Thermally Stable Merocyanine Form of Photochromic Spiropyran with Aluminum Ion as a Reversible Photo-driven Sensor in Aqueous Solution
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents
2.2. Instrumentation
2.3. Photochromic experiments
3. Results and Discussion
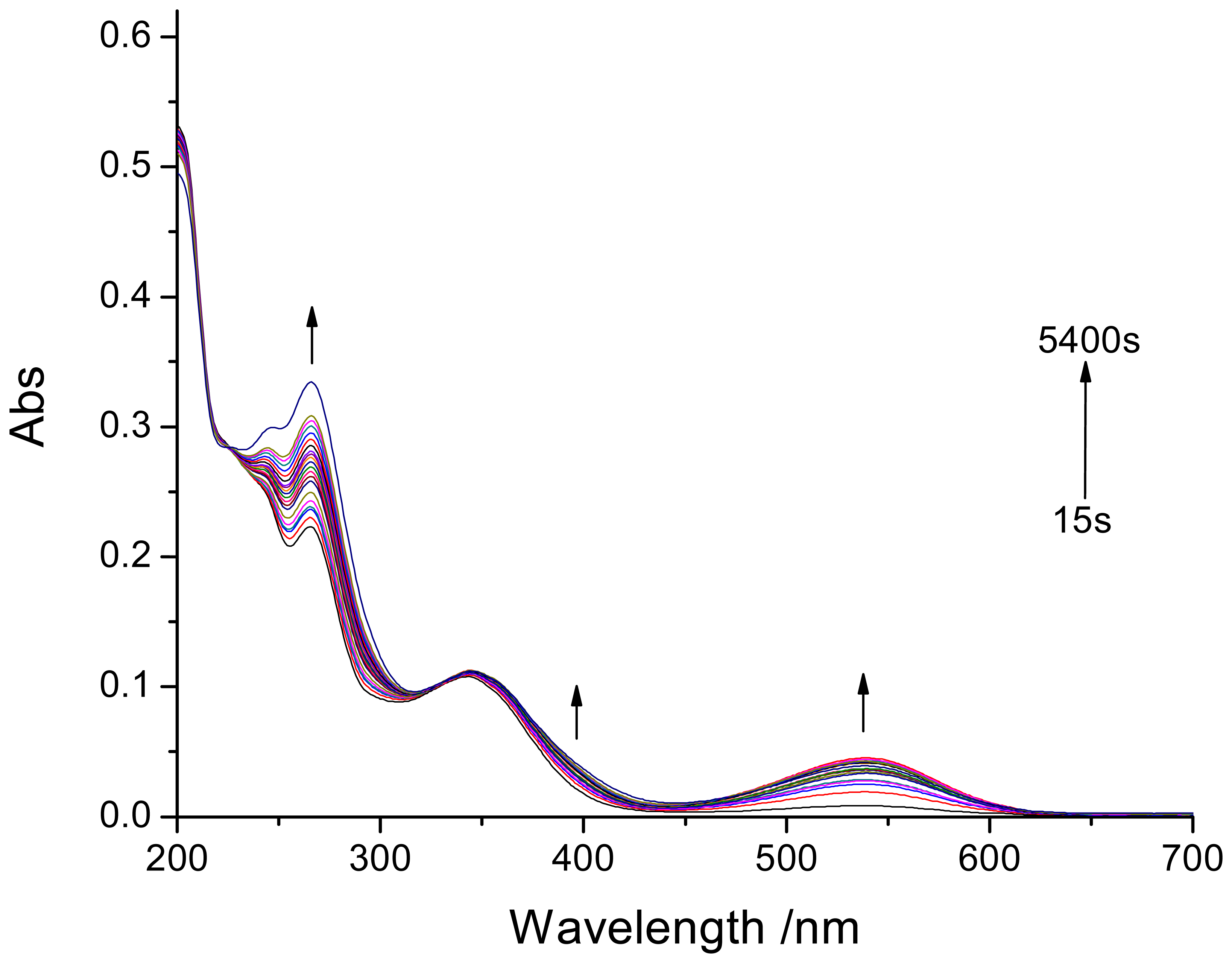
3.1. Photochromic characteristics
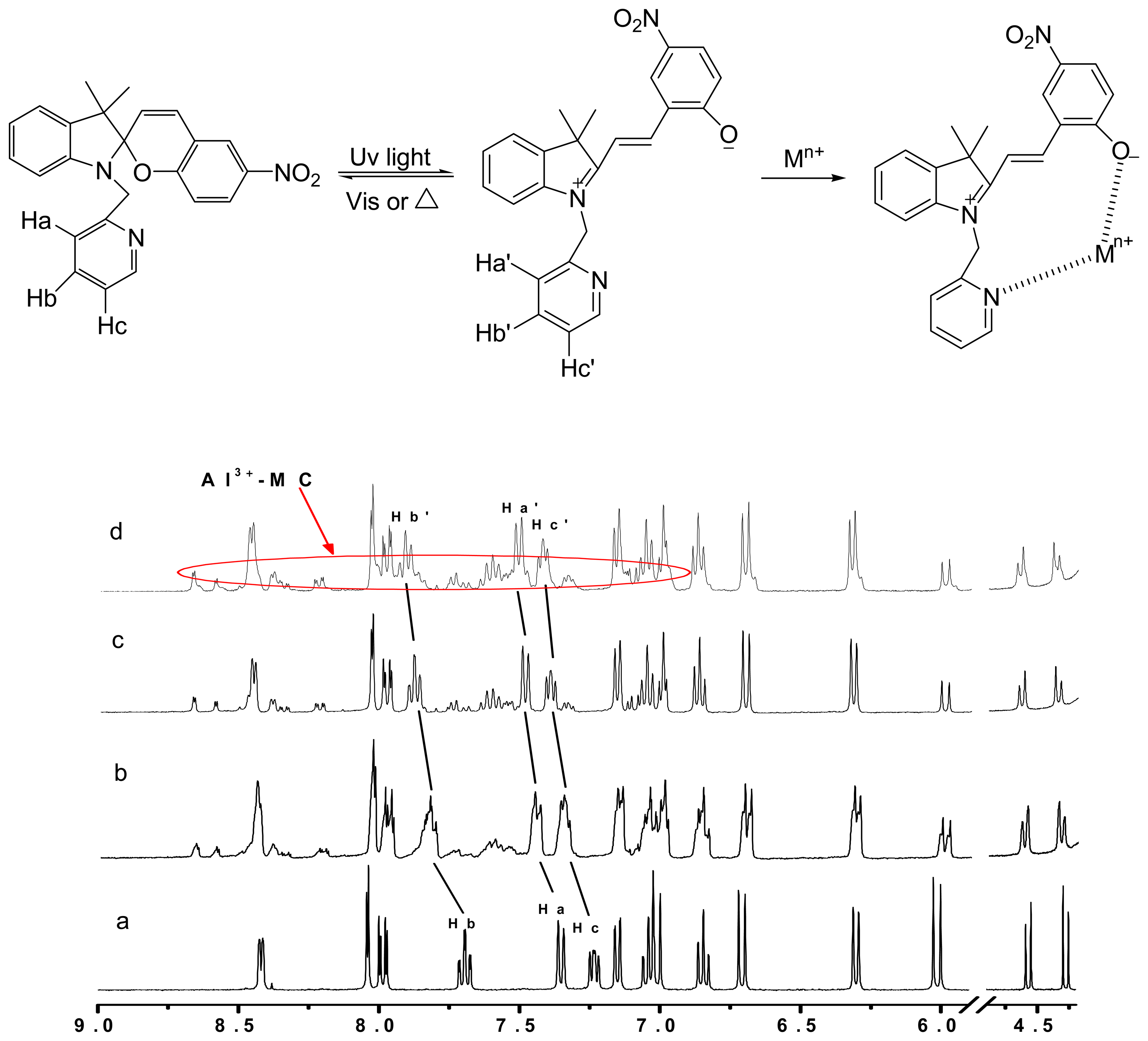
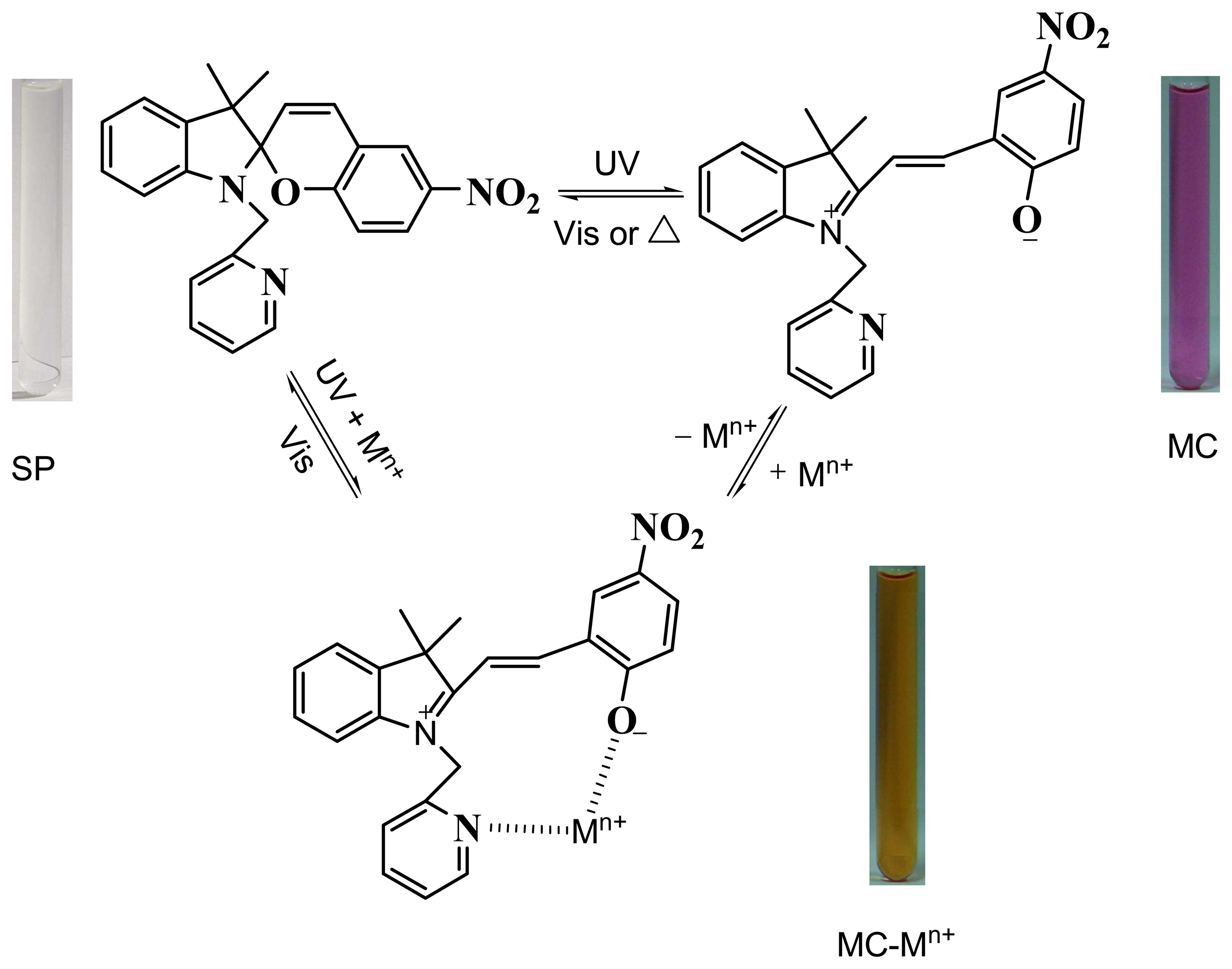
3.2. Recognition mechanism
3.3. Optimization of experimental parameters
3.4. Response time
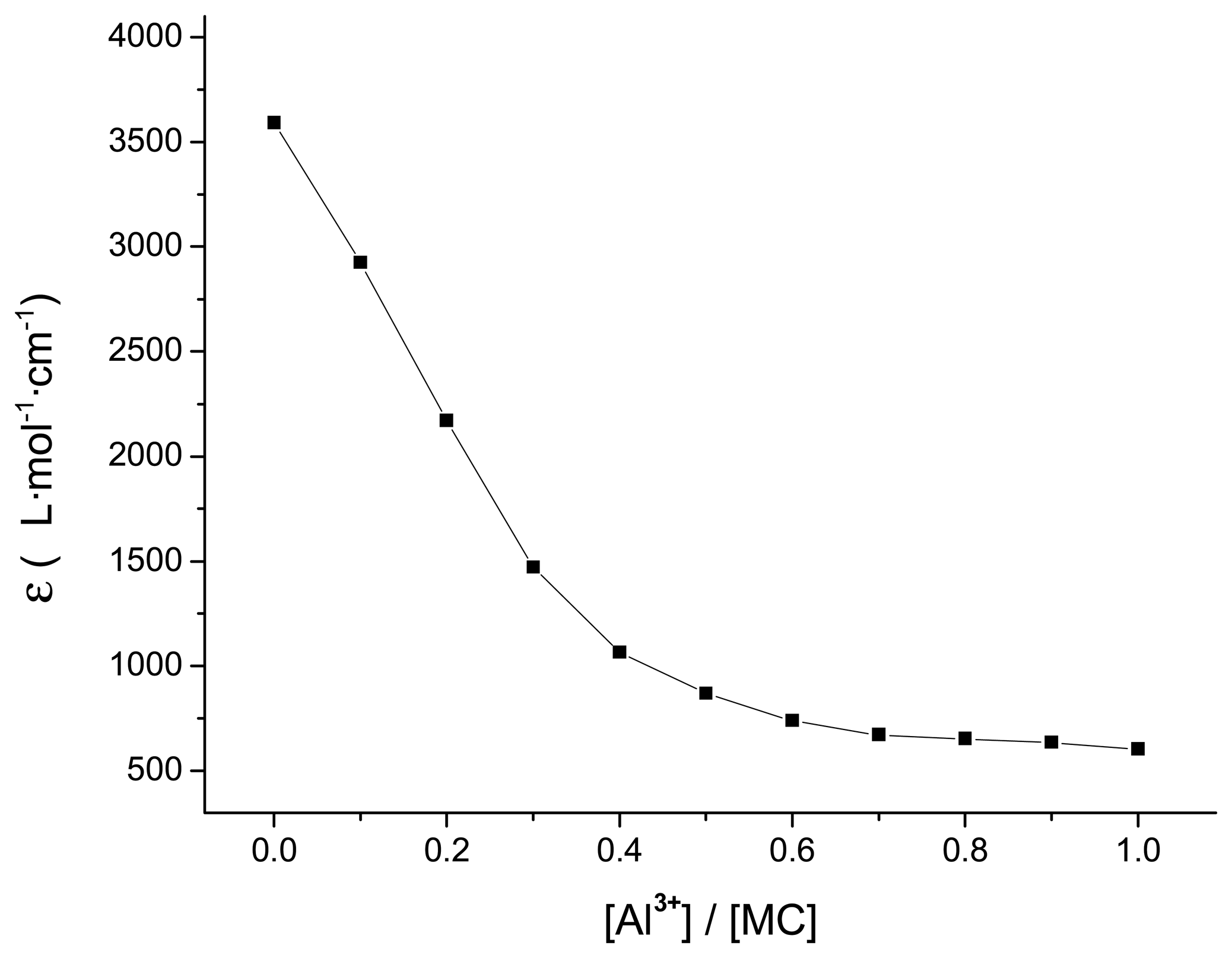
3.5. Detection limits
3.6. Reversibility and stability
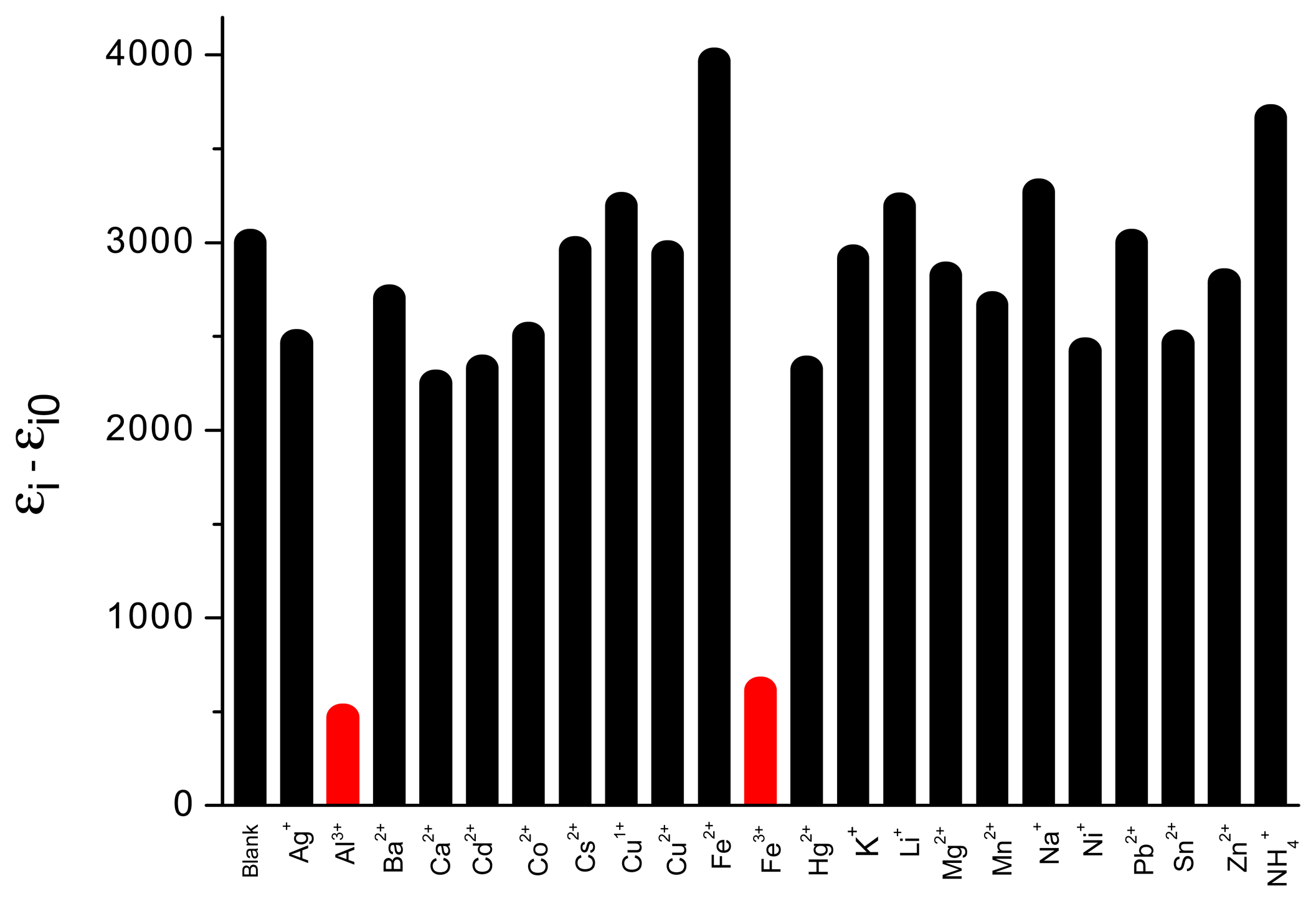
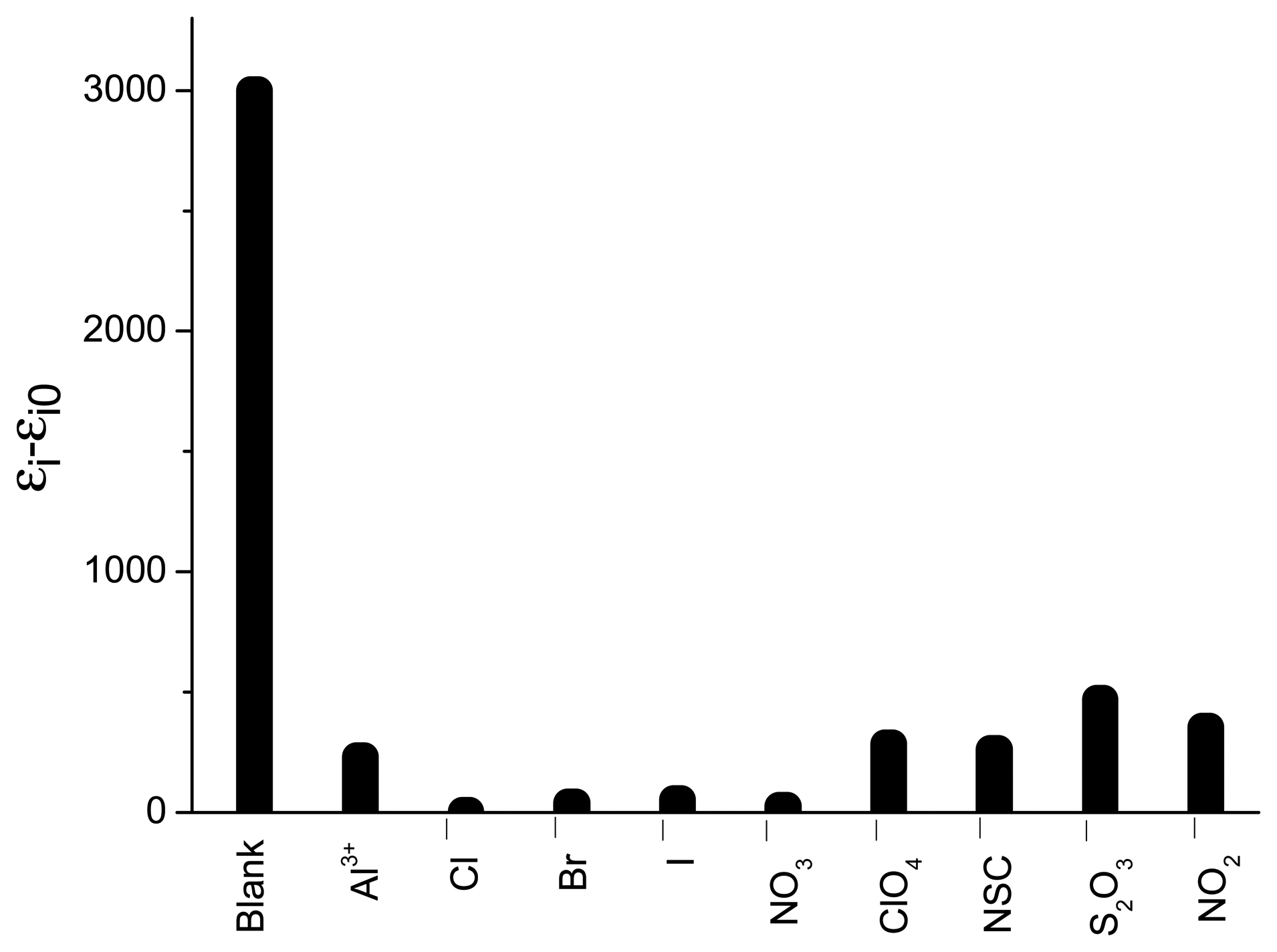
3.7. Selectivity
4. Conclusion
Supplementary data
Acknowledgments
References and Notes
- Gensemer, R.W.; Playle, R.C. The bioavailability and toxicity of aluminum in aquatic environments. Crit. Rev. Environ. Sci. Technol. 1999, 29, 315–450. [Google Scholar]
- Rondeau, V.; Commenges, D. Exley, C., Ed.; Aluminum and Alzheimer's Disease: The Science that Describes the Link; Elsevier Press: London, 2001; pp. p. 59–73. [Google Scholar]
- Flaten, T.P. Aluminium as a risk factor in Alzheimer's disease, with emphasis on drinking water. Brain Res. Bull. 2001, 55, 187–196. [Google Scholar]
