Utilizing of Square Wave Voltammetry to Detect Flavonoids in the Presence of Human Urine
Abstract
:1. Introduction
2. Experimental Section
2.1 Chemicals
2.2 Electrochemical measurements
2.3 Preparation of carbon paste electrode
2.4 Preparation of human urine
2.5 Animal treatment and preparation of microsomes
2.6 Preparation of anti-rat CYP1A1
2.7 Western blot analysis of hepatic and colon microsomes
2.8 Statistical analysis
3. Results
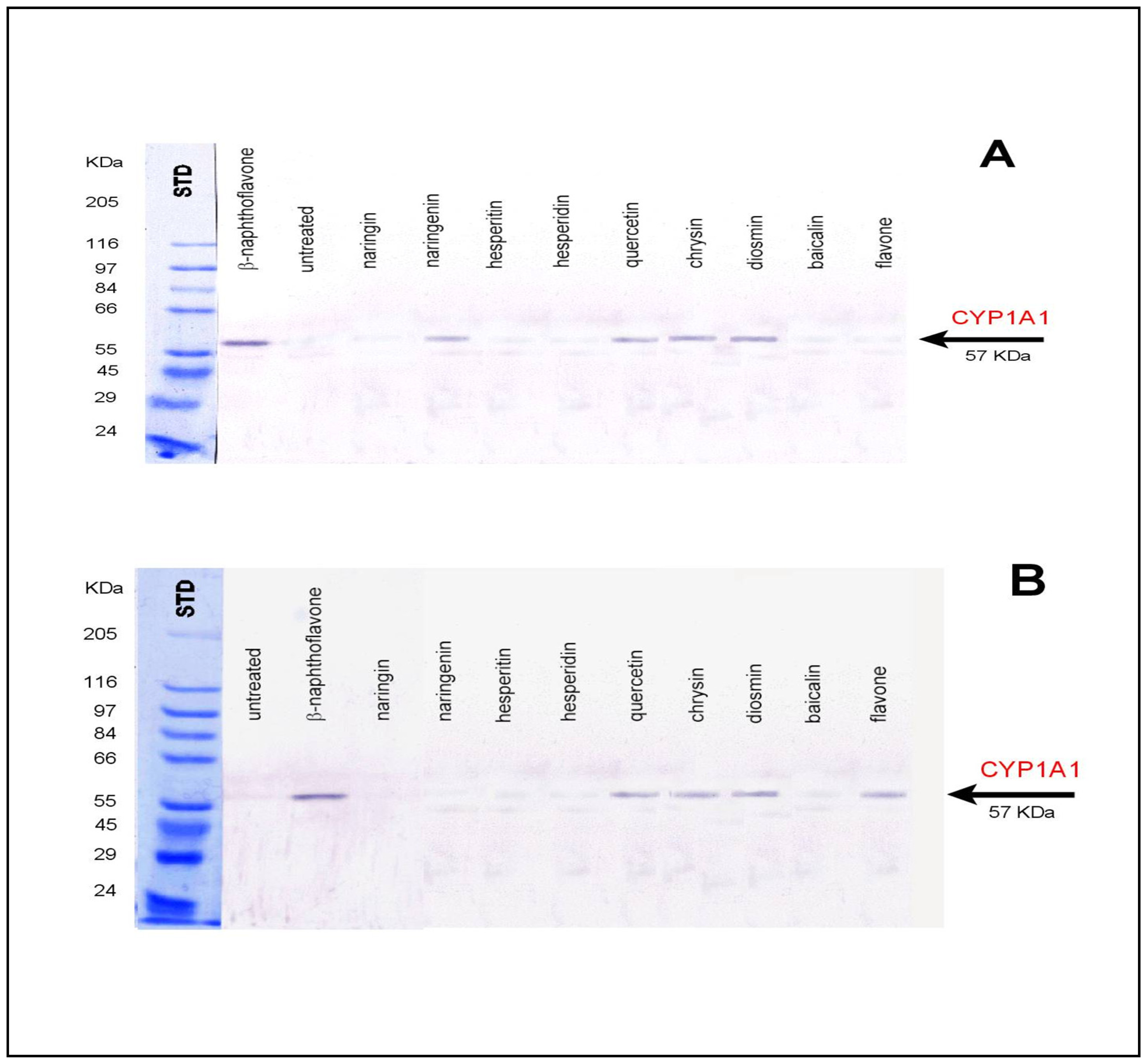
3.1 Biological affecting of certain flavonoids on rats
3.2 Electroanalytical analysis of flavonoids
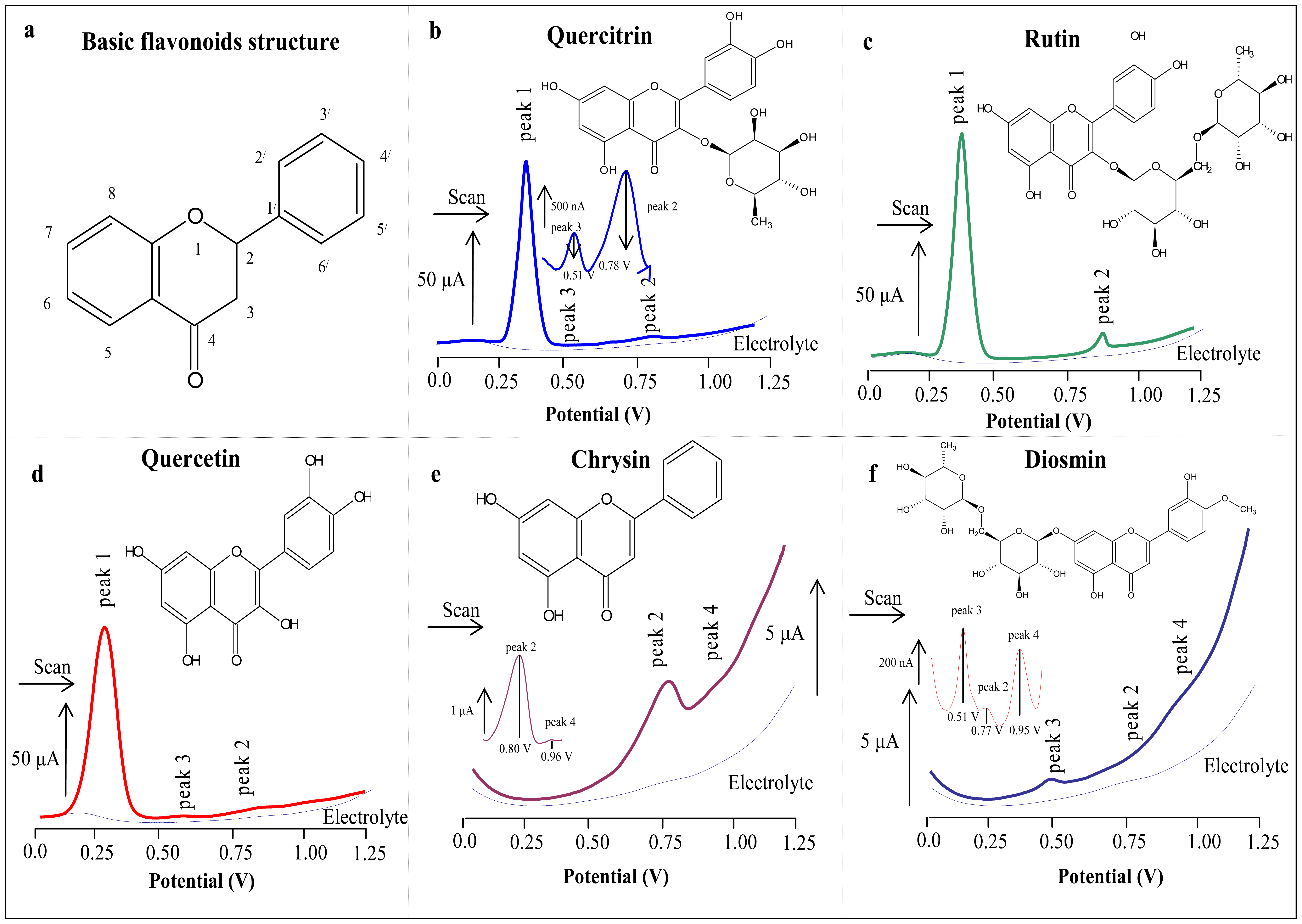
3.2.1 Basic electrochemical behaviour of flavonoids measured on the surface of carbon paste electrode
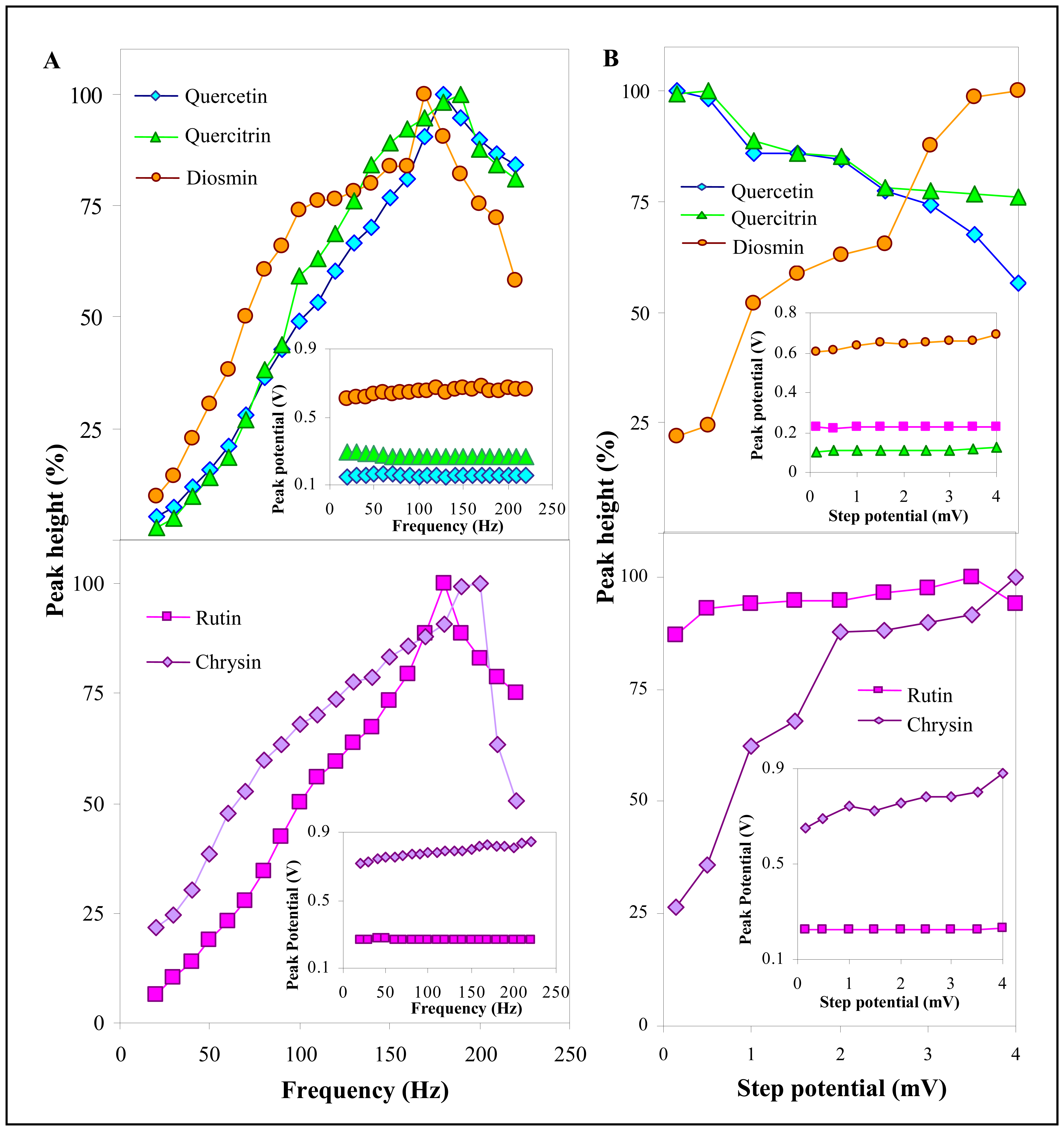
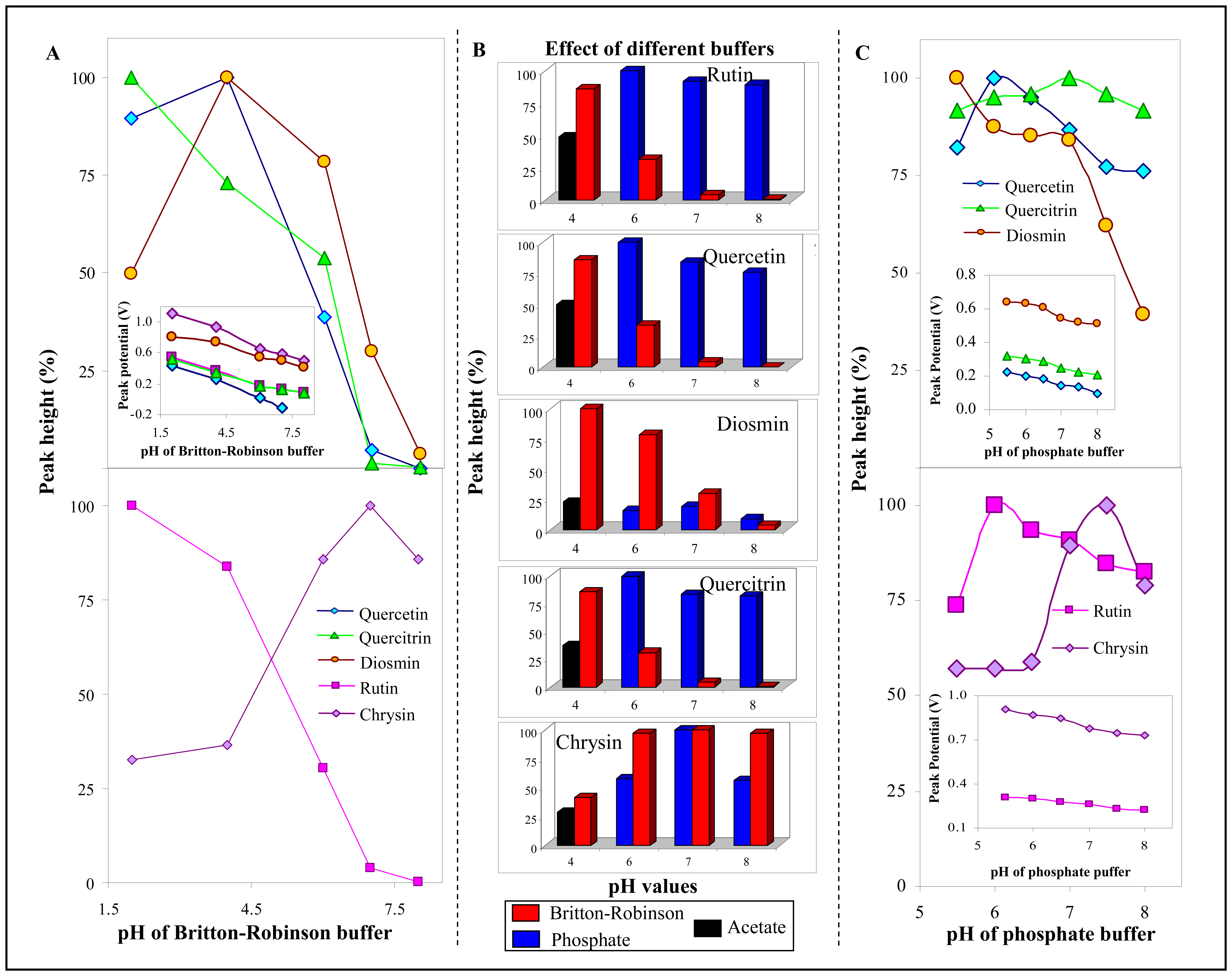
3.2.2 Influence of frequency, step potential and supporting electrolyte on SWV determination of flavonoids
3.2.3 SWV study of influence of different flavonoids' concentrations on their electrochemical behaviour
3.3 An analysis of the flavonoids in the presence of urine
4. Conclusion
References
Acknowledgments
- Taylor, L.P.; Grotewold, E. Flavonoids as developmental regulators. Curr. Opin. Plant Biol. 2005, 8, 317–323. [Google Scholar]
- Woo, H.H.; Jeong, B.R.; Hawes, M.C. Flavonoids: from cell cycle regulation to biotechnology. Biotechnol. Lett. 2005, 27, 365–374. [Google Scholar]
- Williams, C.A.; Grayer, R.J. Anthocyanins and other flavonoids. Nat. Prod. Rep. 2004, 21, 539–573. [Google Scholar]
- Di Carlo, G.; Mascolo, N.; Izzo, A.A.; Capasso, F. Flavonoids: Old and new aspects of a class of natural therapeutic drugs. Life Sci. 1999, 65, 337–353. [Google Scholar]
- Klejdus, B.; Sterbova, D.; Stratil, P.; Kuban, V. Identification and characterization of isoflavones in plant material by HPLC-DAD-MS tandem. Chem. Listy 2003, 97, 530–539. [Google Scholar]
- Zand, R.S.R.; Jenkins, D.J.A.; Diamandis, E.P. Flavonoids and steroid hormone-dependent cancers. J. Chrom. B 2002, 777, 219–232. [Google Scholar]
- Yao, L.H.; Jiang, Y.M.; Shi, J.; Tomas-Barberan, F.A.; Datta, N.; Singanusong, R.; Chen, S.S. Flavonoids in food and their health benefits. Plant Foods for Human Nutrition 2004, 59, 113–122. [Google Scholar]
