Shapes of Differential Pulse Voltammograms and Level of Metallothionein at Different Animal Species
Abstract
:1. Introduction
2. Experimental Section
2.1 Chemicals
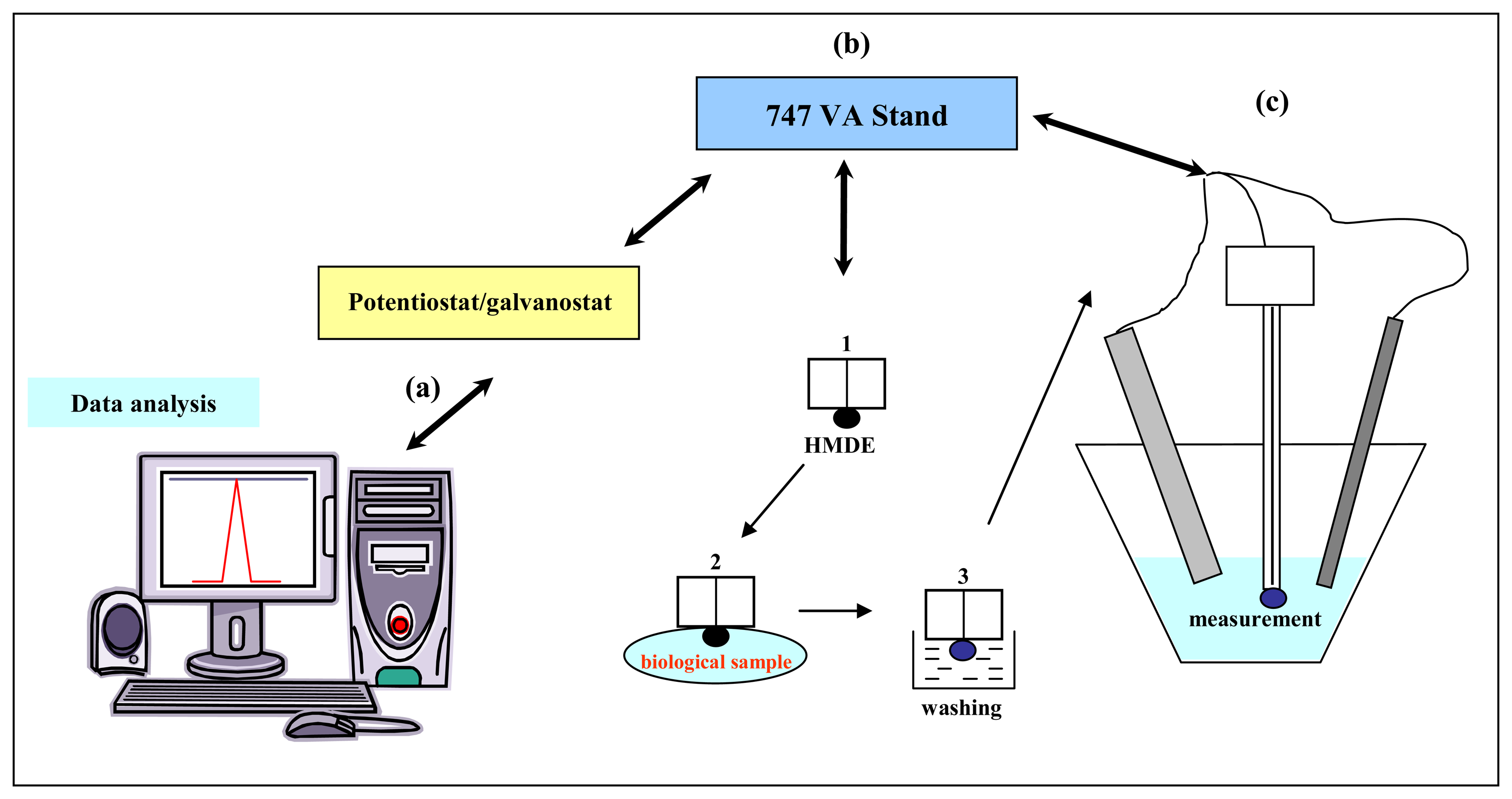
2.2 Electrochemical measurements
2.2.1 Adsorptive transfer stripping technique
2.2.2 AdTS DPV Brdicka reaction of MT
2.3 pH measurement
2.4 Species examined
2.5. Samples and their preparation prior metallothionein analysis
2.6 Statistical analysis
3. Results
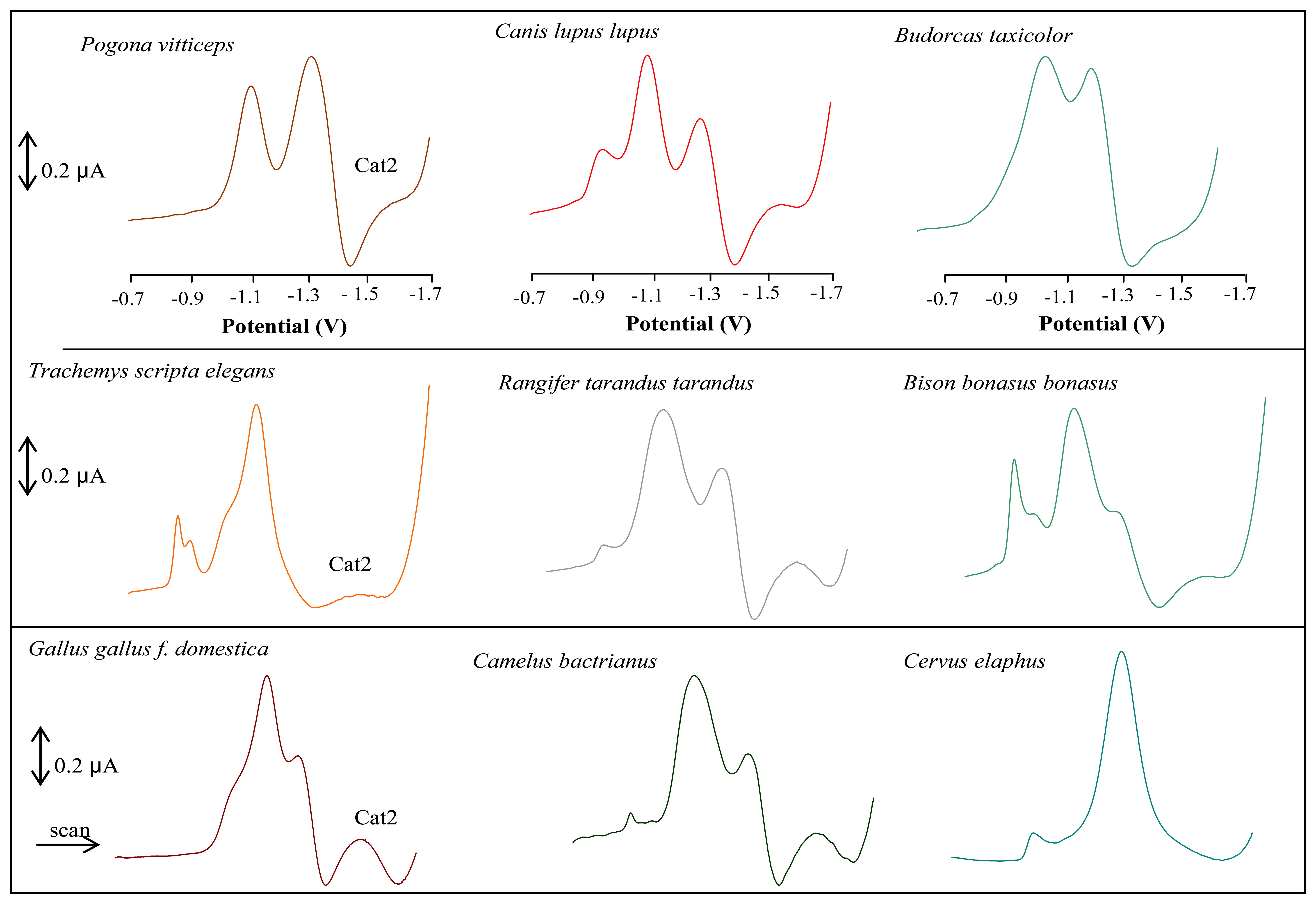
3.1 Shape of DP voltammetric curves of mammalian species analysed
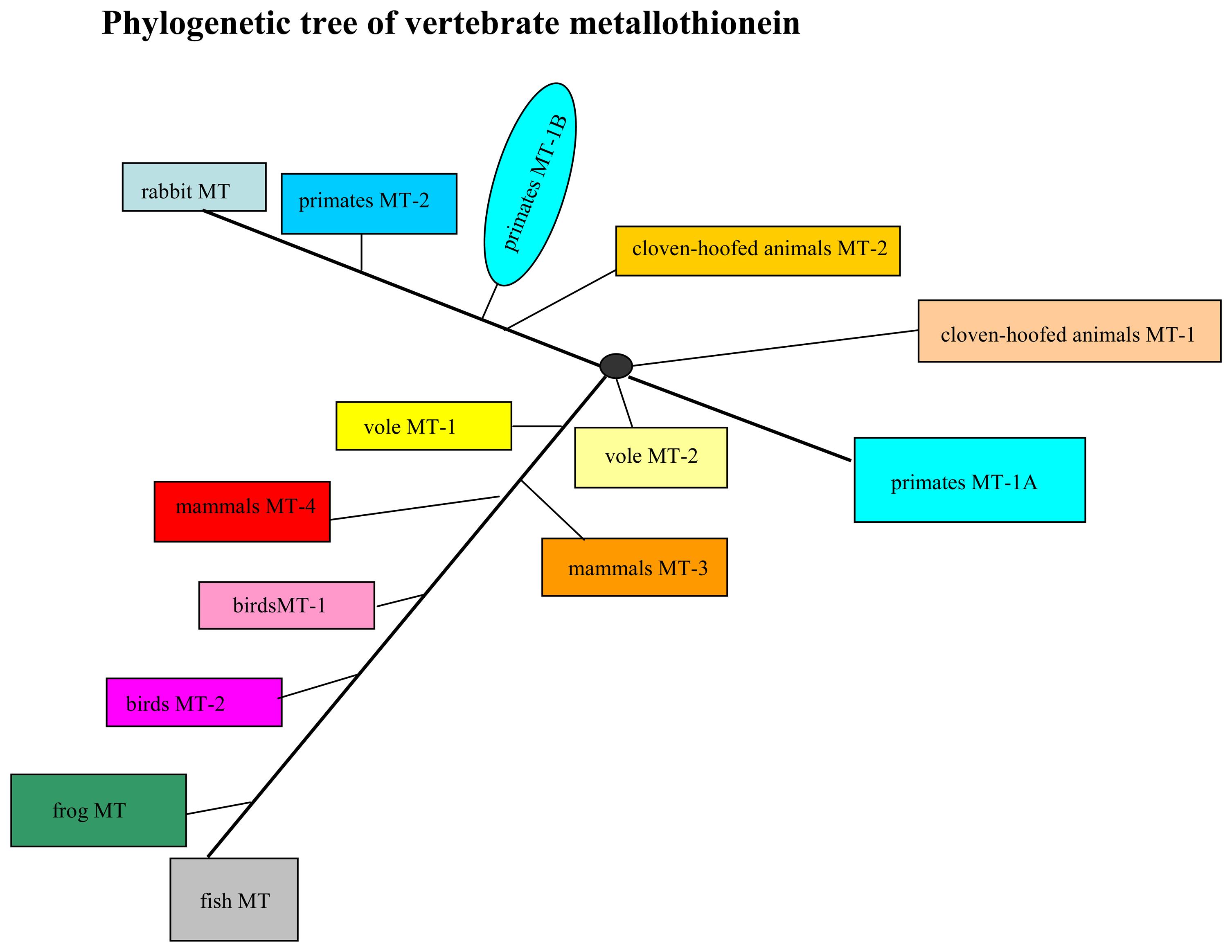
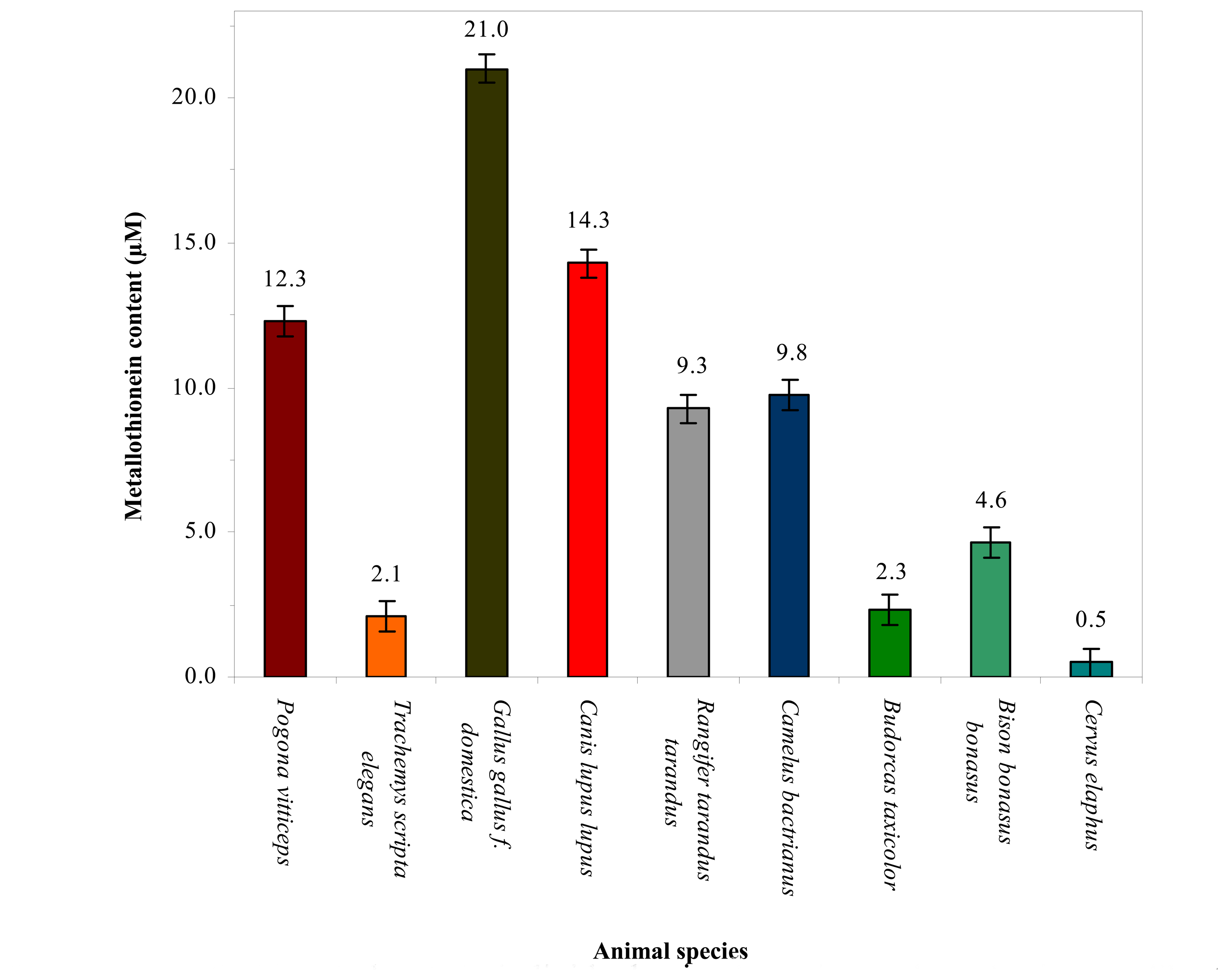
3.2 Association of changes in metallothionein level in blood of the vertebrates and their taxonomic group
4. Conclusion
Acknowledgments
References
- Bremner, I.; Beattie, J.H. Metallothionein and the Trace Minerals. Annu. Rev. Nutr. 1990, 10, 63–83. [Google Scholar]
- Brady, F.O. The Physiological-Function of Metallothionein. Trends Biochem. Sci. 1982, 7, 143–145. [Google Scholar]
- Meneghini, R. Iron homeostasis, oxidative stress, and DNA damage. Free Radic. Biol. Med. 1997, 23, 783–792. [Google Scholar]
- Margoshes, M.; Vallee, B.L.A. A cadmium protein from equine kidney cortex. J. Am. Chem. Soc. 1957, 79, 4813–4814. [Google Scholar]
- Kagi, J.H.R.; Schaffer, A. Biochemistry of Metallothionein. Biochemistry 1988, 27, 8509–8515. [Google Scholar]
- Hamer, D.H. Metallothionein. Annu. Rev. Biochem. 1986, 55, 913–951. [Google Scholar]
- Dunn, M.A.; Blalock, T.L.; Cousins, R.J. Metallothionein. Proc. Soc. Exp. Biol. Med. 1987, 185, 107–119. [Google Scholar]
