3.1. The general pattern of CO2 circulation in the container
In order to investigate the dynamic equilibration pattern of the enclosure system investigated in this study, a series of preliminary tests were conducted initially using CO
2-free air. As shown in
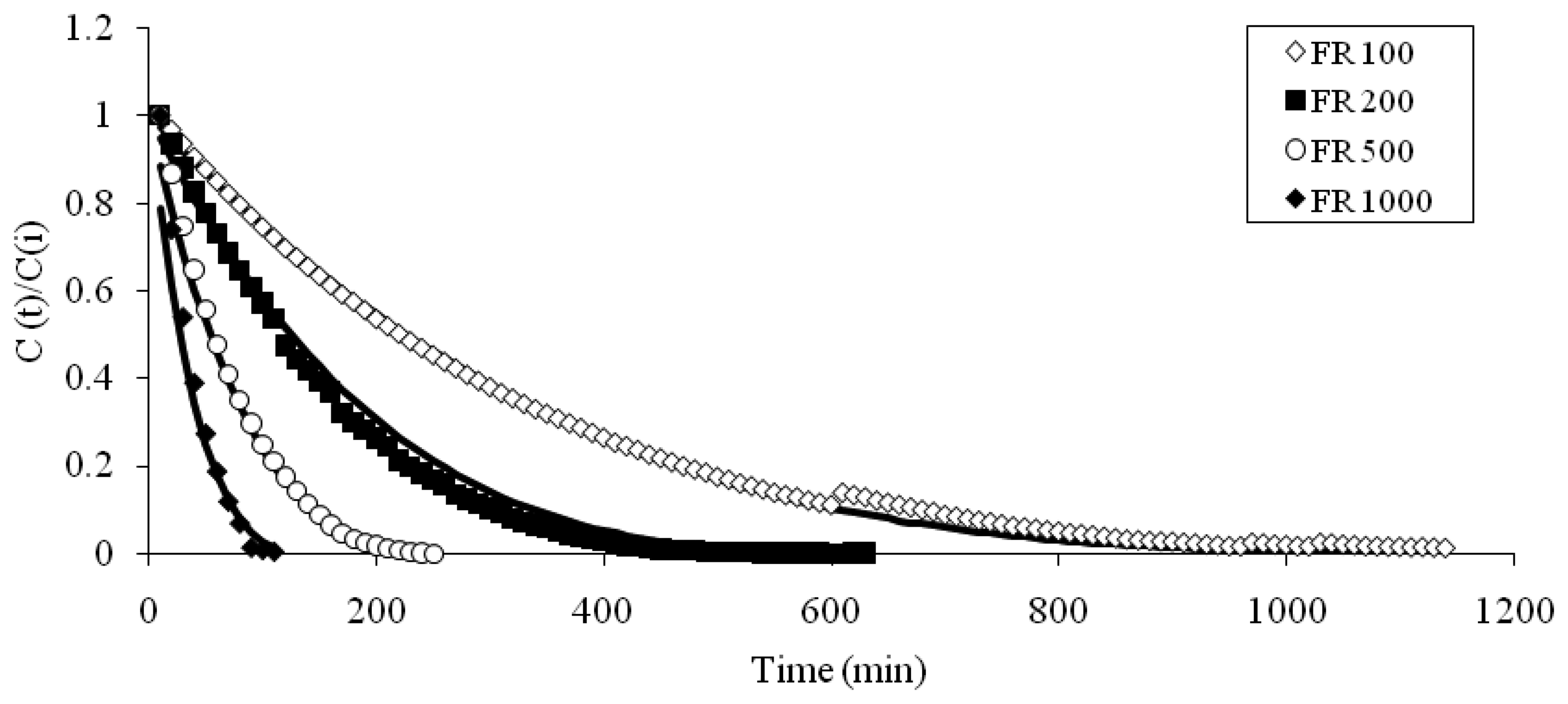
Table 1, the results of this test conducted at four different flow rates (i.e., 100, 200, 500, and 1000 mL min
-1) demonstrated that all the sensor units exhibited highly systematic patterns at each individual flow rate (
Fig 2). As shown in
Fig 2, log (CO
2) values tend to maintain strong inverse correlations with time. Hence, to learn more about the fundamental features of CO
2 circulation in an enclosure system, these experimental results were utilized to derive the empirical relationship between all the experimental variables.