- Ren, W.Y.; Qiao, Z.H.; Wang, H.W.; Zhu, L.; Zhang, L. Flavonoids: Promising anticancer agents. Med. Res. Rev. 2003, 23, 519–534. [Google Scholar]
- Sukardiman; Darwanto, A.; Tanjung, M.; Darmadi, M.O. Cytotoxic mechanism of flavonoid from Temu Kunci (Kaempferia pandurata) in cell culture of human mammary carcinoma. Clin. Hemorheol. Microcirc. 2000, 23, 185–190. [Google Scholar]
- Constantinou, A.; Mehta, R.; Runyan, C.; Rao, K.; Vaughan, A.; Moon, R. Flavonoids as DNA Topoisomerase Antagonists and Poisons - Structure-Activity-Relationships. J. Nat. Prod. 1995, 58, 217–225. [Google Scholar]
- Primiano, T.; Yu, R.; Kong, A.N.T. Signal transduction events elicited by natural products that function as cancer chemopreventive agents. Pharm. Biol. 2001, 39, 83–107. [Google Scholar]
- Kohn, M.C.; Walker, N.J.; Kim, A.H.; Portier, C.J. Physiological modeling of a proposed mechanism of enzyme induction by TCDD. Toxicology 2001, 162, 193–208. [Google Scholar]
- Omiecinski, C.J.; Remmel, R.P.; Hosagrahara, V.P. Concise review of the cytochrome P450s and their roles in toxicology. Toxicol. Sci. 1999, 48, 151–156. [Google Scholar]
- Hodek, P.; Trefil, P.; Stiborova, M. Flavonoids-potent and versatile biologically active compounds interacting with cytochromes P450. Chem.-Biol. Interact. 2002, 139, 1–21. [Google Scholar]
- Naczk, M.; Shahidi, F. Extraction and analysis of phenolics in food. J. Chromatogr. A 2004, 1054, 95–111. [Google Scholar]
- Jac, P.; Polasek, M.; Pospisilova, M. Recent trends in the determination of polyphenols by electromigration methods. J. Pharm. Biomed. Anal. 2006, 40, 805–814. [Google Scholar]
- Bedgood, D.R.; Bishop, A.G.; Prenzler, P.D.; Robards, K. Analytical approaches to the determination of simple biophenols in forest trees such as Acer (maple), Betula (birch), Coniferus, Eucalyptus, Juniperus (cedar), Picea (spruce) and Quercus (oak). Analyst 2005, 130, 809–823. [Google Scholar]
- Smyth, W.F.; Brooks, P. A critical evaluation of high performance liquid chromatography-electrospray ionisation-mass pectrometry and capillary electrophoresis-electrospray-mass spectrometry for the detection and determination of small molecules of significance in clinical and forensic science. Electrophoresis 2004, 10-11, 1413–1446. [Google Scholar]