- Kizek, R.; Trnkova, L.; Palecek, E. Determination of metallothionein at the femtomole level by constant current stripping chronopotentiometry. Anal. Chem. 2001, 73, 4801–4807. [Google Scholar]
- Prusa, R.; Svoboda, M.; Blastik, O.; Adam, V.; Zitka, O.; Beklova, M.; Eckschlager, T.; Kizek, R. Increase in content of metallothionein as marker of resistence to cisplatin treatment. Clin. Chem. 2006, 52, A174–A175. [Google Scholar]
- Strouhal, M.; Kizek, R.; Vecek, J.; Trnkova, L.; Nemec, M. Electrochemical study of heavy metals and metallothionein in yeast Yarrowia lipolytica. Bioelectrochemistry 2003, 60, 29–36. [Google Scholar]
- Trnkova, L.; Kizek, R.; Vacek, J. Catalytic signal of rabbit liver metallothionein on a mercury electrode: a combination of derivative chronopotentiometry with adsorptive transfer stripping. Bioelectrochemistry 2002, 56, 57–61. [Google Scholar]
- Miura, N.; Koizumi, S. Heavy metal responses of the human metallothionein isoform genes. Yakugaku Zasshi-J. Pharm. Soc. Jpn. 2007, 127, 665–673. [Google Scholar]
- Otsuka, F.; Ohno, S.; Suzuki, K.; Takahashi, K.; Ohsawa, M.; Koizumi, S. Mechanism of metallothionein gene activation mediated by heavy-metal dependent transcription factor MTF-1. Yakugaku Zasshi-J. Pharm. Soc. Jpn. 2007, 127, 675–684. [Google Scholar]
- Itoh, N.; Kimura, T. Cytokine-induced metallothionein expression and modulation of cytokine expression by metallothionein. Yakugaku Zasshi-J. Pharm. Soc. Jpn. 2007, 127, 685–694. [Google Scholar]
- Min, K.S. The physiological significance of metallothionein in oxidative stress. Yakugaku Zasshi-J. Pharm. Soc. Jpn. 2007, 127, 695–702. [Google Scholar]
- Sato, M.; Suzuki, S. Endoplasmic reticulum stress and metallothionein. Yakugaku Zasshi-J. Pharm. Soc. Jpn. 2007, 127, 703–708. [Google Scholar]
