Micro Semiconductor CO Sensors Based on Indium-Doped Tin Dioxide Nanocrystalline Powders
Abstract
:Introduction
Experimental
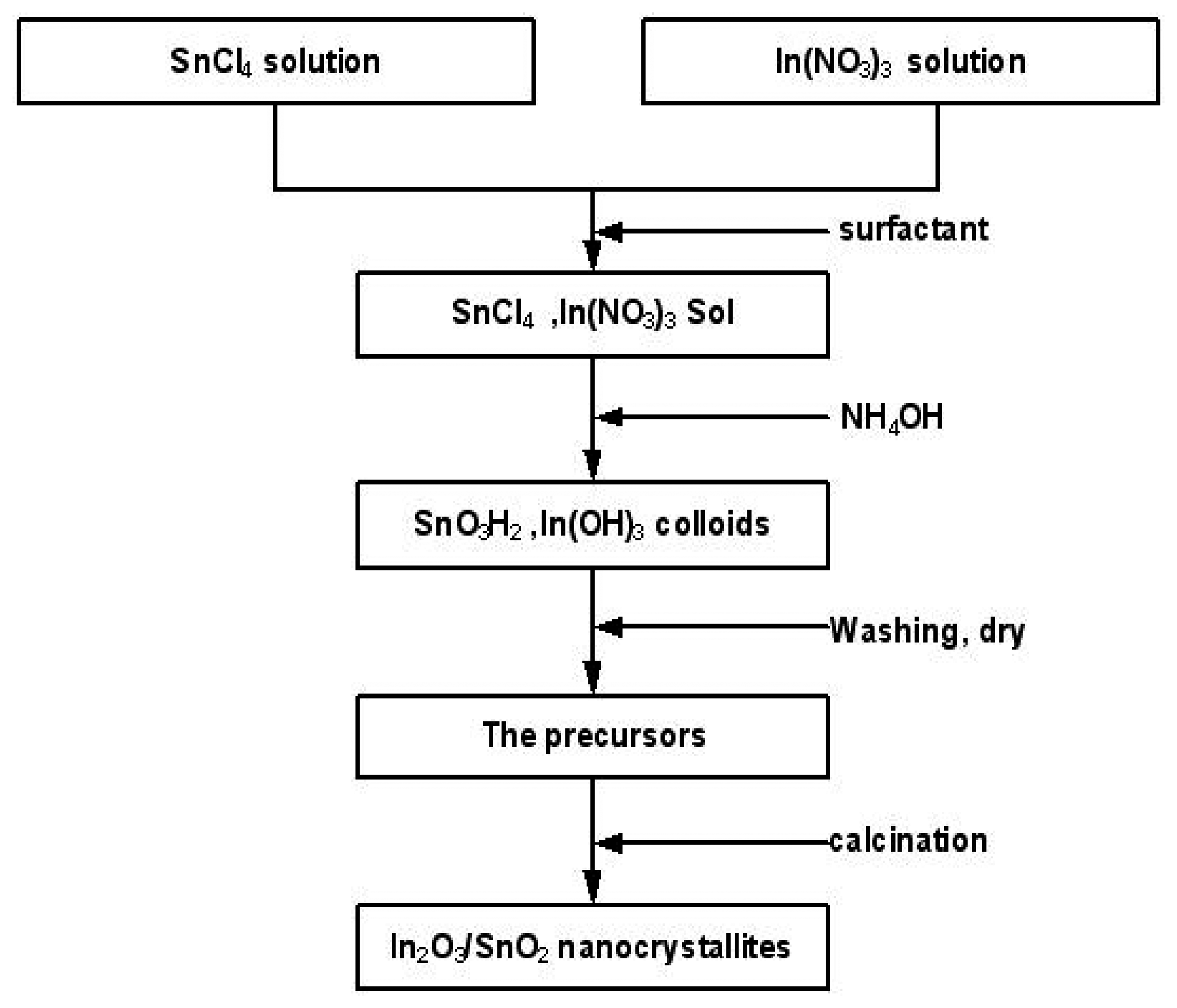
Sensing materials preparation
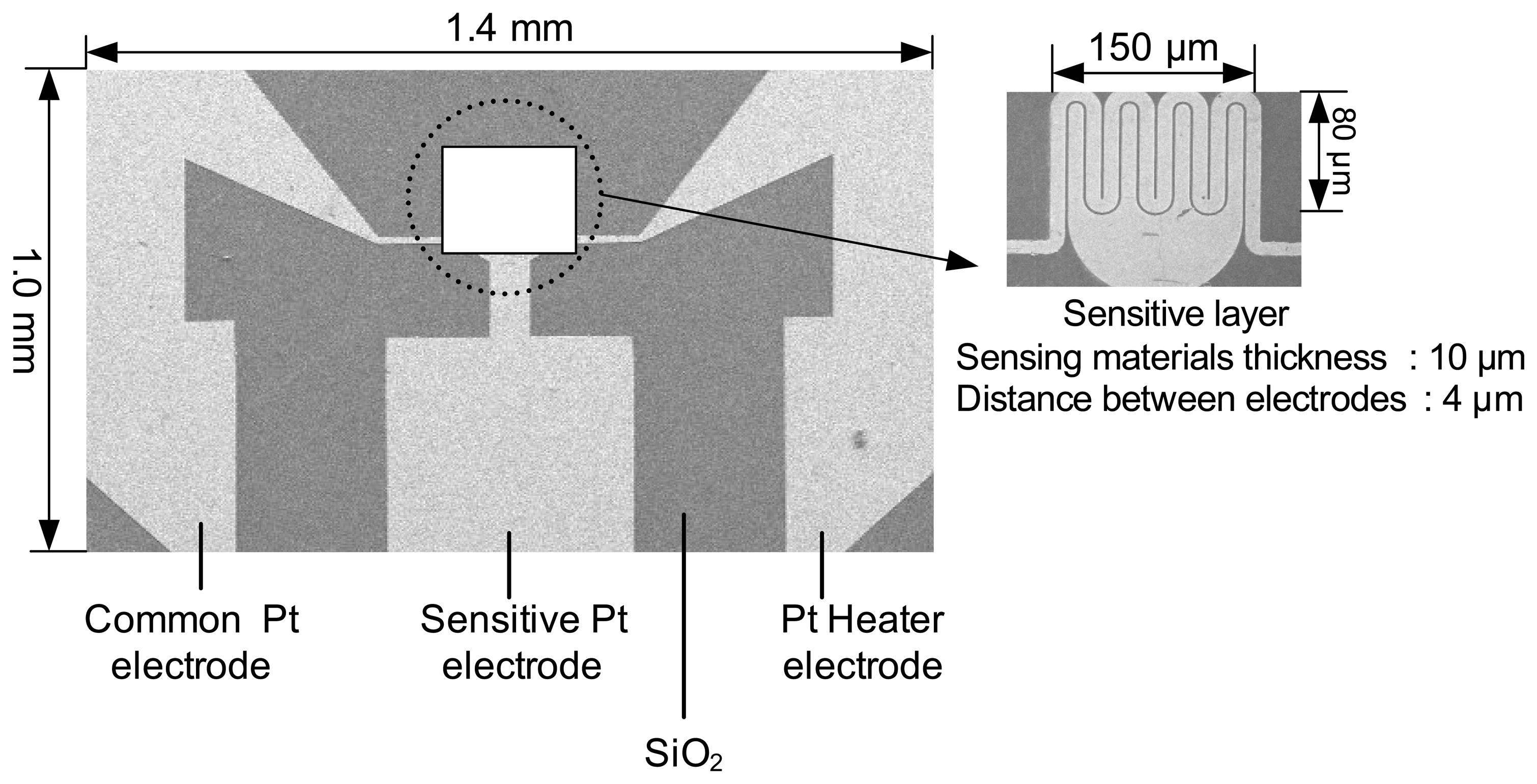
Sensor fabrication
Measurement of sensor resistance
Results and discussion
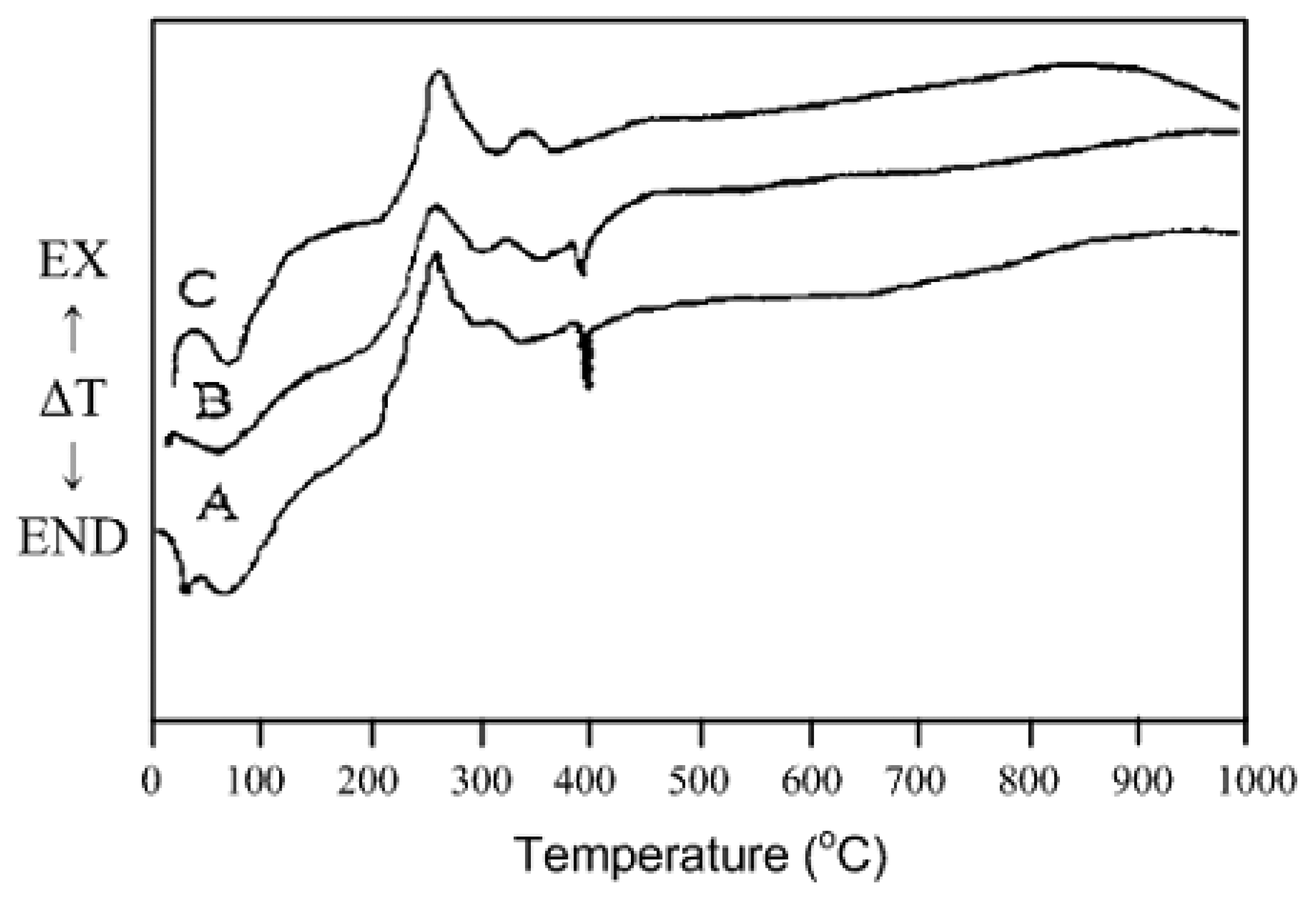
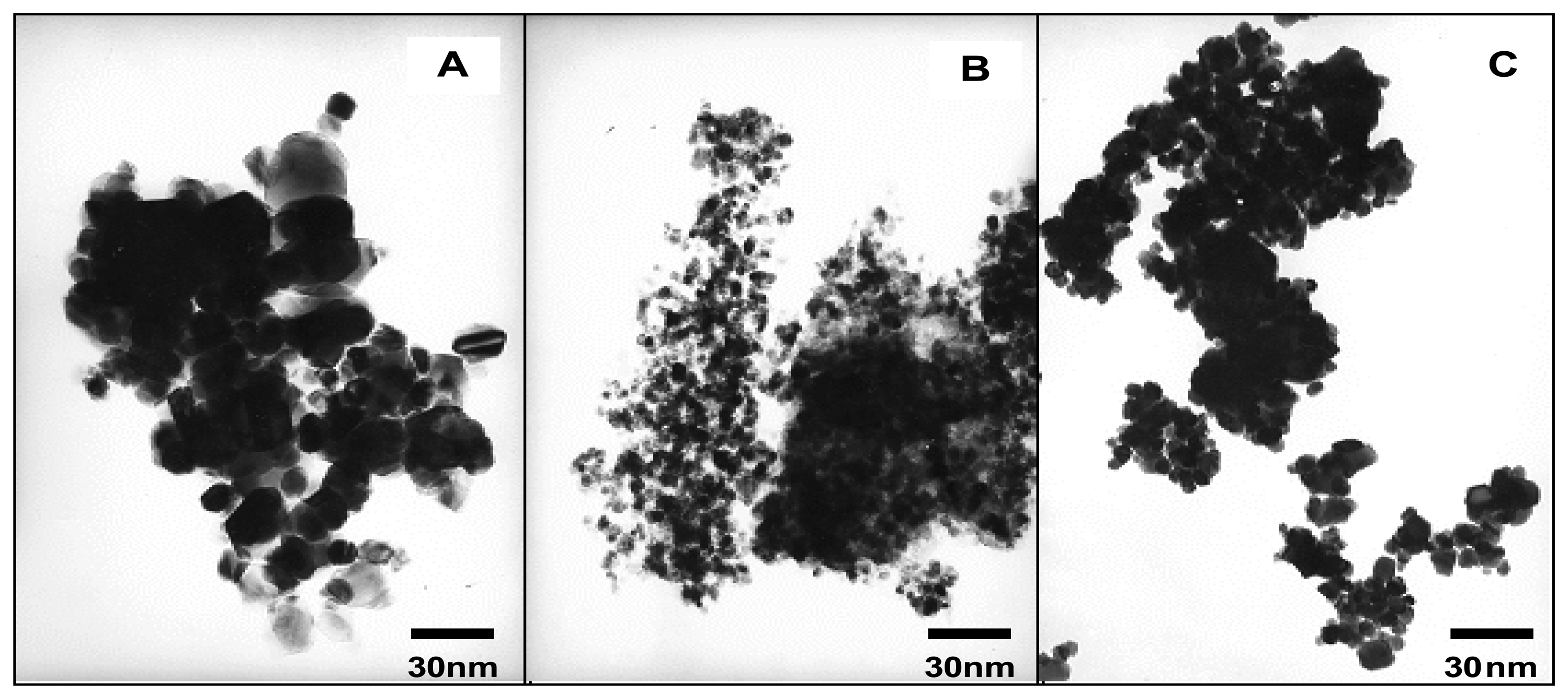
Structural characteristics of sensing materials
- SnO3H2→Sn2O5H2→Sn4O9H3→SnO2
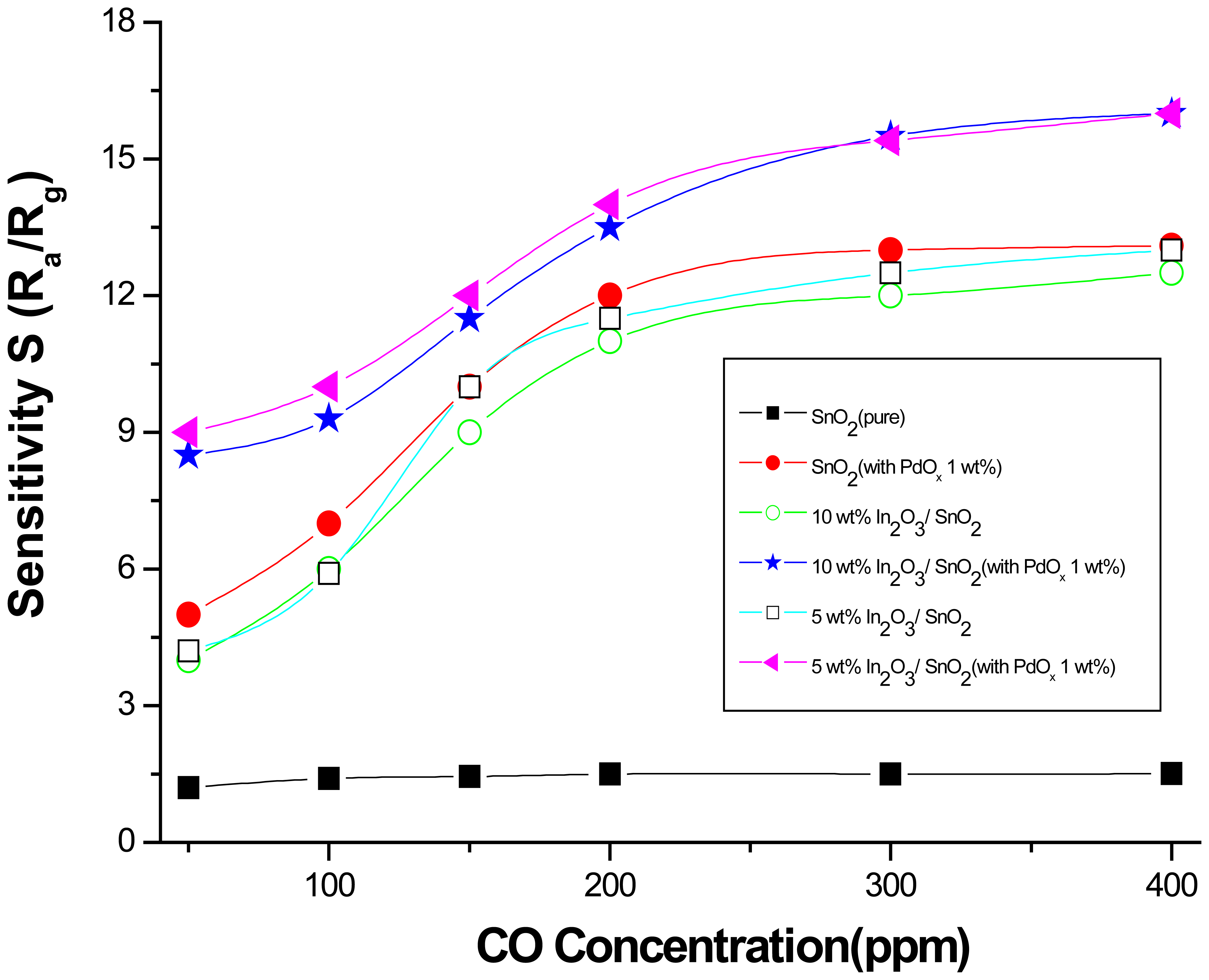
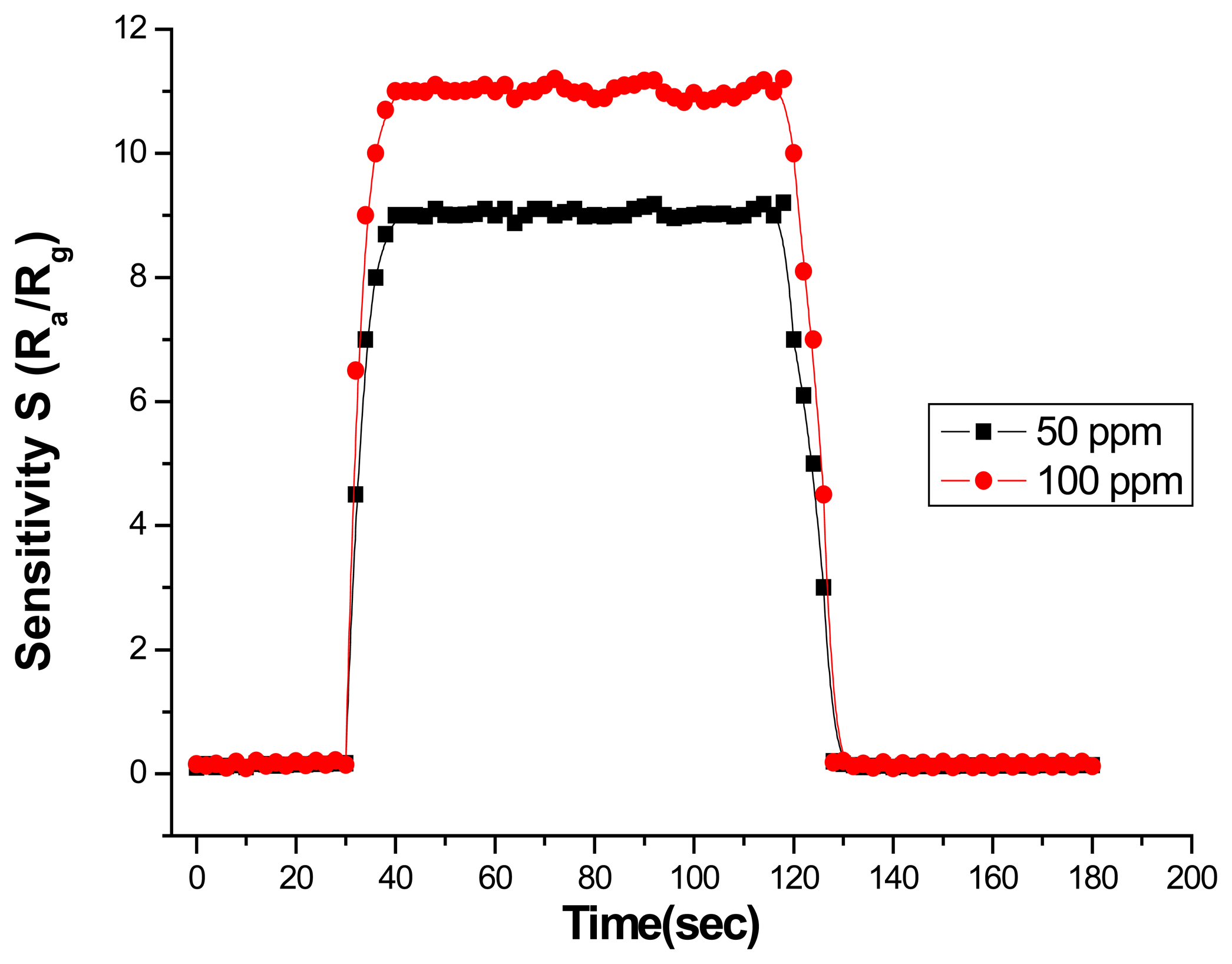
Gas sensing property
- –
- The application of nano-scaled SnO2 or In2O3/ SnO2 particles enables exposure of the gas to the maximum surface area.