In order to describe the exchange dynamics of CO
2 gas in a quantitative manner, the experimental data acquired from the CO
2-free gas were used to fit the non-linear equations via a trial and error. As a result, the
equation (1) was derived to equally consider the major parameters involved in this exchange process:
where [
1] the concentration of CO
2 expressed as the ratio between a given time (CO
2 (t)) and the initial time (CO
2 (i)) (unitless), [
2] flow rate (FR) of CO
2-free gas (mL min
-1), and [
3] time (t) for the equilibration (min).
From this equation, the equilibration time to reach the CO
2 concentration (of zero or any other concentration values) at a given flow rate (mL min
-1) can be computed by the following equation:
According to the above formula, the equilibration times for all four flow rates (100, 200, 500, and 1000 mL min
-1) are computed as 1283, 710, 303, and 155 minutes, respectively (
Table 2).
To evaluate the efficacy of these model-fit equations, a parallel comparison was made between observed and predicted values of CO
2 ratio (
Fig 3). As seen in the
Fig 3, the observed route of CO
2 equilibration in the enclosure system shows an excellent agreement with the predicted pattern at each of all flow rates.
3.2. The accuracy of NDIR-CO2 analysis
In an attempt to investigate the analytical bias arising from the application of the NDIR-system, the accuracy of the NDIR method was assessed using CO
2 standards prepared at the two concentration levels of 500 and 1000 ppm. Based on our initial equilibration experiments using CO
2-free air, all experiments representing CO
2 levels of 500 and 1000 ppm were conducted at a moderate flow rate of 500 mL min
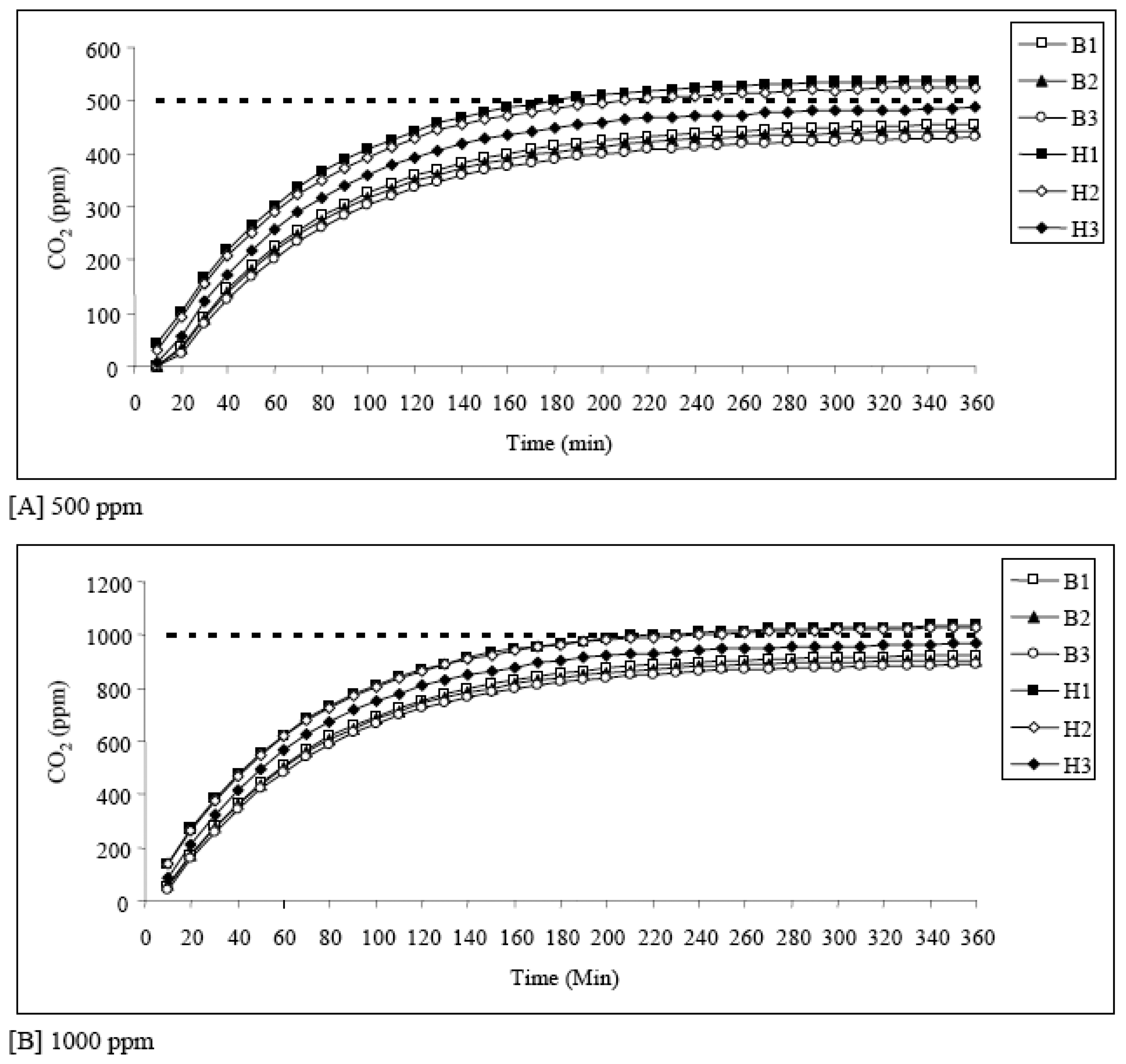
-1. As shown in