- Satoh, M. Analysis of toxicity using metallothionein knockout mice. Yakugaku Zasshi-J. Pharm. Soc. Jpn. 2007, 127, 709–717. [Google Scholar]
- Piskorova, L.; Vasilkova, Z.; Krupicer, I. Heavy metal residues in tissues of wild boar (Sus scrofa) and red fox (Vulpes vulpes) in the Central Zemplin region of the Slovak Republic. Czech J. Anim. Sci. 2003, 48, 134–138. [Google Scholar]
- Hejtmankova, A.; Kucerova, J.; Miholova, D.; Kolihova, D.; Orsak, M. Levels of selected macro- and microelements in goat milk from farms in the Czech Republic. Czech J. Anim. Sci. 2002, 47, 253–260. [Google Scholar]
- Spurny, P.; Mares, J.; Hedbavny, J.; Sukop, I. Heavy metal distribution in the ecosystems of the upper course of the Jihlava River. Czech J. Anim. Sci. 2002, 47, 160–167. [Google Scholar]
- Rous, P.; Jelinek, P. The effect of increased soil contamination with heavy metals on their content in some rabbit tissues. Czech J. Anim. Sci. 2000, 45, 319–324. [Google Scholar]
- Thirumoorthy, N.; Kumar, K.T.M.; Sundar, A.S.; Panayappan, L.; Chatterjee, M. Metallothionein: An overview. World J. Gastroenterol. 2007, 13, 993–996. [Google Scholar]
- Amiard, J.C.; Amiard-Triquet, C.; Barka, S.; Pellerin, J.; Rainbow, P.S. Metallothioneins in aquatic invertebrates: Their role in metal detoxification and their use as biomarkers. Aquat. Toxicol. 2006, 76, 160–202. [Google Scholar]
- Coyle, P.; Philcox, J.C.; Carey, L.C.; Rofe, A.M. Metallothionein: The multipurpose protein. Cell. Mol. Life Sci. 2002, 59, 627–647. [Google Scholar]
- Vasak, M.; Hasler, D.W. Metallothioneins: new functional and structural insights. Curr. Opin. Chem. Biol. 2000, 4, 177–183. [Google Scholar]