- –
- The compatibility of noble metal salt with the surfactant may result in a well-dispersed distribution of nano sized catalyst particles over the sensing films.
Conclusion
Acknowledgments
References
- Birringer, R. Nanocrystalline materials. Mater. Sci. Eng. A 1989, 117, 33–43. [Google Scholar]
- Glieter, H. Nanostructured materials. Adv. Mater. 1992, 4, 474–481. [Google Scholar]
- Han, S.D.; Huang, S.Y.; Campet, G.; Kennard, M.A. Influence of the pH values of the sol-gel state on the properties of SnO2 nanocrystalline powders obtained from a sol-gel route. Active Passive Elec. Comp. 1995, 18, 53–60. [Google Scholar]
- Dawar, A.L.; Joshi, J.C. Review semiconducting transparent tin films: their properties and applications. J. Appl. Phys. 1980, 59, 6243–6251. [Google Scholar]
- Zhang, D.Y.; Wang, D.Z.; Wu, Y.H.; Wang, A. Investigation of nanocrystalline SnO2. Mater. Sci. Eng. B 1991, 8, 189–192. [Google Scholar]
- Ihokura, K.; Watson, J. The Stannic Oxide Gas Sensor; CRC Press: Boca Raton, 1994; ISBN 0-8493 2604-4. [Google Scholar]
- Heilig, A.; Barsan, N.; Weimer, U.; Schweizer-Berberich, M.; Gardner, J.W.; Göpel, W. Gas identification by modulating temperatures of SnO2-based thick-film sensors. Sensors and Actuators B 1997, 43, 45–51. [Google Scholar]
- Heilig, A.; Barsan, N.; Weimar, U.; Göpel, W. Selectivity enhancement of SnO2 gas sensors: Simultaneous monitoring of resistances and temperatures. Sens. Actuators B 1999, 58, 302–309. [Google Scholar]