- Lin, T.W.; Huang, S.D. Direct and simultaneous determination of copper, chromium, aluminum, and manganese in urine with a multielement graphite furnace atomic absorption spectrometer. Anal. Chem. 2001, 73, 4319–4325. [Google Scholar]
- Tangen, G.; Wickstrom, T.; Lierhagen, S.; Vogt, R.; Lund, W. Fractionation and determination of aluminum and iron in soil water samples using SPE cartridges and ICP-AES. Environ. Sci. Technol. 2002, 36, 5421–5425. [Google Scholar]
- Bocca, B.; Alimonti, A.; Petrucci, F.; Violante, N.; Sancesario, G.; Forte, G.; Senofonte, O. Quantification of trace elements by sector field inductively coupled plasma mass spectrometry in urine, serum, blood and cerebrospinal fluid of patients with Parkinson's disease. Spectrochimica Acta Part B. 2004, 59, 559–566. [Google Scholar]
- Measures, C.I.; Edmond, J.M. Shipboard determination of aluminum in seawater at the nanomolar level by electron capture detection gas chromatography. Anal. Chem. 1989, 61, 544–547. [Google Scholar]
- Hara, H.; Kobayashi, H.; Maeda, M.; Ueno, A.; Kobayashi, Y. Speciation of aluminum in rainwater using a fluoride ion-selective electrode and ion-exchange chromatography with fluorometric detection of the aluminum-lumogallion complex. Anal. Chem. 2001, 73, 5590–5595. [Google Scholar]