- Henry, R.B.; Liu, J.; Choudhuri, S.; Klaassen, C.D. Species Variation in Hepatic Metallothionein. Toxicol. Lett. 1994, 74, 23–33. [Google Scholar]
- Adam, V.; Petrlova, J.; Potesil, D.; Zehnalek, J.; Sures, B.; Trnkova, L.; Jelen, F.; Kizek, R. Study of metallothionein modified electrode surface behavior in the presence of heavy metal ions-biosensor. Electroanalysis 2005, 17, 1649–1657. [Google Scholar]
- Vacek, J.; Petrek, J.; Kizek, R.; Havel, L.; Klejdus, B.; Trnkova, L.; Jelen, F. Electrochemical determination of lead and glutathione in a plant cell culture. Bioelectrochemistry 2004, 63, 347–351. [Google Scholar]
- Kizek, R.; Vacek, J.; Trnkova, L.; Jelen, F. Cyclic voltammetric study of the redox system of glutathione using the disulfide bond reductant tris(2-carboxyethyl)phosphine. Bioelectrochemistry 2004, 63, 19–24. [Google Scholar]
- Hubalek, J.; Hradecky, J.; Adam, V.; Krystofova, O.; Huska, D.; Masarik, M.; Trnkova, L.; Horna, A.; Klosova, K.; Adamek, M.; Zehnalek, J.; Kizek, R. Spectrometric and voltammetric analysis of urease - nickel nanoelectrode as an electrochemical sensor. Sensors 2007, 7, 1238–1255. [Google Scholar]
- Adam, V.; Zehnalek, J.; Petrlova, J.; Potesil, D.; Sures, B.; Trnkova, L.; Jelen, F.; Vitecek, J.; Kizek, R. Phytochelatin modified electrode surface as a sensitive heavy-metal ion biosensor. Sensors 2005, 5, 70–84. [Google Scholar]
- Petrlova, J.; Potesil, D.; Mikelova, R.; Blastik, O.; Adam, V.; Trnkova, L.; Jelen, F.; Prusa, R.; Kukacka, J.; Kizek, R. Attomole voltammetric determination of metallothionein. Electrochim. Acta 2006, 51, 5112–5119. [Google Scholar]