- Robards, K. Strategies for the determination of bioactive phenols in plants, fruit and vegetables. J. Chrom. A 2003, 1-2, 657–691. [Google Scholar]
- Suntornsuk, L. Capillary electrophoresis of phytochemical substances. J. Pharmaceut. Biomed. 2002, 5, 679–698. [Google Scholar]
- Franke, A.A.; Custer, L.J.; Arakaki, C.; Murphy, S.P. Vitamin C and flavonoid levels of fruits and vegetables consumed in Hawaii. J. Food Compos. Anal. 2004, 1, 1–35. [Google Scholar]
- Escarpa, A.; Gonzales, M.C. An overview of analytical chemistry of phenolic compounds in foods. Crit. Rev. Anal. Chem. 2001, 2, 57–139. [Google Scholar]
- Kizek, R.; Trnkova, L.; Palecek, E. Determination of metallothionein at the femtomole level by constant current stripping chronopotentiometry. Anal. Chem. 2001, 73, 4801–4807. [Google Scholar]
- Petrlova, J.; Potesil, D.; Mikelova, R.; Blastik, O.; Adam, V.; Trnkova, L.; Jelen, F.; Prusa, R.; Kukacka, J.; Kizek, R. Attomole voltammetric determination of metallothionein. Electrochim. Acta 2006, 51, 5112–5119. [Google Scholar]
- Palecek, E.; Masarik, M.; Kizek, R.; Kuhlmeier, D.; Hassmann, J.; Schulein, J. Sensitive electrochemical determination of unlabeled MutS protein and detection of point mutations in DNA. Anal. Chem. 2004, 76, 5930–5936. [Google Scholar]
- Masarik, M.; Kizek, R.; Kramer, K.J.; Billova, S.; Brazdova, M.; Vacek, J.; Bailey, M.; Jelen, F.; Howard, J.A. Application of avidin-biotin technology and adsorptive transfer stripping square-wave voltammetry for detection of DNA hybridization and avidin in transgenic avidin maize. Anal. Chem. 2003, 75, 2663–2669. [Google Scholar]
- Palecek, E.; Kizek, R.; Havran, L.; Billova, S.; Fojta, M. Electrochemical enzyme-linked immunoassay in a DNA hybridization sensor. Anal. Chim. Acta 2002, 469, 73–83. [Google Scholar]
- Labuda, J.; Buckova, M.; Heilerova, L.; Silhar, S.; Stepanek, I. Evaluation of the redox properties and anti/pro-oxidant effects of selected flavonoids by means of a DNA-based electrochemical biosensor. Anal. Bioanal. Chem. 2003, 376, 168–173. [Google Scholar]
- Labuda, J.; Buckova, M.; Heilerova, L.; Caniova-Ziakova, A.; Brandsteterova, E.; Mattusch, J.; Wennrich, R. Detection of antioxidative activity of plant extracts at the DNA-modified screen-printed electrode. Sensors 2002, 2, 1–10. [Google Scholar]