- Lian, H.; Kang, Y.; Bi, S.; Arkin, Y.; Shao, D.; Li, D.; Chen, Y.; Dai, L.; Gan, N.; Tian, L. Direct determination of trace aluminum with quercetin by reversed-phase high performance liquid chromatography. Talanta 2004, 62, 43–50. [Google Scholar]
- Yari, A.; Darvishi, L.; Shamsipur, M. Al(III)-selective electrode based on newly synthesized xanthone derivative as neutral ionophore. Anal. Chim. Acta. 2006, 555, 329–335. [Google Scholar]
- Di, J.; Bi, S.; Yang, T.; Zhang, M. Voltammetric determination of aluminum(III) using a reagentless sensor fabricated by sol–gel process. Sens. Actuators, B 2004, 99, 468–473. [Google Scholar]
- Gupta, V.K.; Jain, A.K.; Maheshwari, G. Aluminum(III) selective potentiometric sensor based on morin in poly(vinyl chloride) matrix. Talanta 2007, 72, 1469–1473. [Google Scholar]
- Carroll, M.K.; Bright, F.V.; Hieftje, G.M. Fiber-optic time-resolved fluorescence sensor for the simultaneous determination of Al3+ and Ga3+ or In3+. Anal. Chem. 1989, 61, 1768–1772. [Google Scholar]
- Zhang, J.; Xu, H.; Ren, J.L. Fluorimetric determination of dissolved aluminium in natural waters after liquid–liquid extraction into n-hexanol. Anal. Chim. Acta. 2000, 405, 31–42. [Google Scholar]
