Fiber-Optic Biosensor Employing Alexa-Fluor Conjugated Antibody for Detection of Escherichia coli O157:H7 from Ground Beef in Four Hours
Abstract
:Introduction
Results and Discussion
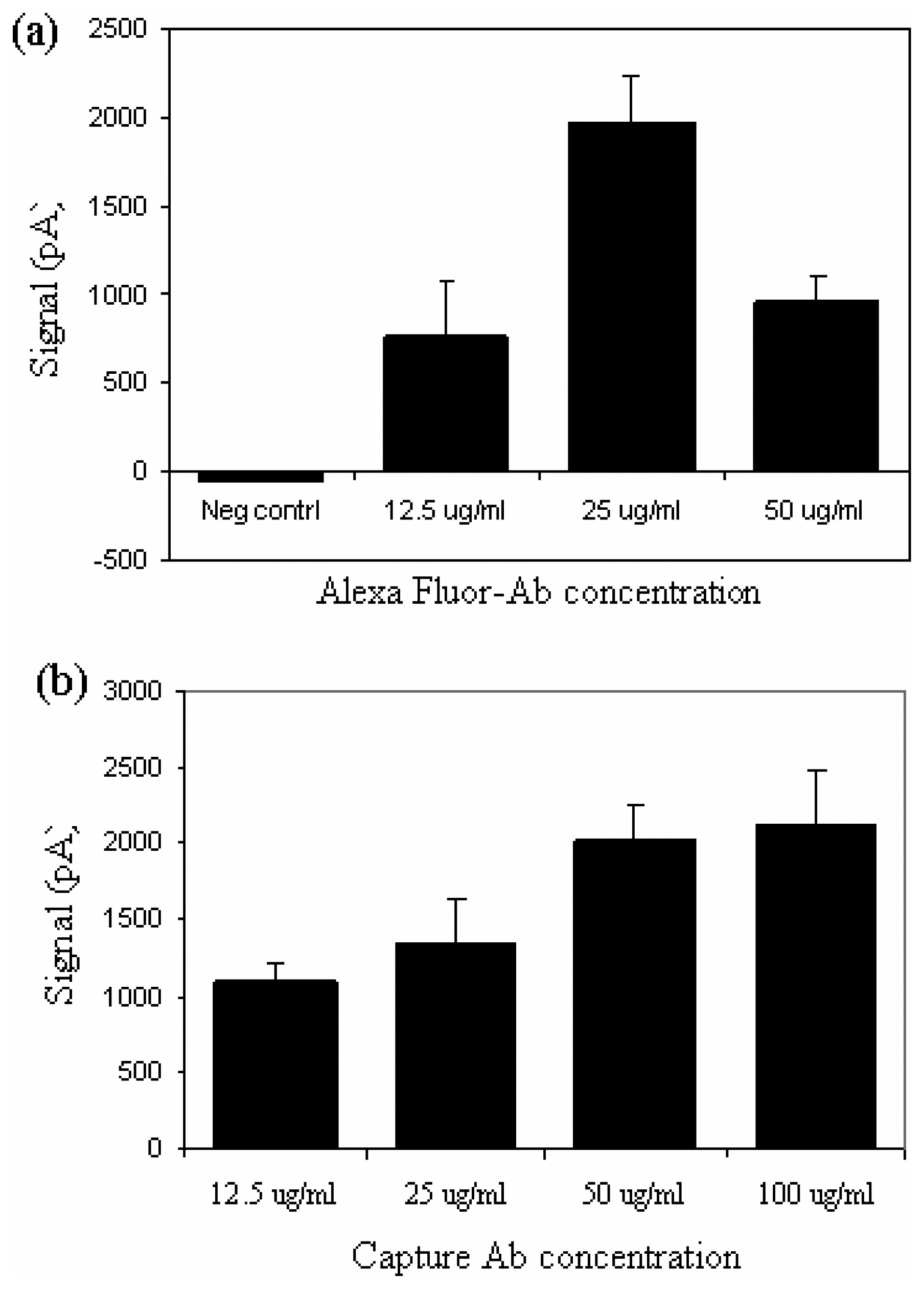
Determination of optimal concentrations of capture and detection antibodies
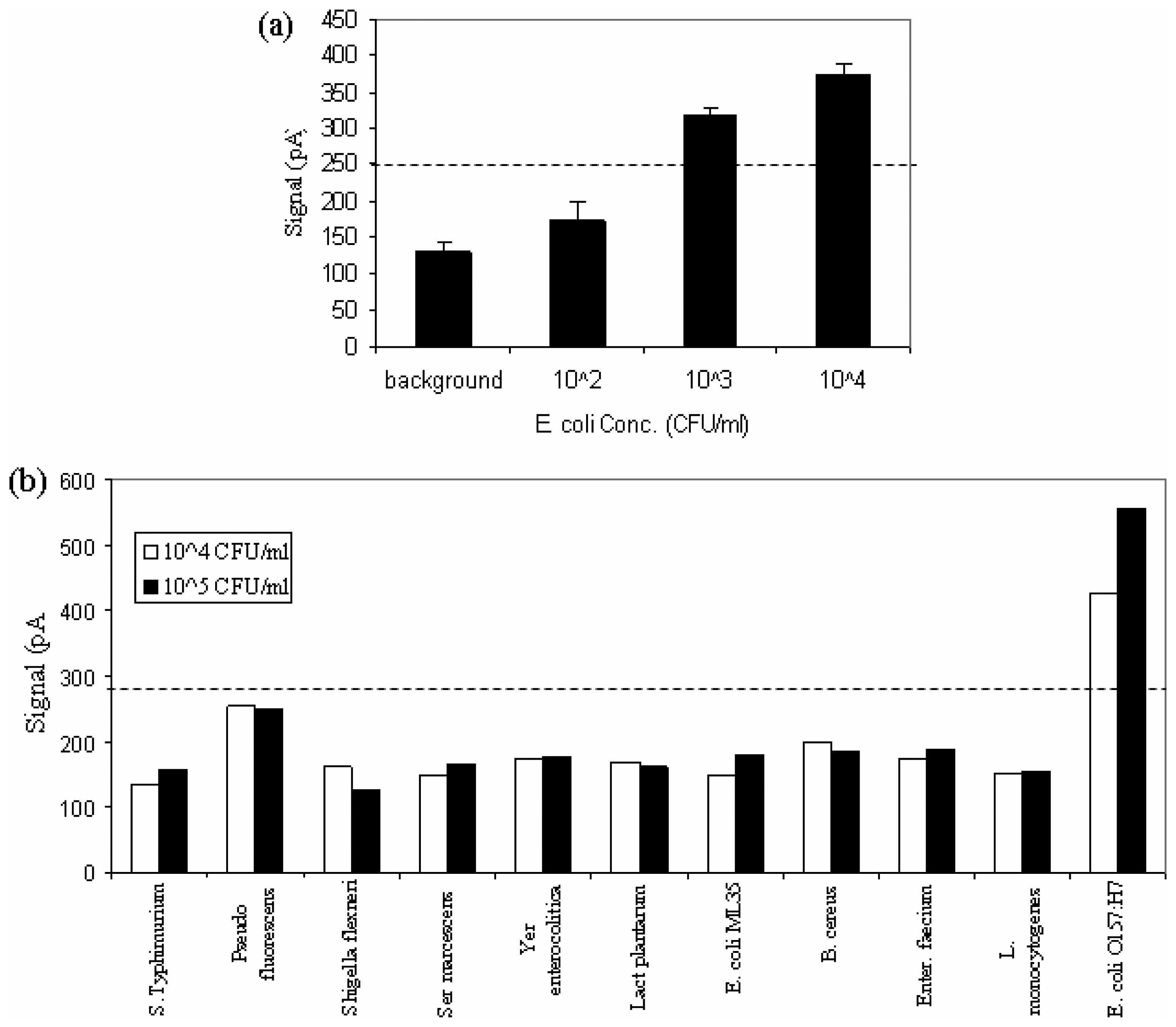
Detection limit and specificity of the biosensor for E. coli O157:H7
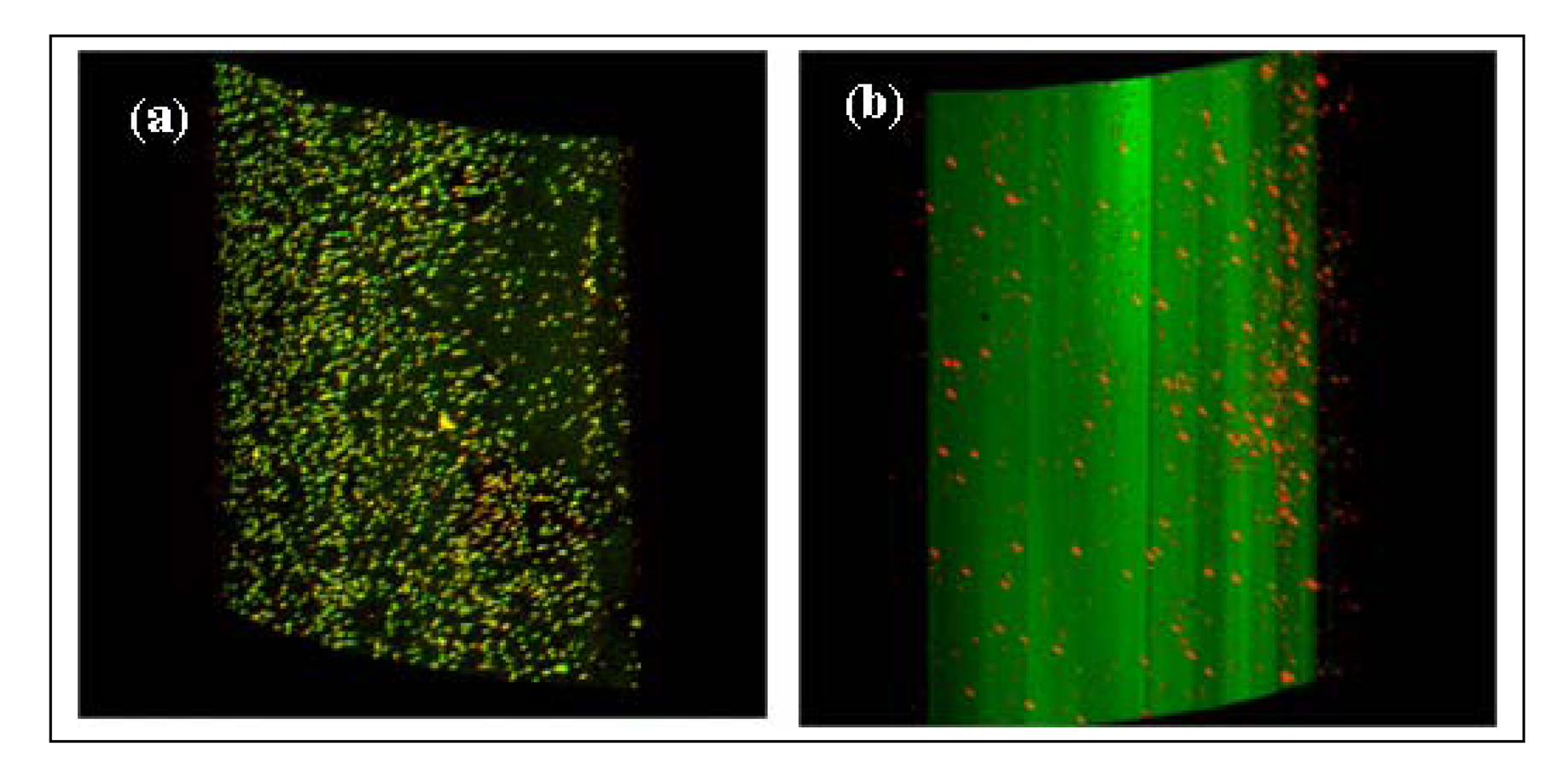
Confocal laser scanning microscopic patterns of fibers
Detection of E. coli O157:H7 in spiked meat
Experimental Section
Bacteria and media
Antibodies and labeling
Immobilization of antibody on fibers
Blocking and background reading
Fiber-optic assays
Determination of optimal concentrations of capture and detection antibodies
Limit of detection and specificity of the biosensor
Detection of E. coli grown in different broths and meat
Confocal laser scanning microscopy
Acknowledgments
References
- Mead, P. S.; Slutsker, L.; Dietz, V.; McCaig, L. F.; Bresee, J. S.; Shapiro, C.; Griffin, P. M.; Tauxe, R. V. Food-related illness and death in the United States. Emerg. Infect. Dis. 1999, 5, 607–625. [Google Scholar]
- Smith, J. L.; Fratamico, P. Foodborne Pathogens: Microbiology and Molecular Biology; Fratamico, P., Bhunia, A. K., Smith, J. L., Eds.; Caister Academic Press: Norfolk, UK, 2005; pp. p 357–382. [Google Scholar]
- Deisingh, A. K.; Thompson, M. Biosensors for the detection of bacteria. Can. J. Microbiol. 2004, 50, 69–77. [Google Scholar]