- Korbut, O.; Buckova, M.; Labuda, J.; Grundler, P. Voltammetric detection of antioxidative properties of flavonoids using electrically heated DNA modified carbon paste electrode. Sensors 2003, 3, 1–10. [Google Scholar]
- Bukova, M.; Labuda, J.; Sandula, J.; Krizkova, L.; Stepanek, I.; Durackova, Z. Detection of damage to DNA and antioxidative activity of yeast polysaccharides at the DNA-modified screen-printed electrode. Talanta 2002, 56, 939–947. [Google Scholar]
- Ovadekova, R.; Jantova, S.; Letasiova, S.; Stepanek, I.; Labuda, J. Nanostructured electrochemical DNA biosensors for detection of the effect of berberine on DNA from cancer cells. Anal. Bioanal. Chem. 2006, 386, 2055–2062. [Google Scholar]
- Hodek, P.; Hanustiak, P.; Krizkova, J.; Mikelova, R.; Krizkova, S.; Stiborova, M.; Trnkova, L.; Horna, A.; Beklova, M.; Kizek, R. Toxicological aspects of flavonoid interaction with biomacromolecules. Neuroendocrinol. Lett. 2006, 27, 14–17. [Google Scholar]
- Mikelova, R.; Hodek, P.; Hanustiak, P.; Adam, V.; Krizkova, S.; Havel, L.; Stiborova, M.; Horna, A.; Beklova, M.; Trnkova, L.; Kizek, R. Determination of isoflavones using liquid chromatography with electrochemical detection. Acta Chim. Slov. 2007, 54, 92–97. [Google Scholar]
- Zitka, O.; Huska, D.; Krizkova, S.; Adam, V.; Chavis, G.J.; Trnkova, L.; Horna, A.; Hubalek, J.; Kizek, R. An investigation of glutathione-platinum(II) interactions by means of the flow injection analysis using glassy carbon electrode. Sensors 2007, 7, 1256–1270. [Google Scholar]
- Vitecek, J.; Petrlova, J.; Adam, V.; Havel, L.; Kramer, K.J.; Babula, P.; Kizek, R. A fluorimetric sensor for detection of one living cell. Sensors 2007, 7, 222–238. [Google Scholar]
- Trnkova, L.; Jelen, F.; Petrlova, J.; Adam, V.; Potesil, D.; Kizek, R. Elimination voltammetry with linear scan as a new detection method for DNA sensors. Sensors 2005, 5, 448–464. [Google Scholar]
- Supalkova, V.; Petrek, J.; Havel, L.; Krizkova, S.; Petrlova, J.; Adam, V.; Potesil, D.; Babula, P.; Beklova, M.; Horna, A.; Kizek, R. Electrochemical sensors for detection of acetylsalicylic acid. Sensors 2006, 11, 1483–1497. [Google Scholar]