Fig 4, the concentration of CO
2 increased systematically with time to reach their respective equilibration concentrations (i.e., near 500 and 1000 ppm) after the equilibration point (approx. 300 min). The observed pattern suggests that the NDIR sensors behave systematically to detect the changes in the CO
2 concentrations. As summarized in
Table 2A, the results taken from the 500 ppm CO
2 standard gas showed an average CO
2 concentration of 477 ± 44.9 ppm with a range of 427-536 ppm after the equilibration time (using the data taken between 300 and 360 min). Likewise, the experimental data for the 1000 ppm CO
2 standard, taken for the similar duration, averaged 954 ± 62.7 ppm with a range of 885-1031 ppm.
The CO
2 data obtained from these comparative experiments were used to compute the percent deviation (PD) values of each sensor unit. The PD values were calculated by subtracting the CO
2 concentration value for a given unit by the known value of CO
2 for the respective gaseous standard:
where CO
2(obs) is the mean CO
2 value observed by all sensor units, and CO
2(known) is the actual CO
2 concentration of standard (i.e., 500 and 1000 ppm). The PD values obtained using the CO
2 values in the equilibrium stage (during the 300 to 360 min of the study duration) are summarized in
Table 2.
As shown in
Table 2B, the sensor units of the model type B-530 showed a negative bias for the 500 ppm CO
2 measurement in terms of their PD values (ranging from -14.6 % (B3) to -9.39 % (B1)). In contrast, H-500 units generally exhibited a positive bias, with PD values of +7.30 (H1) and +4.83% (H2) (with the exception of the H3 unit). The results of 1000 ppm experiment in fact showed patterns that are highly analogous to the 500 ppm experiment.