- Park, C.I.; Cha, K.W. Spectrofluorimetric method for determination of aluminium with chromotropic acid and its application to water samples. Talanta 2000, 51, 769–774. [Google Scholar]
- Tóth, I.V.; Rangel, A.O.S.S.; Santos, J. L. M.; Lima, J.L.F.C. Determination of aluminum(III) in crystallized fruit samples using a multicommutated flow system. J. Agric. Food Chem. 2004, 52, 2450–2454. [Google Scholar]
- Albendín, G.; Míánuel-Vez, M.P.; Moreno, C.; García-Vargas, M. Reverse flow-injection manifold for spectrofluorimetric determination of aluminum in drinking water. Talanta 2003, 60, 425–431. [Google Scholar]
- Szunerits, S.; Walt, D.R. Aluminum surface corrosion and the mechanism of inhibitors using pH and metal ion selective imaging fiber bundles. Anal. Chem. 2002, 74, 886–894. [Google Scholar]
- Joshi, B. P.; Cho, W.M.; Kim, J.; Yoon, J.; Lee, K.H. Design, synthesis, and evaluation of peptidyl fluorescent probe for Zn2+in aqueous solution. Bioorg. Med. Chem. Lett. 2007. [Google Scholar] [CrossRef]
- Ha-Thi, M.H.; Penhoat, M.; Michelet, V.; Leray, I. Highly selective and sensitive phosphane sulfide derivative for the detection of Hg2+ in an organoaqueous medium. Org. Lett. 2007, 9, 1133–1136. [Google Scholar]