- Supalkova, V.; Petrek, J.; Baloun, J.; Adam, V.; Bartusek, K.; Trnkova, L.; Beklova, M.; Diopan, V.; Havel, L.; Kizek, R. Multi-instrumental investigation of affecting of early somatic embryos of Spruce by cadmium(II) and lead(II) ions. Sensors 2007, 7, 743–759. [Google Scholar]
- Supalkova, V.; Huska, D.; Diopan, V.; Hanustiak, P.; Zitka, O.; Stejskal, K.; Baloun, J.; Pikula, J.; Havel, L.; Zehnalek, J.; Adam, V.; Trnkova, L.; Beklova, M.; Kizek, R. Electroanalysis of plant thiols. Sensors 2007, 7, 932–959. [Google Scholar]
- Prasek, J.; Adamek, M.; Hubalek, J.; Adam, V.; Trnkova, L.; Kizek, R. New hydrodynamic electrochemical arrangement for cadmium ions detection using thick-film chemical sensor electrodes. Sensors 2006, 11, 1498–1512. [Google Scholar]
- Krizkova, S.; Beklova, M.; Pikula, J.; Adam, V.; Horna, A.; Kizek, R. Hazards of secondary bromadiolone intoxications evaluated using high-performance liquid chromatography with electrochemical detection. Sensors 2007, 7, 1271–1286. [Google Scholar]
- Hubalek, J.; Hradecky, J.; Adam, V.; Krystofova, O.; Huska, D.; Masarik, M.; Trnkova, L.; Horna, A.; Klosova, K.; Adamek, M.; Zehnalek, J.; Kizek, R. Spectrometric and voltammetric analysis of urease – Nickel nanoelectrode as an electrochemical sensor. Sensors 2007, 7, 1238–1255. [Google Scholar]
- Babula, P.; Huska, D.; Hanustiak, P.; Baloun, J.; Krizkova, S.; Adam, V.; Hubalek, J.; Havel, L.; Zemlicka, M.; Horna, A.; Beklova, M.; Kizek, R. Flow injection analysis coupled with carbon electrodes as the tool for analysis of naphthoquinones with respect to their content and functions in biological samples. Sensors 2006, 11, 1466–1482. [Google Scholar]
- Adam, V.; Zehnalek, J.; Petrlova, J.; Potesil, D.; Sures, B.; Trnkova, L.; Jelen, F.; Vitecek, J.; Kizek, R. Phytochelatin modified electrode surface as a sensitive heavy metal ions biosensor. Sensors 2005, 5, 70–84. [Google Scholar]
- Brett, A.M.O.; Ghica, M.E. Electrochemical oxidation of quercetin. Electroanalysis 2003, 15, 1745–1750. [Google Scholar]
- Corduneanu, O.; Janeiro, P.; Brett, A.M.O. On the electrochemical oxidation of resveratrol. Electroanalysis 2006, 18, 757–762. [Google Scholar]
- Janeiro, P.; Corduneanu, O.; Brett, A.M.O. Chrysin and (+/−)-taxifolin electrochemical oxidation mechanisms. Electroanalysis 2005, 17, 1059–1064. [Google Scholar]