- Barsan, N.; Stettner, J.R.; Findley, M.; Göpel, W. High performance gas sensing of CO: Comparative tests for (SnO2-based) semiconducting and for electrochemical sensors. Sensors and Actuators B 2000, 66, 31–33. [Google Scholar]
- Shimizu, Y.; Kai, S.; Takao, Y.; Hyodo, T.; Egashira, M. Correlation between methylmercaptan gas-sensing properties and its surface chemistry of SnO2-based sensor materials. Sensors and Actuators B 2000, 65, 349–357. [Google Scholar]
- Becker, T.; Ahlers, S.; Braunmuhl, C.B.; Muller, G.; Kiesewetter, O. Gas sensing properties of thin and thick film tin oxide materials. Sensors and Actuators. B 2001, 77, 55–61. [Google Scholar]
- Starke, T.K.H.; Coles, G.S.V. Laser-ablated nano-crystalline SnO2 material for low-level CO detection. Sensors and Actuators B 2003, 88, 227–233. [Google Scholar]
- Mandayo, G.G.; Castano, E.; Gracia, F.J.; Cirera, A.; Cornet, A.; Morante, J.R. Strategies to enhance the carbon monoxide sensitivity of tin oxide thin film. Sensors and Actuators B 2003, 95, 90–96. [Google Scholar]
- Sako, T.; Ohmi, A.; Yumoto, H.; Nishiyama, K. ITO-film gas sensor for measuring photodecomposition of NO2gas. Surface and Coatings Technology 2001, 142-144, 781–785. [Google Scholar]