- Leonard, P.; Hearty, S.; Brennan, J.; Dunne, L.; Quinn, J.; Chakraborty, T.; O'Kennedy, R. Advances in biosensors for detection of pathogens in food and water. Enz. Microb. Technol. 2003, 32, 3–13. [Google Scholar]
- Taitt, C. R.; Anderson, G. P.; Ligler, F. S. Evanescent wave fluorescence biosensors. Biosens. Bioelectron. 2005, 20, 2470–2487. [Google Scholar]
- Squillante, E. Applications of fiber-optic evanescent wave spectroscopy. Drug Develop. Indust. Pharmacy 1998, 24, 1163–1175. [Google Scholar]
- Anderson, G. P.; Golden, J. P.; Ligler, F. S. A fiber optic biosensor - combination tapered fibers designed for improved signal acquisition. Biosens. Bioelectron. 1993, 8, 249–256. [Google Scholar]
- Lave, W. F.; Button, L. J.; Slovacek, R. E. Biosensors with Fiberoptics; Wise, D. L., Wingard, L. B., Eds.; Humana Press: Clifton, NJ, 1991; pp. p 139–180. [Google Scholar]
- Glass, T. R.; Lackie, S.; Hirschfeld, T. Effect of numerical aperture on signal level in cylindrical wave-guide evanescent fluorosensors. Appl. Optics 1987, 26, 2181–2187. [Google Scholar]
- Hirschfeld, T. Instantaneous source wavelength identification with a minimal number of detectors. Appl. Optics 1985, 24, 2484–2485. [Google Scholar]
- Bakaltcheva, I. B.; Ligler, F. S.; Patterson, C. H.; Shriver-Lake, L. C. Multi-analyte explosive detection using a fiber optic biosensor. Anal. Chim. Acta 1999, 399, 13–20. [Google Scholar]