- Ghica, M.E.; Brett, A.M.O. Electrochemical oxidation of rutin. Electroanalysis 2005, 17, 313–318. [Google Scholar]
- Janeiro, P.; Brett, A.M.O. Catechin electrochemical oxidation mechanisms. Anal. Chim. Acta 2004, 518, 109–115. [Google Scholar]
- Malagutti, A.R.; Zuin, V.G.; Cavalheiro, E.T.G.; Mazo, L.H. Determination of rutin in green tea infusions using square-wave voltammetry with a rigid carbon-polyurethane composite electrode. Electroanalysis 2006, 18, 1028–1034. [Google Scholar]
- Zeng, B.Z.; Wei, S.H.; Xiao, F.; Zhao, F.Q. Voltammetric behavior and determination of rutin at a single-walled carbon nanotubes modified gold electrode. Sens. Actuator B: Chem. 2006, 115, 240–246. [Google Scholar]
- Ensafi, A.A.; Hajian, R. Determination of rutin in pharmaceutical compounds and tea using cathodic adsorptive stripping voltammetry. Electroanalysis 2006, 18, 579–585. [Google Scholar]
- Timbola, A.K.; de Souza, C.D.; Giacomelli, C.; Spinelli, A. Electrochemical oxidation of quercetin in hydro-alcoholic solution. J. Braz. Chem. Soc. 2006, 17, 139–148. [Google Scholar]
- Temerk, Y.M.; Ibrahim, H.S.M.; Schuhmann, W. Cathodic adsorptive stripping voltammetric determination of the antitumor drug rutin in pharmaceuticals, human urine, and blood serum. Microchim. Acta 2006, 153, 7–13. [Google Scholar]
- Firuzi, O.; Lacanna, A.; Petrucci, R.; Marrosu, G.; Saso, L. Evaluation of the antioxidant activity of flavonoids by “ ferric reducing antioxidant power” assay and cyclic voltammetry. Biochim. Biophys. Acta-Gen. Subj. 2005, 1721, 174–184. [Google Scholar]
- Zoulis, N.E.; Efstathiou, C.E. Preconcentration at a carbon-paste electrode and determination by adsorptive-stripping voltammetry of rutin and other flavonoids. Anal. Chim. Acta 1996, 320, 255–261. [Google Scholar]
- Janeiro, P.; Brett, A.M.O. Solid state electrochemical oxidation mechanisms of morin in aqueous media. Electroanalysis 2005, 17, 733–738. [Google Scholar]
- Oliveira-Brett, A.M.; Diculescu, V.C. Electrochemical study of quercetin-DNA interactions: Part I. Analysis in incubated solutions. Bioelectrochemistry 2004, 64, 133–141. [Google Scholar]
