Development of an In-Fiber Nanocavity Towards Detection of Volatile Organic Gases
Abstract
:1. Introduction
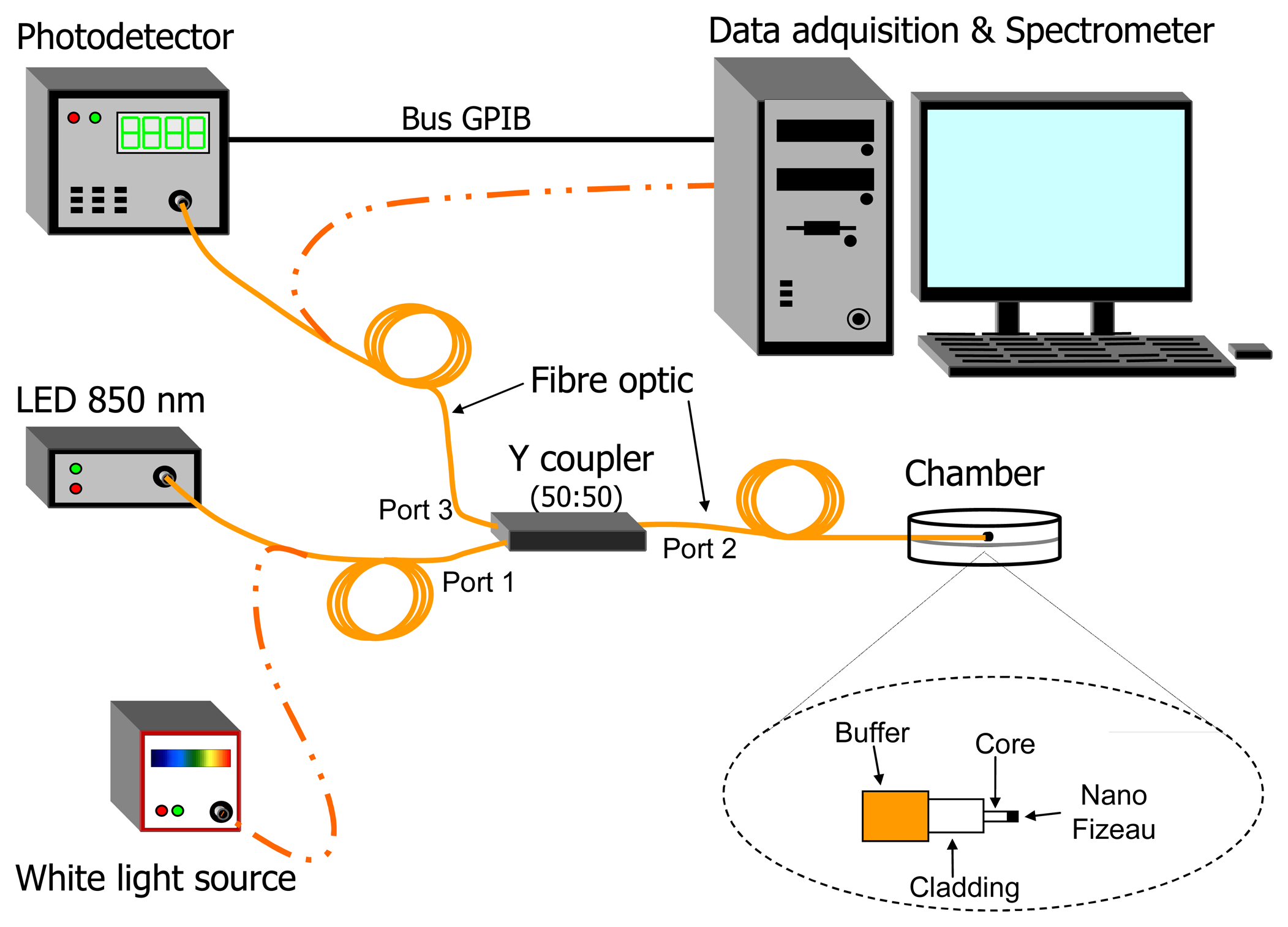
2. Experimental Set-up
3. Nanosensor construction
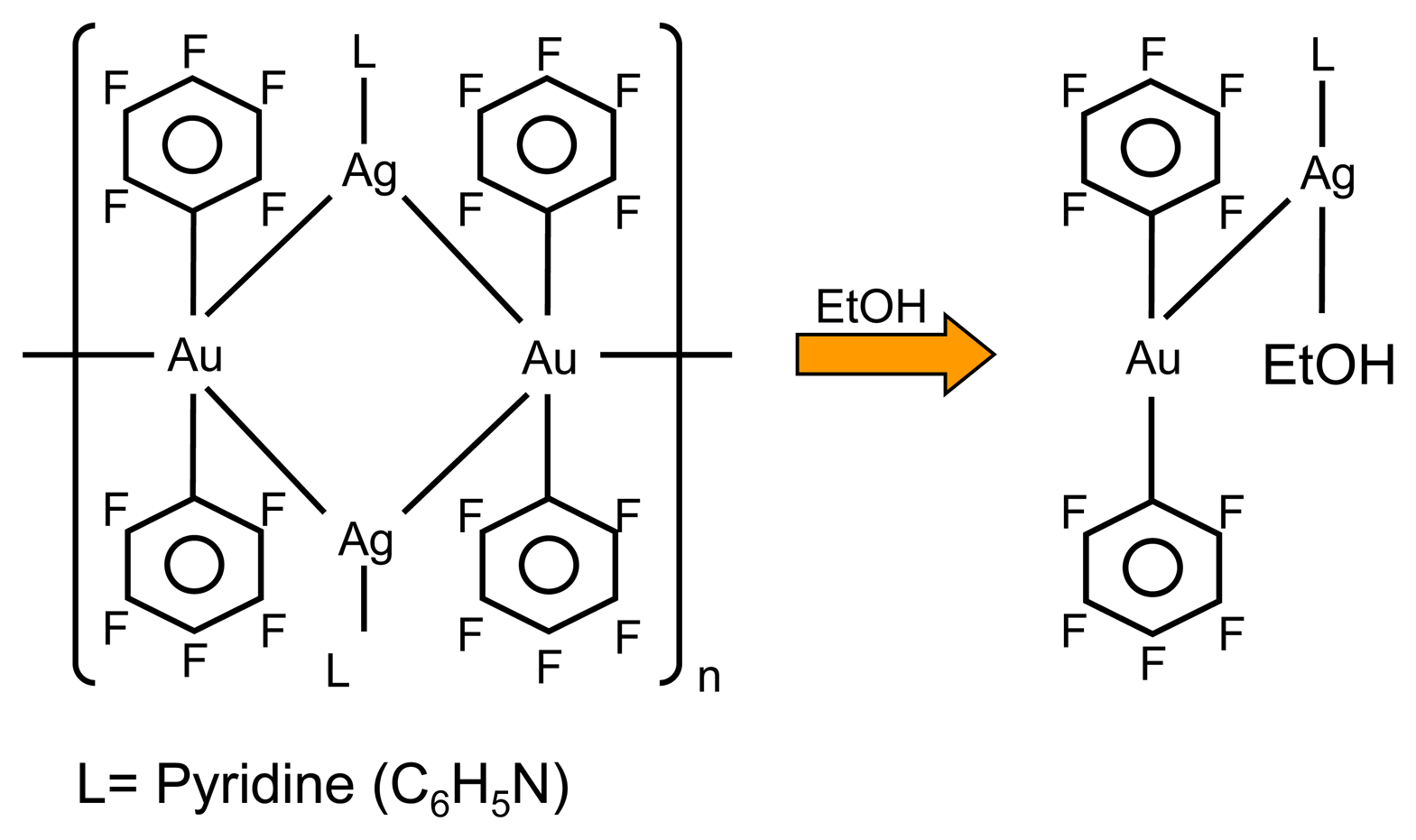
3.1. Vapochromic material
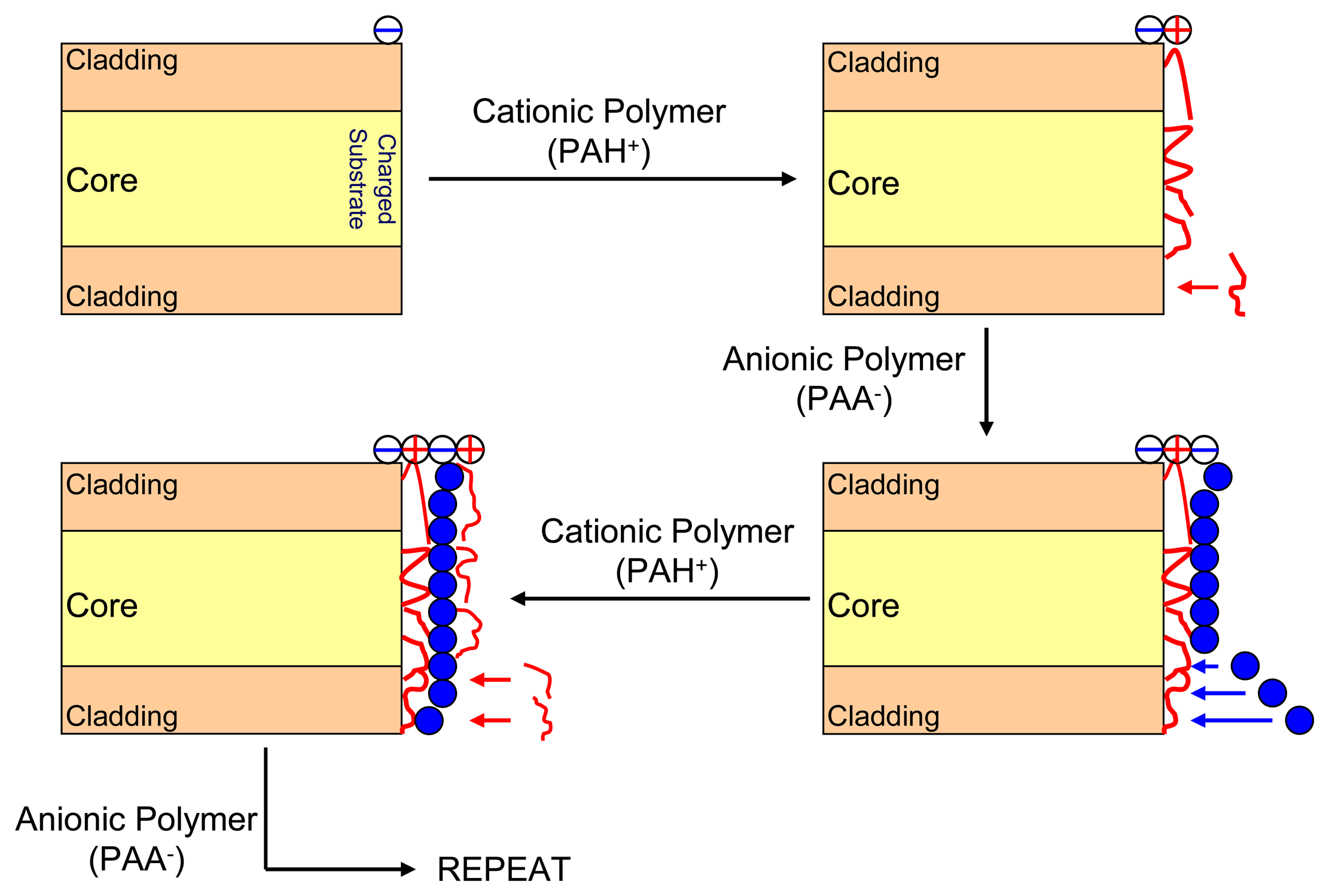
3.2. Electrostatic self-assembly method
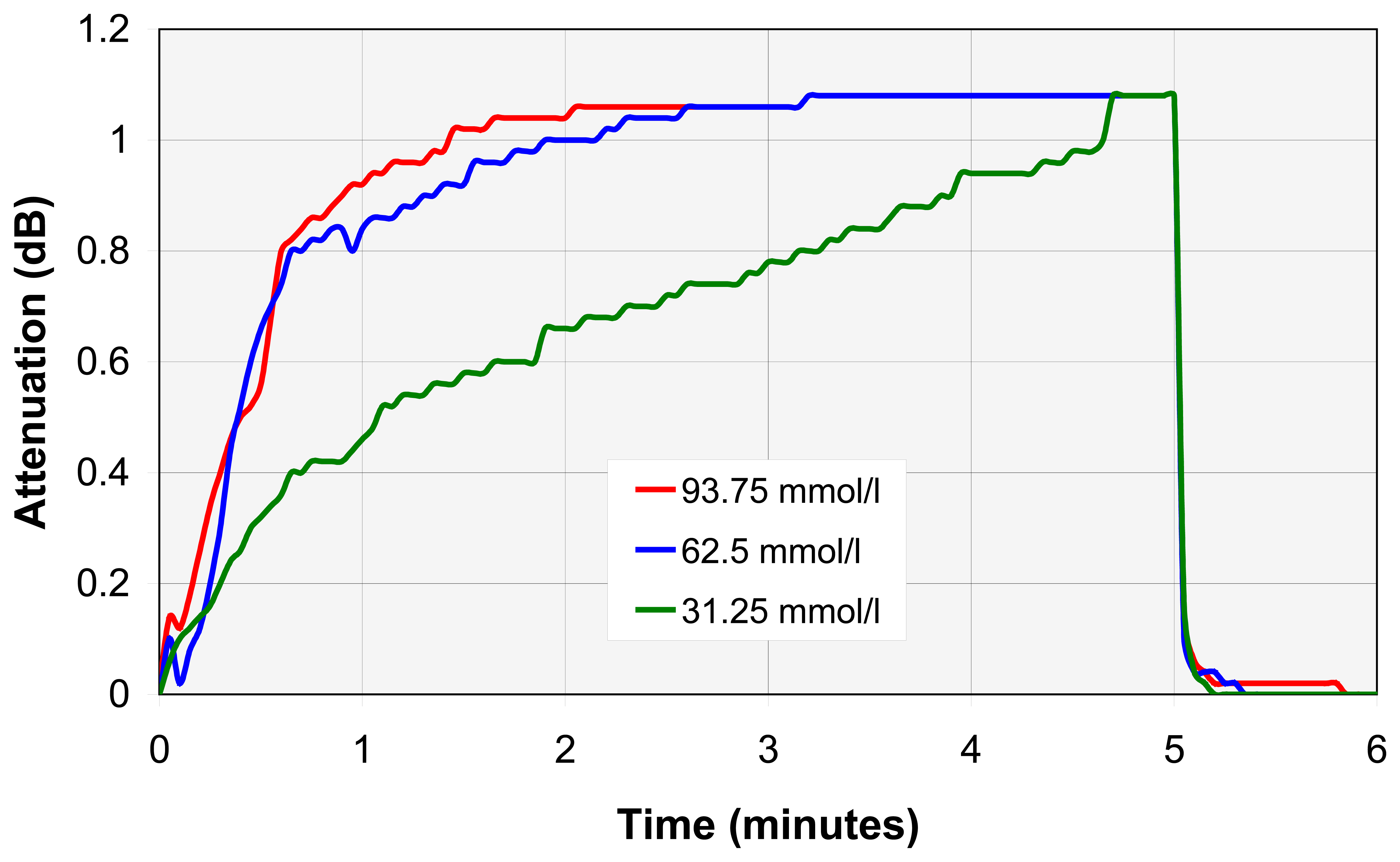
3.3. Nanocavity construction
4. Characteristics of the sensor
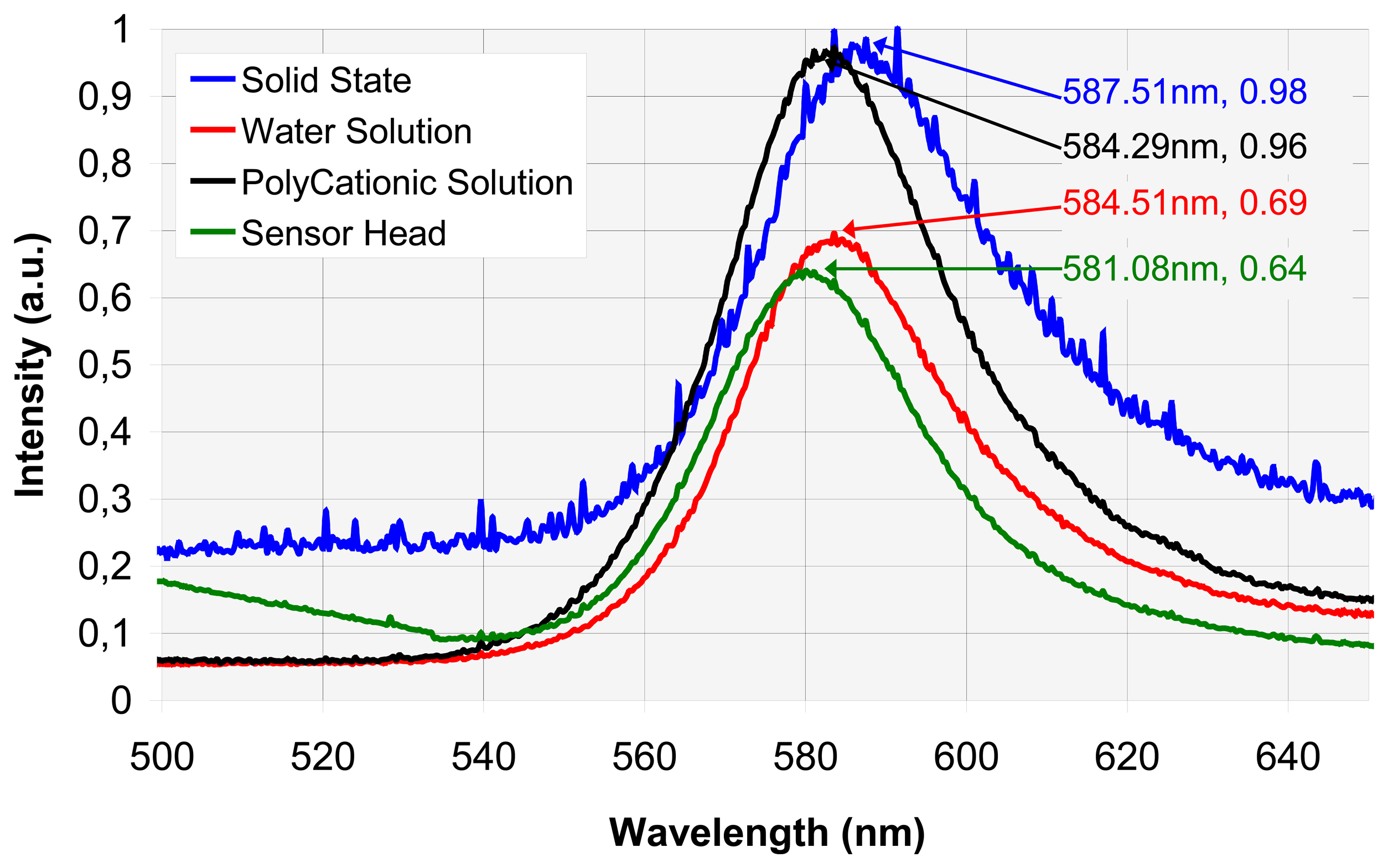
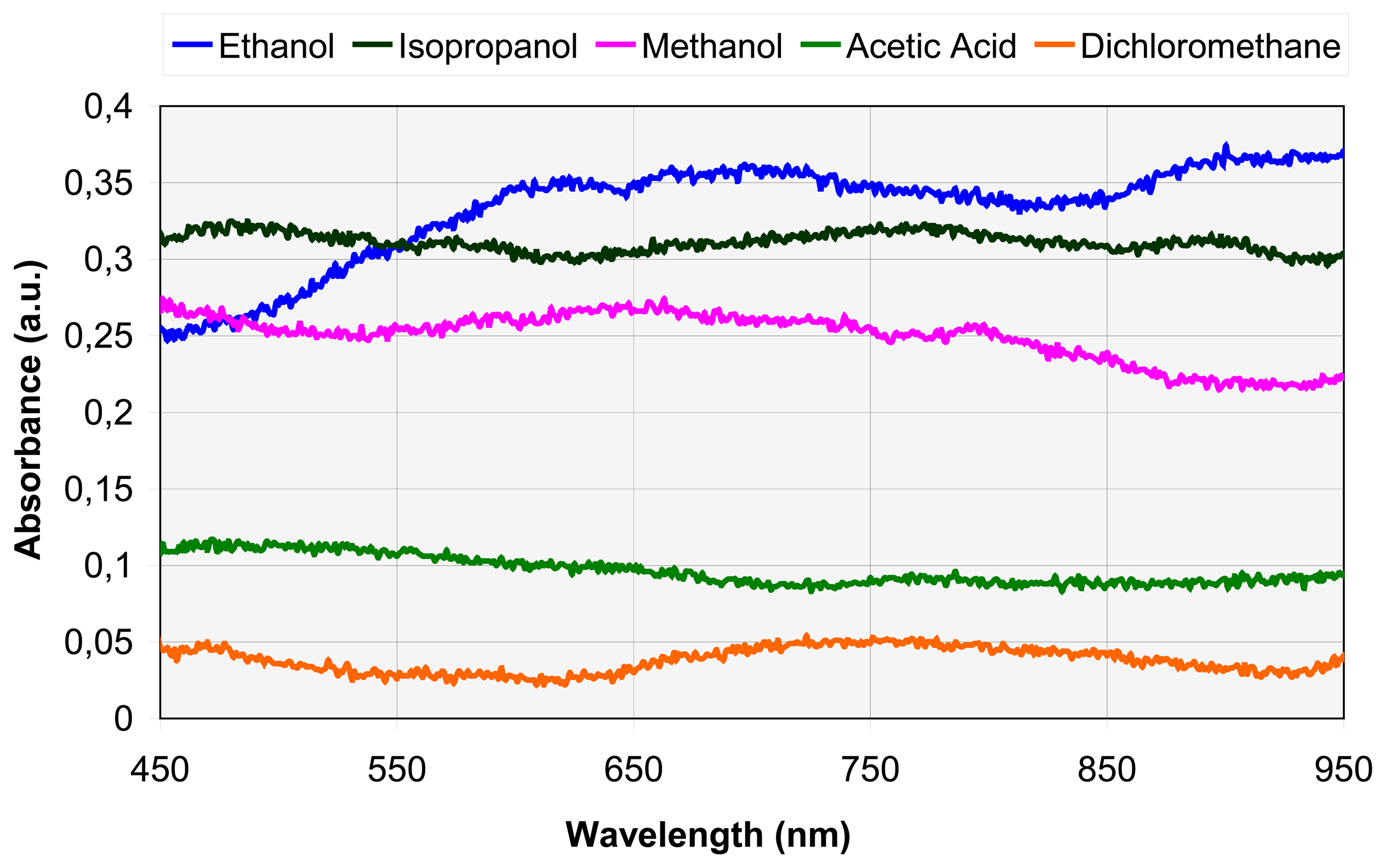