- Tempelman, L. A.; King, K. D.; Anderson, G. P.; Ligler, F. S. Quantitating staphylococcal enterotoxin B in diverse media using a portable fiber-optic biosensor. Anal. Biochem. 1996, 233, 50–57. [Google Scholar]
- Ogert, R. A.; Brown, J. E.; Singh, B. R.; Shriver-Lake, L. C.; Ligler, F. S. Detection of Clostridium botulinum toxin A using a fiber optic-based biosensor. Anal. Biochem. 1992, 205, 306–312. [Google Scholar]
- Zhou, C. H.; Pivarnik, P.; Auger, S.; Rand, A.; Letcher, S. A compact fiber-optic immunosensor for Salmonella based on evanescent wave excitation. Sens. Actuators B-Chem. 1997, 42, 169–175. [Google Scholar]
- Kramer, M. F.; Lim, D. V. A rapid and automated fiber optic-based biosensor assay for the detection of Salmonella in spent irrigation water used in the sprouting of sprout seeds. J. Food Prot. 2004, 67, 46–52. [Google Scholar]
- Geng, T.; Morgan, M. T.; Bhunia, A. K. Detection of low levels of Listeria monocytogenes cells by using a fiber-optic immunosensor. Appl. Environ. Microbiol. 2004, 70, 6138–6146. [Google Scholar]
- DeMarco, D. R.; Saaski, E. W.; McCrae, D. A.; Lim, D. V. Rapid detection of Escherichia coli O157:H7 in ground beef using a fiber-optic biosensor. J. Food Prot. 1999, 62, 711–716. [Google Scholar]
- Anderson, G. P.; Nerurkar, N. L. Improved fluoroimmunoassays using the dye Alexa Fluor 647 with the RAPTOR, a fiber optic biosensor. J. Immunol. Methods 2002, 271, 17–24. [Google Scholar]