- Zheng, Y.; Orbulescu, J.; Ji, X.; Andreopoulos, F.M.; Pham, S.M.; Leblanc, R.M. Development of fluorescent film sensors for the detection of divalent copper. J. Am. Chem. Soc. 2003, 125, 2680–2686. [Google Scholar]
- Collins, G.E.; Choi, L.S.; Ewing, K.J.; Michelet, V.; Bowen, C.M.; Winkler, J.D. Photoinduced switching of metal complexation by quinolinospiropyranindolines in polar solvents. Chem. Commun. 1999, 321–322. [Google Scholar]
- Winkler, J.D.; Bowen, C.M.; Michelet, V. Photodynamic fluorescent metal ion sensors with parts per billion sensitivity. J. Am. Chem. Soc. 1998, 120, 3237–3242. [Google Scholar]
- Berkovic, G.; Krongauz, V.; Weiss, V. Spiropyrans and spirooxazines for memories and switches. Chem. Rev. 2000, 100, 1741–1753. [Google Scholar]
- Kawata, S.; Kawata, Y. Three-dimensional optical data storage using photochromic materials. Chem. Rev. 2000, 100, 1777–1788. [Google Scholar]
- Shipway, A. N.; Willner, I. Electronically transduced molecular mechanical and information functions on surfaces. Acc. Chem. Res. 2001, 34, 421–432. [Google Scholar]
- Raymo, F.M.; Giordani, S. Signal processing at the molecular level. J. Am. Chem. Soc. 2001, 123, 4651–4652. [Google Scholar]