- Adam, V.; Hanustiak, P.; Krizkova, S.; Beklova, M.; Zehnalek, J.; Trnkova, L.; Horna, A.; Sures, B.; Kizek, R. Palladium biosensor. Electroanalysis 2007, 19, 1909–1914. [Google Scholar]
- Adam, V.; Krizkova, S.; Zitka, O.; Trnkova, L.; Petrlova, J.; Beklova, M.; Kizek, R. Determination of apo-metallothionein using adsorptive transfer stripping technique in connection with differential pulse voltammetry. Electroanalysis 2007, 19, 339–347. [Google Scholar]
- Huska, D.; Zitka, O.; Adam, V.; Beklova, M.; Krizkova, S.; Zeman, L.; Horna, A.; Havel, L.; Zehnalek, J.; Kizek, R. A sensor for investigating the interaction between biologically important heavy metals and glutathione. Czech J. Anim. Sci. 2007, 52, 37–43. [Google Scholar]
- Krizkova, S.; Adam, V.; Petrlova, J.; Zitka, O.; Stejskal, K.; Zehnalek, J.; Sures, B.; Trnkova, L.; Beklova, M.; Kizek, R. A suggestion of electrochemical biosensor for study of platinum(II)-DNA interactions. Electroanalysis 2007, 19, 331–338. [Google Scholar]
- Petrlova, J.; Potesil, D.; Zehnalek, J.; Sures, B.; Adam, V.; Trnkova, L.; Kizek, R. Cisplatin electrochemical biosensor. Electrochim. Acta 2006, 51, 5169–5173. [Google Scholar]
- Trnkova, L.; Jelen, F.; Petrlova, J.; Adam, V.; Potesil, D.; Kizek, R. Elimination voltammetry with linear scan as a new detection method for DNA sensors. Sensors 2005, 5, 448–464. [Google Scholar]
- Palecek, E.; Postbieglova, I. Adsorptive Stripping Voltammetry of Biomacromolecules with Transfer of the Adsorbed Layer. J. Electroanal. Chem. 1986, 214, 359–371. [Google Scholar]