4.1. Spectral analysis
A. Vapochromic complex fluorescence
B. Absorbance spectra
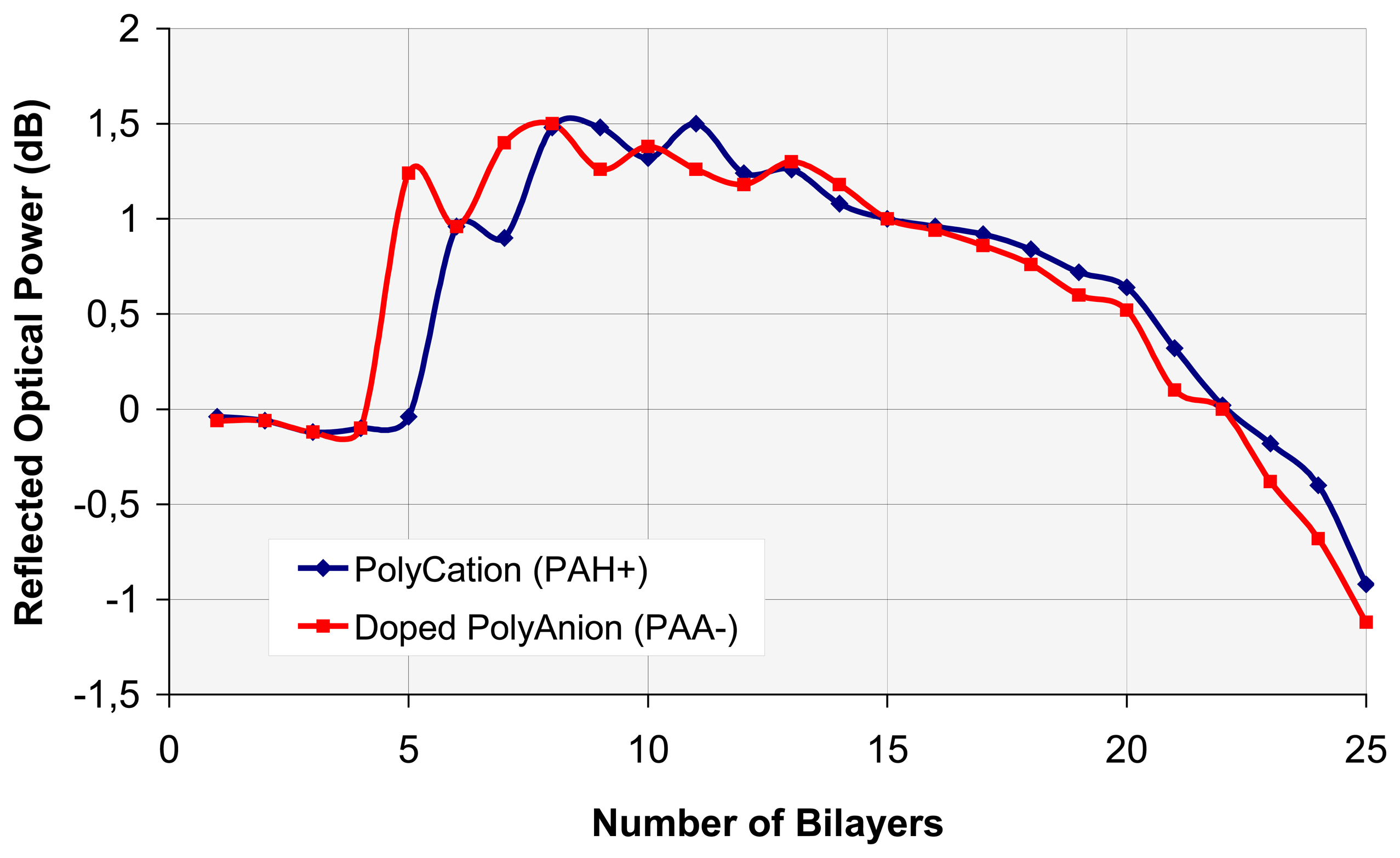
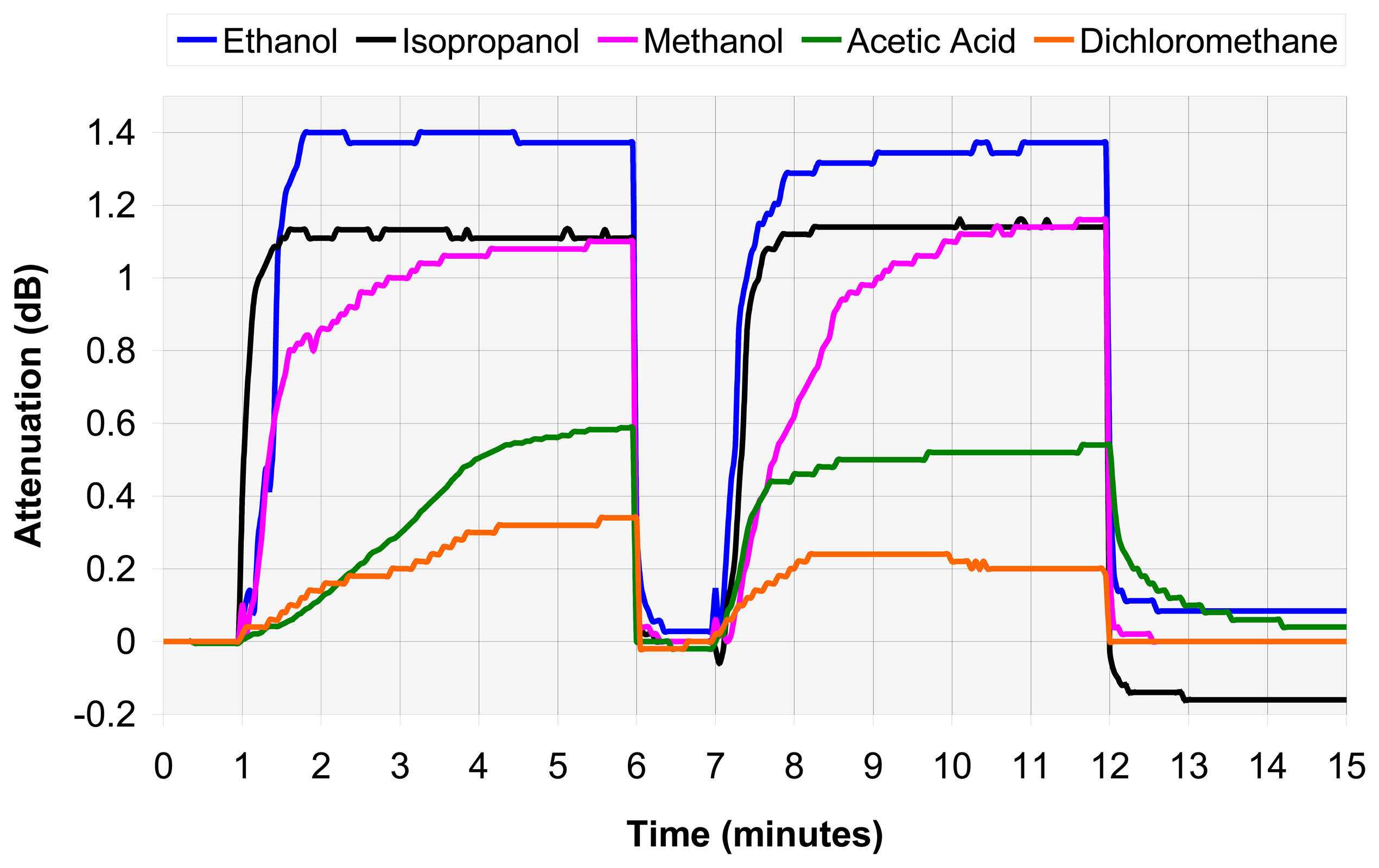
4.2. VOC assembly analysis
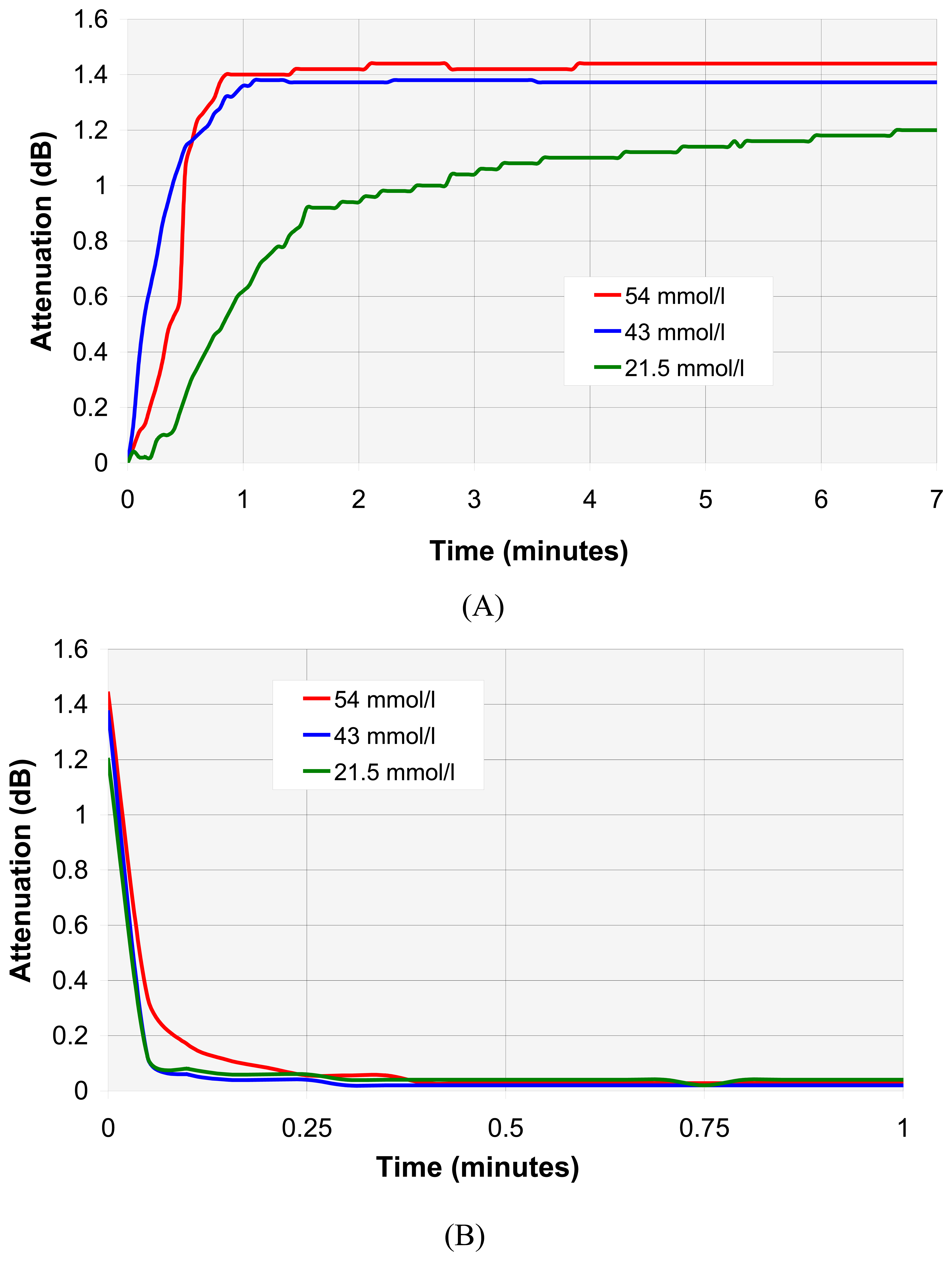
4.3. VOC individual analyse
5. Conclusions
Acknowledgments
References and Notes
- Delpha, C.; Lumbreras, M.; Siadat, M. Discrimination and identification of a refrigerant gas in a humidity controlled atmosphere containing or not carbon dioxide: application to the electronic nose. Sensors and Actuators B 2004, 98, 46–53. [Google Scholar]