- Wojtyk, J.T.C.; Kazmaier, P.M.; Buncel, E. Modulation of the spiropyran-merocyanine reversion via metal-ion selective complexation: trapping of the “transient” cis-merocyanine. Chem. Mater. 2001, 13, 2547–2551. [Google Scholar]
- Raymo, F. M. Digital processing and communication with molecular switches. Adv. Mater. 2002, 14, 401–414. [Google Scholar]
- Inouye, M.; Akamatsu, K.; Nakzumi, H. New crown spirobenzopyrans as light- and ion-responsive dual-mode signal transducers. J. Am. Chem. Soc. 1997, 119, 9160–9165. [Google Scholar]
- Wojtyk, J.T.C.; Kazmaier, P.M.; Buncel, E. Effects of metal ion complexation on the spiropyran–merocyanine interconversion: development of a thermally stable photo-switch. Chem. Commun. 1998, 1703–1704. [Google Scholar]
- Andréasson, J.; Straight, S. D.; Kodis, G.; Park, C. D.; Hambourger, M.; Gervaldo, M.; Albinsson, B.; Moore, T. A.; Moore, A. L.; Gust, D. All-photonic molecular half-adder. J. Am. Chem. Soc. 2006, 128(50), 16259–16265. [Google Scholar]
- Guo, X.; Zhou, Y.; Zhang, D.; Yin, B.; Liu, Z.; Liu, C.; Lu, Z.; Huang, Y.; Zhu, D. 7-Trifluoromethylquinoline-functionalized luminescent photochromic spiropyran with the stable merocyanine species both in solution and in the solid state. J. Org. Chem. 2004, 69, 8924–8931. [Google Scholar]