- Patel, N.G.; Patel, P.D.; Vaishnav, V.S. Indium tin oxide (ITO) thin film gas sensor for detection of methanol at room temperature. Sensors and Actuators B 2003, 96, 180–189. [Google Scholar]
- Wang, Y.; Wu, X.; Li, Y.; Zhou, Z. Meso-structural SnO2 as sensing material for gas sensors. Solid State Elect. 2004, 48, 627–632. [Google Scholar]
- Seal, S.; Shukla, S. Nanocrystalline SnO2 gas sensor in view of the surface reactions and modifications. JOM 2002, 54, 35–38. [Google Scholar]
- Shukla, S.; Patil, S.; Kuiry, S.C.; Rahman, Z.; Du, T.; Ludwig, L.; Parish, C.; Seal, S. Synthesis and characterization of sol-gel derived nanocrystalline tin oxide thin film as hydrogen sensor. Sensors and Actuators B 2003, 96, 343–353. [Google Scholar]
- Schweizer-Berberich, M.; Strathmann, S.; Weimar, U.; Sharma, R.; Seube, A.; Peyre-Lavigne, A.; Gopel, W. Strategies to avoid VOC cross-sensitivity of SnO2-based CO sensors. Sensors and Actuators B 1999, 58, 318–324. [Google Scholar]
- Shimizu, Y.; Egashira, M. Basic concepts and challenges of semiconductor gas sensors. MRS Bulletin 1999, 24, 18–24. [Google Scholar]
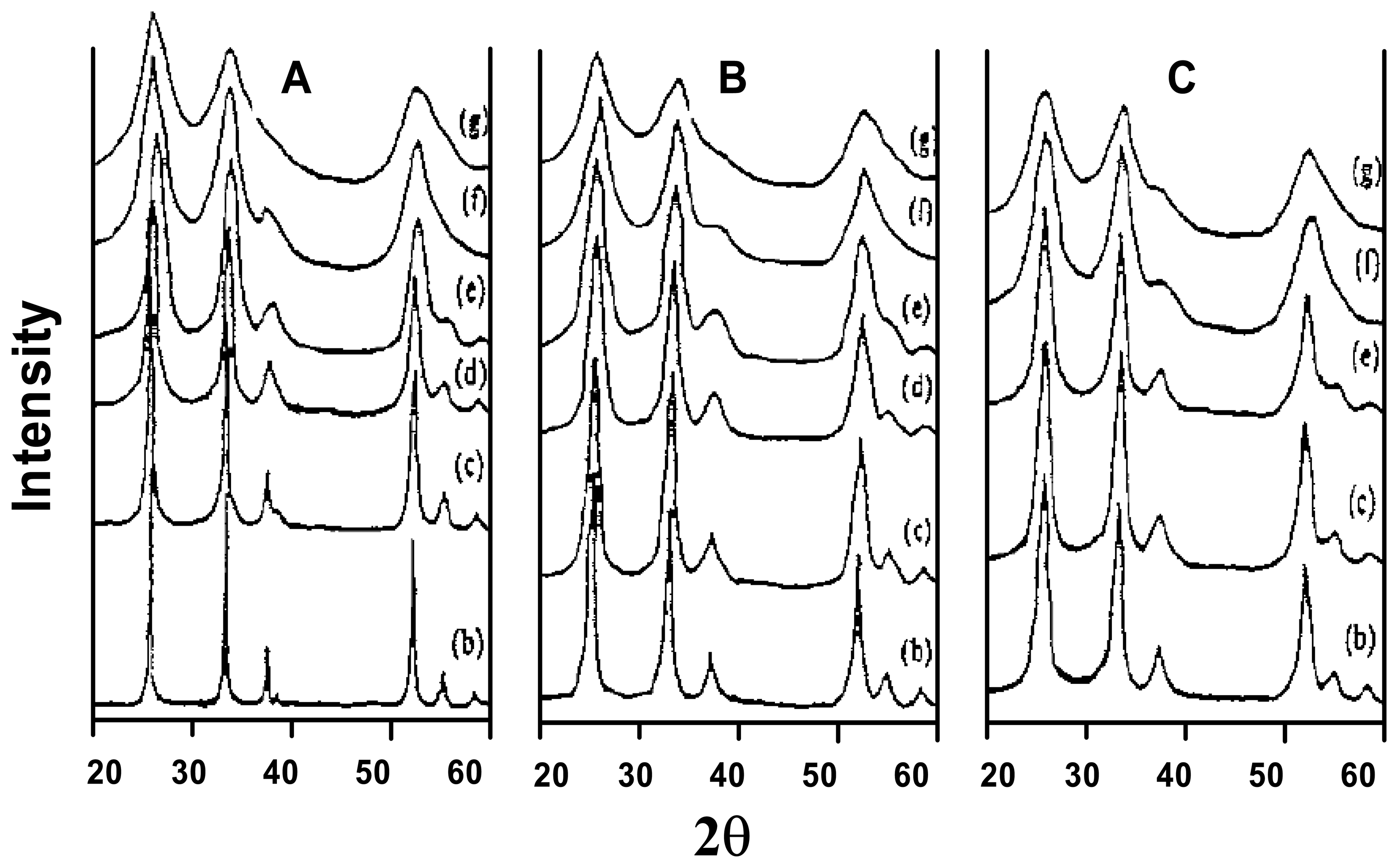
| No. | a | b | c | d | e | f | g |
|---|---|---|---|---|---|---|---|
| T(°C) | 80 | 200 | 300 | 400 | 500 | 600 | 800 |
| SnO2 | 2.9 | 3.1 | 3.8 | 5.3 | 7.8 | 13.2 | 24.5 |
| D(nm) 5% In-SnO2 | 3.1 | 3.5 | 4.5 | 6.4 | 7.9 | 10.5 | |
| D(nm) 10% In-SnO2 | 3.1 | 3.7 | 5.7 | 6.6 | 8.7 | ||
| D(nm) |
© 2006 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Kim, I.J.; Han, S.D.; Han, C.H.; Gwak, J.; Lee, H.D.; Wang, J.S. Micro Semiconductor CO Sensors Based on Indium-Doped Tin Dioxide Nanocrystalline Powders. Sensors 2006, 6, 526-535. https://doi.org/10.3390/s6050526
Kim IJ, Han SD, Han CH, Gwak J, Lee HD, Wang JS. Micro Semiconductor CO Sensors Based on Indium-Doped Tin Dioxide Nanocrystalline Powders. Sensors. 2006; 6(5):526-535. https://doi.org/10.3390/s6050526
Chicago/Turabian StyleKim, Il Jin, Sang Do Han, Chi Hwan Han, Jihye Gwak, Hi Doek Lee, and Jin Suk Wang. 2006. "Micro Semiconductor CO Sensors Based on Indium-Doped Tin Dioxide Nanocrystalline Powders" Sensors 6, no. 5: 526-535. https://doi.org/10.3390/s6050526