- D'Amico, A.; Di Natale, C.; Macagnano, A.; Davide, F.; Mantini, A.; Tarizzo, E.; Paolesse, R.; Boschi, T. Technologies and tools for mimicking olfaction: status of the Rome “Tor Vergata” electronic nose. Biosensors & Bioelectronics 1998, 13, 711–721. [Google Scholar]
- Ampuero, S.; Bosset, J.O. The electronic nose applied to dairy products: a review. Sensors and Actuators B 2003, 94, 1–12. [Google Scholar]
- Gouws, G.J.; Gouws, D.J. Analyte identification using concentration modulation and wavelet analysis of QCM sensors. Sensors and Actuators B 2003, 91, 326–332. [Google Scholar]
- Brezmes, J.; Llobet, E.; Vilanova, X.; Saiz, G.; Correig, X. Fruit ripeness monitoring using an Electronic Nose. Sensors and Actuators B 2000, 69, 223–229. [Google Scholar]
- Lonergan, M.C.; Severin, E.J.; Doleman, B.J.; Beaber, S.A.; Grubbs, R.H.; Lewis, N.S. Array-based vapor sensing using chemically sensitive carbon black-polymer resistors. Chemical Materials 1996, 8, 2298–2312. [Google Scholar]
- Yang, Y.; Yang, P.; Wang, X. Electronic nose based on SAWS array and its odor identification capability. Sensors and Actuators B 2000, 66, 167–170. [Google Scholar]
- Delapierre, G.; Grange, H.; Chambaz, B.; Destannes, L. Polymer-based capacitive humidity sensor: characteristics and experimental results. Sensors and Actuators B 1983, 4, 97–104. [Google Scholar]
- El-Sherif, M. A. On-Fiber sensor and modulator. IEEE Transactions on Instrumentation and Measurement 1989, 38, 595–598. [Google Scholar]