- Guo, X.; Zhang, D.; Zhu, D. Logic control of the fluorescence of a new dyad, spiropyran-perylene diimide-spiropyran, with light, ferric ion, and proton: construction of a new three- input “AND” logic gate. Adv. Mater. 2004, 16, 125–130. [Google Scholar]
- Roxburgh, C. J.; Sammes, P.G. Synthesis of some new substituted photochromic N, N'-bis (spiro[1-benzopyran-2, 2′-indolyl]) diazacrown systems with substituent control over ion chelation. Eur. J. Org. Chem. 2006, 1050–1056. [Google Scholar]
- Shao, N.; Zhang, Y.; Cheung, S.; Yang, R.; Chan, W.; Mo, T.; Li, K.; Liu, F. Copper ion-selective fluorescent sensor based on the inner filter effect using a spiropyran derivative. Anal. Chem. 2005, 77, 7294–7303. [Google Scholar]
- Nakamura, M.; Fujioka, T.; Sakamoto, H.; Kimura, K. High stability constants for multivalent metal ion complexes of crown ether derivatives incorporating two spirobenzopyran moieties. New J. Chem. 2002, 26, 554–559. [Google Scholar]
- Yuan, W.; Sun, L.; Tang, H.; Wen, Y.; Jiang, G.; Huang, W.; Jiang, L.; Song, Y.; Tian, H.; Zhu, D. A novel thermally stable spironaphthoxazine and its application in rewritable high density optical data storage. Adv. Mater. 2005, 17, 156–160. [Google Scholar]