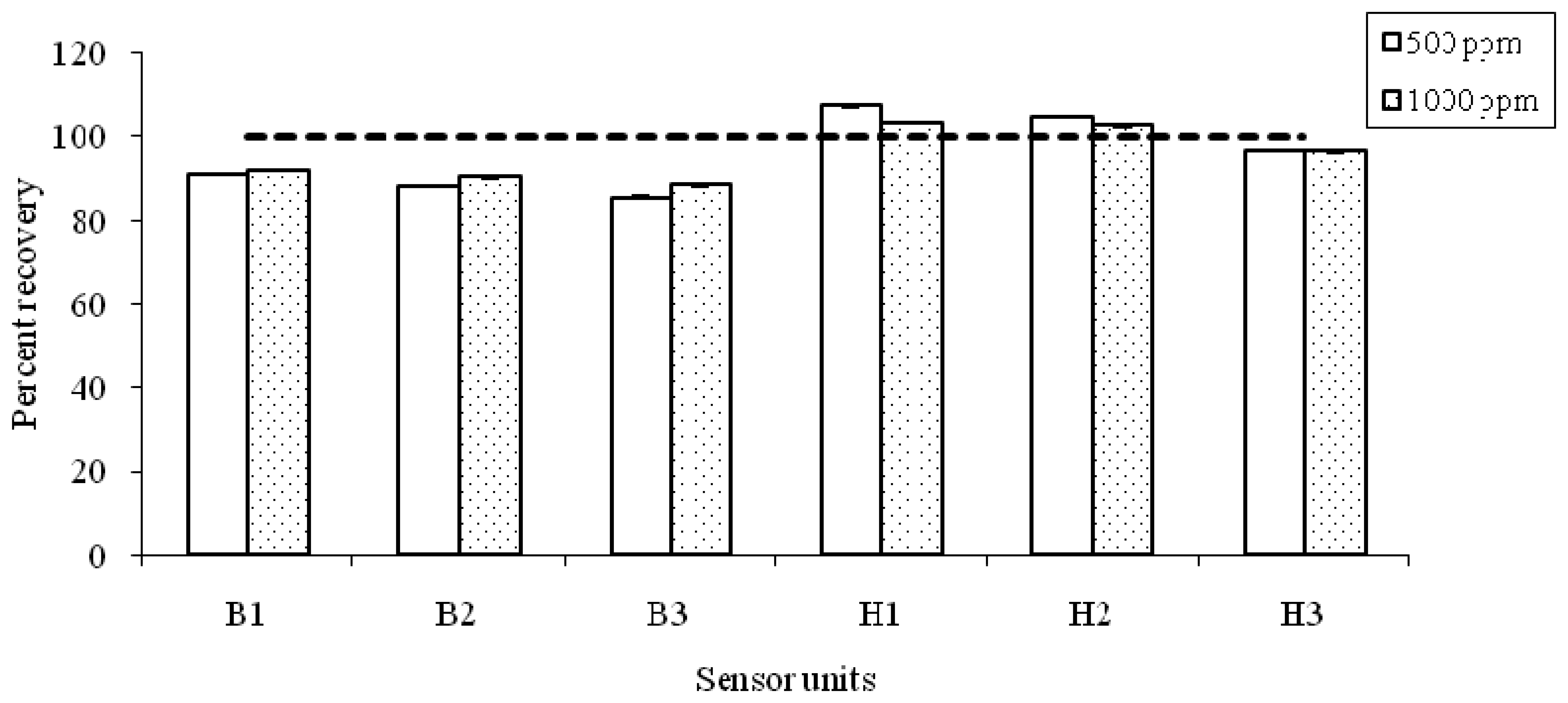
As another means to assess the analytical bias of NDIR sensor units, the percent recovery (PR) rates of each sensor unit were computed and plotted for CO
2 standards at both concentration levels (500 and 1000 ppm) (
Fig 5). The PR is calculated by dividing the mean CO
2 concentration (after the equilibration time) with the known standard concentration (i.e., after approx. 300 min onwards for 60 min; refer to
Table 2 for details). The PR value of the 500 ppm CO
2 standard averaged 95.4 ± 8.95 % with a range of about. 86 to 107 %. Similarly, the PR values for the 1000 ppm test averaged 95.4 ± 6.27 % with a range of about 88 to 103 %. As such, the accuracy of NDIR sensor investigated in this study seems to approach the 5% level, if the accuracy is expressed as the difference between the unity and the recovery ratio. Although the absolute accuracy of the NDIR-based CO
2 sensors seemed to improve slightly at the 1000 ppm level, their variabilities do not appear to be significant with increasing concentration levels. The results of the present study thus indicate that the performance of the NDIR method is fairly stable and reproducible in the tested concentration ranges in terms of its precision and accuracy.