- Achaerandio, E.; Jarabe, S.; Abad, S.; Lopez-Amo, M. New WDM Amplified Network for Optical Sensor Multiplexing. IEEE Photonics Technology Letters 1999, 11, 1644–1946. [Google Scholar]
- Decher, G. Fuzzy Nano Assemblies: toward layered polymeric multicomposites. Science 1997, 277, 1232–1237. [Google Scholar]
- Kolle, C.; Gruber, W.; Trettnak, W.; Biebernik, K.; Dolezal, C.; Reininger, F. Fast optochemical sensor for continuous monitoring of oxygen in breath–gas analysis. Sensors and Actuators B 1997, 38, 141–149. [Google Scholar]
- Grattan, K.T.V.; Sun, T. Fibre optic sensor technology: an overview. Sensors and Actuators A 2000, 82, 40–61. [Google Scholar]
- Usón, R.; Laguna, A.; Laguna, M.; Manzano, B. R. Syntesis and Reactivity of Bimetallic Polyfluorophenyl Complexes; Crystal and Molecular Structures of [{AuAg(C6F5)2(SC4H8)}n] and [{AuAg(C5F5)2(C6H6)}n]. Chemical Society Dalton Trans 1984, 285–292. [Google Scholar]
- Nagel, C.C. U.S. Patent 4,826,774.Nagel, C.C. U.S. Patent 48,349,093.