- Dürr, H. Perspectives in Photochromism: A novel system based on the 1, 5-electrocyclization of heteroanalogous pentadienyl anions. Angew. Chem., Int. Ed. Engl. 1989, 28, 413–431. [Google Scholar]
- Mousavi, M. F.; Arvand-Barmchi, M.; Zanjanchi, M. A. Al(III)–selective electrode based on furil as neutral carrier. Electroanalysis. 2001, 13, 1125–1128. [Google Scholar]

© 2007 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Ren, J.; Tian, H. Thermally Stable Merocyanine Form of Photochromic Spiropyran with Aluminum Ion as a Reversible Photo-driven Sensor in Aqueous Solution. Sensors 2007, 7, 3166-3178. https://doi.org/10.3390/s7123166
Ren J, Tian H. Thermally Stable Merocyanine Form of Photochromic Spiropyran with Aluminum Ion as a Reversible Photo-driven Sensor in Aqueous Solution. Sensors. 2007; 7(12):3166-3178. https://doi.org/10.3390/s7123166
Chicago/Turabian StyleRen, Jiaqiang, and He Tian. 2007. "Thermally Stable Merocyanine Form of Photochromic Spiropyran with Aluminum Ion as a Reversible Photo-driven Sensor in Aqueous Solution" Sensors 7, no. 12: 3166-3178. https://doi.org/10.3390/s7123166