- Oliveira-Brett, A.M.; Diculescu, V.C. Electrochemical study of quercetin-DNA interactions - Part II. In situ sensing with DNA biosensors. Bioelectrochemistry 2004, 64, 143–150. [Google Scholar]
- Kizek, R.; Masarik, M.; Kramer, K.J.; Potesil, D.; Bailey, M.; Howard, J.A.; Klejdus, B.; Mikelova, R.; Adam, V.; Trnkova, L.; Jelen, F. An analysis of avidin, biotin and their interaction at attomole levels by voltammetric and chromatographic techniques. Anal. Bioanal. Chem. 2005, 381, 1167–1178. [Google Scholar]
- Stiborova, M.; Asfaw, B.; Frei, E.; Schmeiser, H.H.; Wiessler, M. Benzenediazonium Ion Derived from Sudan-I Forms an 8-(Phenylazo)Guanine Adduct in DNA. Chem. Res. Toxicol. 1995, 8, 489–498. [Google Scholar]
- Wiechelman, K.J.; Braun, R.D.; Fitzpatrick, J.D. Investigation of the Bicinchoninic Acid Protein Assay - Identification of the Groups Responsible for Color Formation. Anal. Biochem. 1988, 175, 231–237. [Google Scholar]
- Omura, T.; Sato, R. The carbon monoxide-binding pigment of liver microsomes. I. Evidence for its hemoprotein nature. J. Biol. Chem. 1964, 239, 2370–2378. [Google Scholar]
- Polson, A.; Vonwechmar, M.B.; Vanregenmortel, M.H.V. Isolation of Viral Igy Antibodies from Yolks of Immunized Hens. Immunol. Commun. 1980, 9, 475–493. [Google Scholar]
- Polson, A.; Vonwechmar, M.B.; Fazakerley, G. Antibodies to Proteins from Yolk of Immunized Hens. Immunol. Commun. 1980, 9, 495–514. [Google Scholar]
- Hodek, P.; Koblas, T.; Rydlova, H.; Kubickova, B.; Sulc, M.; Hudecek, J.; Stiborova, M. Chicken egg yolk as an excellent source of highly specific antibodies against cytochromes P450. Collect. Czech. Chem. Commun. 2004, 69, 659–673. [Google Scholar]
- Guengerich, F.P.; Wang, P.; Davidson, N.K. Estimation of Isozymes of Microsomal Cytochrome-P-450 in Rats, Rabbits, and Humans Using Immunochemical Staining Coupled with Sodium Dodecyl-Sulfate Polyacrylamide-Gel Electrophoresis. Biochemistry 1982, 21, 1698–1706. [Google Scholar]
- Klejdus, B.; Vacek, J.; Adam, V.; Zehnalek, J.; Kizek, R.; Trnkova, L.; Kuban, V. Determination of isoflavones in soybean food and human urine using liquid chromatography with electrochemical detection. J. Chrom. B 2004, 806, 101–111. [Google Scholar]