To evaluate the compatibility of each sensor unit in the CO
2 measurement, a correlation analysis was performed using the CO
2 concentration data acquired by all sensor units at each flow rate. The results of the correlation analyses for three different concentration levels (i.e., 0, 500, and 1000 ppm) are summarized in
Table 3. As shown in
Table 3, all the sensor units showed highly significant correlations with each other in terms of their correlation strengths (i.e., R
2 > 0.99, p = 0.01). Although the correlation results of the CO
2-free air were the strongest of all, the results of high CO
2 levels (500 or 1000 ppm) were also indistinguishable in that respect; all sensor units showed correlation coefficients approaching the unity (> 0.999), irrespective of the model types. Hence, as seen in our earlier study based on side-by-side monitoring of ambient CO
2 levels under the laboratory conditions [
8], we were able to obtain highly comparable patterns between different sensor units. The findings of relatively enhanced compatibility between different sensor units or with CO
2 standard gases may be ascribable to the use of a stable equilibrium system developed for the comparative test.
3.3 Comparison between different detection techniques
As a simple means to assess the analytical reliability of our sensor method in the CO
2 analysis, the previous records of diverse measurement techniques involved in CO
2 analysis were explored in terms of accuracy and precision (
Table 4). According to this analysis, it is found that many scientists relied on the Gas chromatographic (GC) method for the CO
2 analysis. For instance, Ekeberg et al (2004) attempted to quantify the precision and accuracy of certified CO
2 standards by GC with mass spectrometry (GC-MS) [
9]. The results of their GC-MS analysis revealed accuracy near ± 4% for a 977 μL/L certified CO
2 standard; their results appear to be highly comparable to the overall mean accuracy observed in this study (i.e., 4.60%), which was in fact determined from a comparable CO
2 concentration range (i.e., 1000 ppm) using all six sensor units (
Table 2B). It is also found that the overall mean precision of the repetitive analysis made by GC-MS (2.30%) is highly comparable with those derived by our sensor method (2.68%). In comparison, the GC-TCD method for CO
2 analysis has shown an accuracy of ± 5.3% in the range of 2270-10000 ppm [
10]. The precision of the GC-TCD and GC-FIA techniques was also estimated by the repetitive measurement of CO
2 at a constant concentration level (approx. 340 ppm), and the results were found to be 6.20% and 5.98%, respectively [
11].
In a continuous-flow isotope ratio mass spectrometric (CF-IRMS) analysis of CO
2, the accuracy was estimated in the 0.18-0.38 range as differences between the means of two systems, i.e., dual-inlet isotope ratio mass spectrometry/nondispersive infrared gas analysis system (DI-IRMS/NDIR) and automated system of CF-IRMS in the analysis of five CO
2 test gases covering a concentration range of 328 to 603 μmol mol
-1 [
12]. The overall precision of CF-IRMS was estimated at 0.04% for the five CO
2 test gases covering a concentration range of 328 to 603 μmol mol
-1 [
12]. A comparative study of a CO
2 analysis (in the 800-1000 ppm range), conducted between three brands of detector tubes (Draeger CH 30801, Kitagawa 126, and Gastec 2LL), yielded relative standard error values in the 5-7% range [
13]. In comparison, the relative error in the CO
2 measurement was reported at -3.0% by the continuous-flow method using a conductimetric detector [
14].
Considering the performance of various measurement techniques introduced in the previous studies, the NDIR method investigated in the present study appears to be fairly reliable and capable of the near real-time analysis of CO2 in terms of its accuracy and precision. In view of the fact that other techniques (such as GC-TCD, GC-MS, and CF-IRMS, etc.) should consider the delicate arrangement for instrumental management (complicated operating procedures, high cost, duration for the data acquisition, etc), the simple, economic NDIR sensor technique can be comparatively advantageous from a number of aspects (e.g., affordability, handling, and rapid measurement time).