- Buss, C.E.; Anderson, C.E.; Pomije, M.K.; Luzt, C.M.; Britton, D.; Mann, K.R. Structural investigations of vapochromic behaviour – X-ray single-crystal and powder diffraction studies of [Pt(CN-iso-C3H7)4][M(CN)4] for M= Pt or Pd. Journal of the American Chemical Society 1998, 120, 7783–7790. [Google Scholar]
- Bariain, C.; Matias, I. R.; Fernandez-Valdivielso, C.; Elosua, C.; Luquin, A.; Garrido, J.; Laguna, M. Optical fibre sensors based on vapochromic gold complexes for environmental applications. Sensors and Actuators B 2005, 108, 535–54. [Google Scholar]
- Luquin, A.; Bariain, C.; Vergara, E.; Cerrada, E.; Garrido, J.; Matias, I. R.; Laguna, M. New preparation of gold–silver complexes and optical fibre environmental sensors based on vapochromic[Au2Ag2(C6F5)4(phen)2]n. Applied Organometallic Chemistry 2005, 19, 1232–1238. [Google Scholar]
- Grant, S. A.; Satcher, J.H., Jr.; Bettencourt, K. Development of sol–gel-based fiber optic nitrogen dioxide gas sensors. Sensors and Actuators B 2000, 69, 132–137. [Google Scholar]
- Lobnik, A.; Majcen, N.; Niederreiter, K.; Uray, G. Optical pH sensor based on the absorption of antenna generated europium luminescence by bromothymolblue in a sol - gel membrane. Sensors and Actuators B 2001, 74, 200–206. [Google Scholar]
- Arregui, F.J.; Otano, M.; Fernández-Valdivielso, C.; Matias, I.R. An experimental study about the utilization of Liquicoat® solutions for the fabrication of pH optical fibre sensors. Sensors and Actuators B 2002, 87(2), 291–297. [Google Scholar]
- Bariain, C.; Matias, I.R.; Fernandez-Valdivielso, C.; Arregui, F.J.; Rodriguez-Mendez, M.L.; de Saja, J.A. Optical fiber sensor based on lutetium bisphthalocyanine for the detection of gases using standard telecommunication wavelengths. Sensors and Actuators B 2003, 93, 153–158. [Google Scholar]
- Arregui, F.J.; Latasa, I.; Matias, I.R.; Claus, R.O. An optical fiber pH sensor based on the electrostatic self-assembly method. Proceedings of the Second IEEE Sensors Conference; 2003. [Google Scholar]
- Lenahan, K.M.; Wang, Y.-X.; Liu, Y.; Claus, R.O.; Heflin, J.R.; Marciu, D.; Figura, C. Novel polymer dyes for nonlinear optical applications using ionic self assembled monolayer technology. Advanced Materials 1998, 853–855. [Google Scholar]
- Zhanga, J.; Sengera, B.; Vautiera, D.; Picarta, C.; Schaafc, P.; Voegela, J.C.; Lavallea, P. Natural polyelectrolyte films based on layer-by layer deposition of collagen and hyaluronic acid. Biomaterials 2005, 26, 3353–3361. [Google Scholar]
- Choi, J.; Rubner, M.F. Influence of the degree of ionization on weak polyelectrolyte multilayer assembly. Macromolecules 2005, 38, 124–166. [Google Scholar]
- Arregui, F. J.; Cooper, K. L.; Liu, Y. J. Optical fiber humidity sensor with a fast response time using the ionic self-assembly method. IEICE Transactions on electronics 2000, E83C(3), 360–365. [Google Scholar]
- Arregui, F. J.; Matías, I.R.; Claus, R. O. Optical fiber gas sensors based on hydrophobic alumina thin films formed by the Electrostatic Self-Assembly Monolayer process. IEEE Sensors Journal 2003, 3, 56–61. [Google Scholar]
- Elosua, C.; Bariain, C.; Matias, I. R.; Arregui, F. J.; Luquin, A.; Laguna, M. Volatile alcoholic compunds fibre optic nano sensor. Sensors and Actuators B 2005, 115, 444–449. [Google Scholar]
- Yoshino, T.; Kurosawa, K.; Itoh, K.; Ose, T. Fiber-Optic Fabry-Perot Interferometer and Its Sensor Applications. IEEE Journal of quantum electronics 1982, 18(10), 1624–1633. [Google Scholar]
- Arregui, F. J.; Matias, I. R.; Liu, Y.; Lenahan, K. M.; Claus, R. O. Optical fiber nanometer-scale Fabry–Perot interferometer formed by the ionic self-assembly monolayer process. Optics Letters 1999, 24(9), 596–598. [Google Scholar]
- Arregui, F. J.; Richard, O. C.; Cooper, K. L.; Fernández-Valdivielso, C.; Matias, I. R. Optical Fiber Gas Sensor Based on Self-Assembled Microgratings. IEEE Journal of Lightwave Technology 2001, 19, 1932–1937. [Google Scholar]
- Casado, S.; Elosúa, C.; Bariain, C.; Segura, A.; Matias, I.R.; Fernandez, A.; Luquin, A.; Garrido, J.; Laguna, M. A volatile-organic-compound optic-fibre sensor using a gold-silver vapochromic complex. Optical Engineering 2006, 45(4), 044401. [Google Scholar]

© 2006 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Elosua, C.; Matias, I.R.; Bariain, C.; Arregui, F.J. Development of an In-Fiber Nanocavity Towards Detection of Volatile Organic Gases. Sensors 2006, 6, 578-592. https://doi.org/10.3390/s6060578
Elosua C, Matias IR, Bariain C, Arregui FJ. Development of an In-Fiber Nanocavity Towards Detection of Volatile Organic Gases. Sensors. 2006; 6(6):578-592. https://doi.org/10.3390/s6060578
Chicago/Turabian StyleElosua, Cesar, Ignacio R. Matias, Candido Bariain, and Francisco J. Arregui. 2006. "Development of an In-Fiber Nanocavity Towards Detection of Volatile Organic Gases" Sensors 6, no. 6: 578-592. https://doi.org/10.3390/s6060578