- Potesil, D.; Petrlova, J.; Adam, V.; Vacek, J.; Klejdus, B.; Zehnalek, J.; Trnkova, L.; Havel, L.; Kizek, R. Simultaneous femtomole determination of cysteine, reduced and oxidized glutathione, and phytochelatin in maize (Zea mays L.) kernels using high-performance liquid chromatography with electrochemical detection. J. Chrom. A 2005, 1084, 134–144. [Google Scholar]
- Petrlova, J.; Mikelova, R.; Stejskal, K.; Kleckerova, A.; Zitka, O.; Petrek, J.; Havel, L.; Zehnalek, J.; Adam, V.; Trnkova, L.; Kizek, R. Simultaneous determination of eight biologically active thiol compounds using gradient elution-Liquid Chromatography with Coul-Array detection. J. Sep. Sci. 2006, 29, 1166–1173. [Google Scholar]
- Jovanovic, S.V.; Steenken, S.; Tosic, M.; Marjanovic, B.; Simic, M.G. Flavonoids as Antioxidants. J. Am. Chem. Soc. 1994, 116, 4846–4851. [Google Scholar]
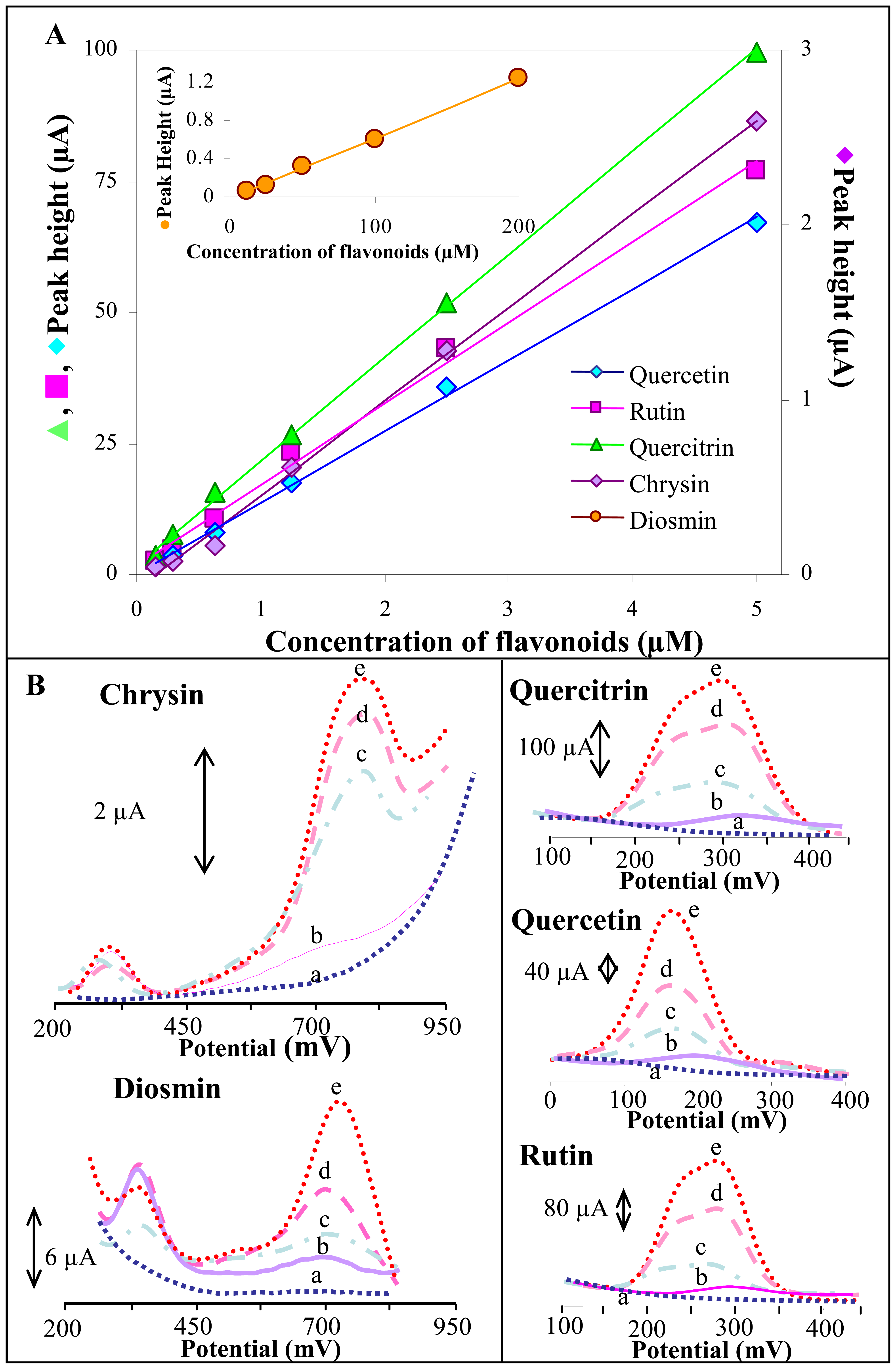
| Flavonoid | Regression equation | Concentration of the flavonoid (μM) | aR2 | bLOD (nM) | cLOQ (nM) | dR.S.D. (%) |
|---|---|---|---|---|---|---|
| Quercetin | y = 13.602x + 0.1072 | 0.15 – 5.0 | 0.9988 | 10 | 33 | 3.7 |
| Quercitrin | y = 19.678x + 1.9359 | 0.15 – 5.0 | 0.9992 | 7 | 23 | 1.9 |
| Rutin | y = 15.443x + 1.5354 | 0.15 – 5.0 | 0.9944 | 9 | 30 | 3.6 |
| Chrysin | y = 0.5367x − 0.0841 | 0.15 – 5.0 | 0.9978 | 42 | 140 | 2.2 |
| Diosmin | y = 0.0063x − 0.0187 | 12.5– 200 | 0.9995 | 2,663 | 8,877 | 3.5 |
© 2007 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Adam, V.; Mikelova, R.; Hubalek, J.; Hanustiak, P.; Beklova, M.; Hodek, P.; Horna, A.; Trnkova, L.; Stiborova, M.; Zeman, L.; et al. Utilizing of Square Wave Voltammetry to Detect Flavonoids in the Presence of Human Urine. Sensors 2007, 7, 2402-2418. https://doi.org/10.3390/s7102402
Adam V, Mikelova R, Hubalek J, Hanustiak P, Beklova M, Hodek P, Horna A, Trnkova L, Stiborova M, Zeman L, et al. Utilizing of Square Wave Voltammetry to Detect Flavonoids in the Presence of Human Urine. Sensors. 2007; 7(10):2402-2418. https://doi.org/10.3390/s7102402
Chicago/Turabian StyleAdam, Vojtech, Radka Mikelova, Jaromír Hubalek, Pavel Hanustiak, Miroslava Beklova, Petr Hodek, Ales Horna, Libuse Trnkova, Marie Stiborova, Ladislav Zeman, and et al. 2007. "Utilizing of Square Wave Voltammetry to Detect Flavonoids in the Presence of Human Urine" Sensors 7, no. 10: 2402-2418. https://doi.org/10.3390/s7102402