- DeMarco, D. R.; Lim, D. V. Direct detection of Escherichia coli O157: H7 in unpasteurized apple juice with an evanescent wave biosensor. J. Rapid Methods Automat. Microbiol. 2001, 9, 241–257. [Google Scholar]
- DeMarco, D. R.; Lim, D. V. Detection of Escherichia coli O157: H7 in 10-and 25-gram ground beef samples with an evanescent-wave biosensor with silica and polystyrene waveguides. J. Food Prot. 2002, 65, 596–602. [Google Scholar]
- Liu, Y. C.; Ye, J. M.; Li, Y. B. Rapid detection of Escherichia coli O157: H7 inoculated in ground beef, chicken carcass, and lettuce samples with an immunomagnetic chemiluminescence fiber-optic biosensor. J. Food Prot. 2003, 66, 512–517. [Google Scholar]
- Tims, T. B.; Lim, D. V. Rapid detection of Bacillus anthracis spores directly from powders with an evanescent wave fiber-optic biosensor. J. Microbiol. Methods 2004, 59, 127–130. [Google Scholar]
- Zourob, M.; Mohr, S.; Brown, B. J. T.; Fielden, P. R.; McDonnell, M. B.; Goddard, N. J. An integrated metal clad leaky waveguide sensor for detection of bacteria. Anal. Chem. 2005, 77, 232–242. [Google Scholar]
- Geng, T.; Kim, K. P.; Gomez, R.; Sherman, D. M.; Bashir, R.; Ladisch, M. R.; Bhunia, A. K. Expression of cellular antigens of Listeria monocytogenes that react with monoclonal antibodies C11E9 and EM-7G1 under acid-, salt- or temperature-induced stress environments. J. Appl. Microbiol. 2003, 95, 762–772. [Google Scholar]