- Supalkova, V.; Huska, D.; Diopan, V.; Hanustiak, P.; Zitka, O.; Stejskal, K.; Baloun, J.; Pikula, J.; Havel, L.; Zehnalek, J.; Adam, V.; Trnkova, L.; Beklova, M.; Kizek, R. Electroanalysis of plant thiols. Sensors 2007, 7, 932–959. [Google Scholar]
- Supalkova, V.; Petrek, J.; Baloun, J.; Adam, V.; Bartusek, K.; Trnkova, L.; Beklova, M.; Diopan, V.; Havel, L.; Kizek, R. Multi-instrumental investigation of affecting of early somatic embryos of spruce by cadmium(II) and lead(II) ions. Sensors 2007, 7, 743–759. [Google Scholar]
- Krizkova, S.; Zitka, O.; Adam, V.; Beklova, M.; Horna, A.; Svobodova, Z.; Sures, B.; Trnkova, L.; Zeman, L.; Kizek, R. Possibilities of electrochemical techniques in metallothionein and lead detection in fish tissues. Czech J. Anim. Sci. 2007, 52, 143–148. [Google Scholar]
- Brdicka, R. Polarographic studies with the dropping mercury kathode. -Part XXXI. - A new test for proteins in the presence of cobalt salts in ammoniacal solutions of ammonium chloride. Coll. Czech. Chem. Commun. 1933, 5, 112–128. [Google Scholar]
- Brdicka, R. Polarographic Studies with the Dropping Mercury Kathode. -Part LXI. - The Effect of buffer Solutions on the Reaction of Proteins. Coll. Czech. Chem. Commun. 1936, 8, 366–376. [Google Scholar]
- Erk, M.; Ivanković, D.; Raspor, B.; Pavičić, J. Evaluation of different purification procedures for the electrochemical quantification of mussel metallothioneins. Talanta 2002, 57, 1211–1218. [Google Scholar]
- Kukacka, J.; Vajtr, D.; Huska, D.; Prusa, R.; Houstava, L.; Samal, F.; Diopan, V.; Kotaska, K.; Kizek, R. Blood metallothionein, neuron specific enolase, and protein S100B in patients with traumatic brain injury. Neuroendocrinol. Lett. 2006, 27, 116–120. [Google Scholar]
