sens. Actuators B 2004, 102, 117–125. [Google Scholar]
© 2006 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Han, C.-H.; Han, S.-D.; Khatkar, S.P. Enhancement of H2-sensing Properties of F-doped SnO2 Sensorby Surface Modification with SiO2. Sensors 2006, 6, 492-502. https://doi.org/10.3390/s6050492
Han C-H, Han S-D, Khatkar SP. Enhancement of H2-sensing Properties of F-doped SnO2 Sensorby Surface Modification with SiO2. Sensors. 2006; 6(5):492-502. https://doi.org/10.3390/s6050492
Chicago/Turabian StyleHan, Chi-Hwan, Sang-Do Han, and S. P. Khatkar. 2006. "Enhancement of H2-sensing Properties of F-doped SnO2 Sensorby Surface Modification with SiO2" Sensors 6, no. 5: 492-502. https://doi.org/10.3390/s6050492