- Hahm, B. K.; Bhunia, A. K. Effect of environmental stresses on antibody-based detection of Escherichia coli O157:H7, Salmonella enterica serovar Enteritidis and Listeria monocytogenes. J. Appl. Microbiol. 2006, 100, 1017–1027. [Google Scholar]
- Tu, S.; Golden, M.; Andreotti, P.; Yu, L.; Irwin, P. Applications of time-resolved fluoroimmunoassay to detect magnetic bead captured Escherichia coli O157:H7. J. Rapid Methods Automat. Microbiol. 2001, 9, 33–51. [Google Scholar]
- Tu, S.; Golden, M.; Andreotti, P.; Irwin, P. The use of time-resolved fluoroimmunoassay to simultaneously detect Escherichia coli O157:H7, Salmonella Typhimurium and Salmonella Enteritidis in foods. J. Rapid Methods Automat. Microbiol. 2002, 10, 37–48. [Google Scholar]

© 2006 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Geng, T.; Uknalis, J.; Tu, S.-I.; Bhunia, A.K. Fiber-Optic Biosensor Employing Alexa-Fluor Conjugated Antibody for Detection of Escherichia coli O157:H7 from Ground Beef in Four Hours. Sensors 2006, 6, 796-807. https://doi.org/10.3390/s6080796
Geng T, Uknalis J, Tu S-I, Bhunia AK. Fiber-Optic Biosensor Employing Alexa-Fluor Conjugated Antibody for Detection of Escherichia coli O157:H7 from Ground Beef in Four Hours. Sensors. 2006; 6(8):796-807. https://doi.org/10.3390/s6080796
Chicago/Turabian StyleGeng, Tao, Joe Uknalis, Su-I Tu, and Arun K. Bhunia. 2006. "Fiber-Optic Biosensor Employing Alexa-Fluor Conjugated Antibody for Detection of Escherichia coli O157:H7 from Ground Beef in Four Hours" Sensors 6, no. 8: 796-807. https://doi.org/10.3390/s6080796