- Palmiter, R.D. The elusive function of metallothioneins. Proc. Natl. Acad. Sci. U. S. A. 1998, 95, 8428–8430. [Google Scholar]
- Garty, J. The Amounts of Heavy-Metals in Some Lichens of the Negev Desert. Environ. Pollut. Ser. B-Chem. Phys. 1985, 10, 287–300. [Google Scholar]
- Garty, J.; Ammann, K. The Amounts of Ni, Cr, Zn, Pb, Cu, Fe and Mn in Some Lichens Growing in Switzerland. Environ. Exp. Bot. 1987, 27, 127–138. [Google Scholar]
- Ikingura, J.R.; Akagi, H. Lichens as a good bioindicator of air pollution by mercury in small-scale gold mining areas, Tanzania. Bull. Environ. Contam. Toxicol. 2002, 68, 699–704. [Google Scholar]
- Pawlik-Skowronska, B.; di Toppi, L.S.; Favali, M.A.; Fossati, F.; Pirszel, J.; Skowronski, T. Lichens respond to heavy metals by phytochelatin synthesis. New Phytol. 2002, 156, 95–102. [Google Scholar]
- Munshower, F.F.; Neuman, D.R. Metals in Soft-Tissues of Mule Deer and Antelope. Bull. Environ. Contam. Toxicol. 1979, 22, 827–832. [Google Scholar]
- Froslie, A.; Norheim, G.; Rambaek, J.P.; Steinnes, E. Levels of Trace-Elements in Liver from Norwegian Moose, Reindeer and Red Deer in Relation to Atmospheric Deposition. Acta Vet. Scand. 1984, 25, 333–345. [Google Scholar]
- Wlostowski, T.; Bonda, E.; Krasowska, A. Free-ranging European bisons accumulate more cadmium in the liver and kidneys than domestic cattle in north-eastern Poland. Sci. Total Environ. 2006, 364, 295–300. [Google Scholar]
- Cibulka, J.; Domazlicka, E.; Kozak, J.; Kubiznakova, J.; Mader, P.; Machalek, E.; Mankovska, B.; Musil, J.; Parizek, J.; Pisasa, J.; Pohunkova, H.; Reisnerova, H.; Svobodova, Z. Movement of lead, cadmium and mercury in the biosphere; Acedemia: Prague, 1991; p. 432. [Google Scholar]
- Svobodova, Z.; Celechovska, O.; Kolarova, J.; Randak, T.; Zlabek, V. Assessment of metal contamination in the upper reaches of the Ticha Orlice River. Czech J. Anim. Sci. 2004, 49, 458–464. [Google Scholar]
- Svobodova, Z.; Zlabek, V.; Celechovska, O.; Randak, T.; Machova, J.; Kolarova, J.; Janouskova, D. Content of metals in tissues of marketable common carp and in bottom sediments of selected ponds of South and West Bohemia. Czech J. Anim. Sci. 2002, 47, 339–350. [Google Scholar]
- Celechovska, O.; Svobodova, Z.; Randak, T. Arsenic content in tissues of fish from the River Elbe. Acta Vet. BRNO 2005, 74, 419–425. [Google Scholar]
- Zlabek, V.; Svobodova, Z.; Randak, T.; Valentova, O. Mercury content in the muscle of fish from the Elbe River and its tributaries. Czech J. Anim. Sci. 2005, 50, 528–534. [Google Scholar]
© 2007 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Adam, V.; Beklova, M.; Pikula, J.; Hubalek, J.; Trnkova, L.; Kizek, R. Shapes of Differential Pulse Voltammograms and Level of Metallothionein at Different Animal Species. Sensors 2007, 7, 2419-2429. https://doi.org/10.3390/s7102419
Adam V, Beklova M, Pikula J, Hubalek J, Trnkova L, Kizek R. Shapes of Differential Pulse Voltammograms and Level of Metallothionein at Different Animal Species. Sensors. 2007; 7(10):2419-2429. https://doi.org/10.3390/s7102419
Chicago/Turabian StyleAdam, Vojtech, Miroslava Beklova, Jiri Pikula, Jaromir Hubalek, Libuse Trnkova, and Rene Kizek. 2007. "Shapes of Differential Pulse Voltammograms and Level of Metallothionein at Different Animal Species" Sensors 7, no. 10: 2419-2429. https://doi.org/10.3390/s7102419



