1. Introduction
The sensitive detection of heavy and transition- metal ions, such as mercury is currently a task of prime importance for environmental or biological applications. Mercury, one of the most toxic elements in the world, represents a major toxicity to microorganism and environment even in low concentration. Inorganic mercury has been reported to produce harmful effects at 5 μg/l in a culture medium.
1 Once introduced into the marine environment, microorganisms convert it into methylmercury, a form of mercury being even more toxic to aquatic organisms and birds than the inorganic mercury, which eventually reaches the top of the food chain and accumulates in higher organisms, especially in large edible fish.
2 When consumed by humans, methylmercury triggers several serious disorders including sensory, motor and neurological damage.
3 The development of methods for the determination mercury is, therefore, of significant importance for environment and human health. In the last decade researchers have done a big effort to develop new mercury sensors. An important number of selective Hg
2+ chemosensors have been devised using redox,
4 chromogenic,
5 or fluorogenic
6 changes in organic media as detection channels. However, Hg
2+ cations are relatively easy to be chelated and detected in organic solvents, they are rather difficult to be recognized directly in aqueous environments due to their strong hydrations. Consequently, developing simple and practical Hg
2+ chemosensors in aqueous media is still a challenge.
7 Although instrumental analyses such as atomic absorption or atomic emission spectroscopy are currently used in the detection of metal ions, there is still a need to develop novel methods for the detection of toxic ions that offer high sensitivity, short response times, and high selectivity.
8 Among all these sensing approaches, the optical detectors that allow on-site, real-time qualitative or semiquantitative detection without the use of any complex spectroscopic instrumentation have received a great deal of attention.
9 Even though there are several molecules detecting mercury ions, only a few of them are available to be anchored or firmly physisorbed to a solid substrate and most of them have important short-comings for real applications, such as a lack of selectivity and high price. Firm fixation on solid substrates of colorimetric or fluorimetric reagents for heavy metal detection has been attempted by several techniques: polymer-bound chemosensors,
10 incorporation into PVC-based liquid membranes, covalent anchoring with cross-linked copolymers, layer-by-layer accumulation methods such as Langmuir-Blodgett films or alternate deposition of oppositely charged polyelectrolytes.
11 However, they have some drawbacks including complicated synthetic procedures, insufficient sensitivity and requirements for auxiliary additives.
Cellulose is an abundant, inexpensive, biodegradable and renewable biopolymer exhibiting very good mechanical properties and is also water-absorbing. Cellulose can be modified to alter and tailor its chemical and physical properties.
12 The use of cellulose-based materials could thus be extended to new applications by the incorporation of functional detecting molecules onto its fiber surface. In this context, paper strips have recently been used in biomedical assays,
13 and for heavy-metal ions detection,
14 although with low selectivity towards mercury ions. We present here a new cellulose based colorimetric mercury indicator using a simple procedure that allow its use for on-site selective mercury detection in mixed water environments which is one of the major drawbacks of most of the previously reported mercury chemosensors.
2. Results and Discussion
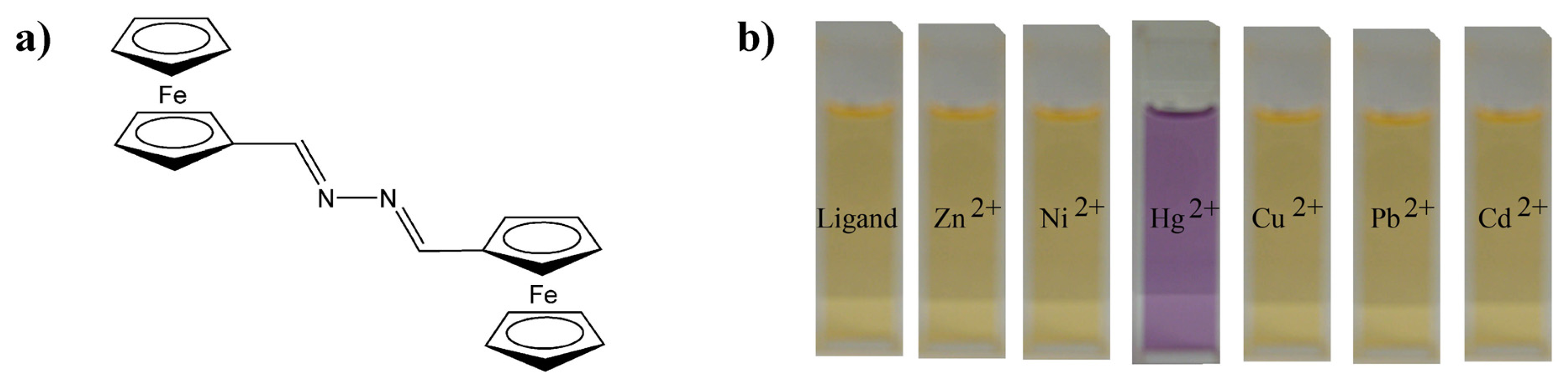
Recently we have reported a highly selective chromogenic mercury detector based on the 1,4-disubstituted azine bearing two end-capped ferrocene groups
1 (
Figure 1). The optical activity of this molecule suffers from important changes when complexed with mercury ions enabling an optical-based mercury detector. A preliminary study
4b in CH
3CN/H
2O mixtures toward Hg
2+ ions have been previously reported showing no interaction with other common cations, such as Mg
2+, Ca
2+, Ni
2+, Zn
2+, Cd
2+, Pb
2+, and Cu
2+ (
Figure 1). Remarkable is this molecular detector since it suffers an important colour change in sensing which can be used for a naked-eye detection of Hg
2+ ions in solution. However, this application is limited to mixtures of polar organic solvents with water because of the poor solubility of
1 in pure water.
To confirm the potential application of this ligand for detecting Hg
2+metal ions on a solid support, cellulose based probes were prepared in two steps. Firstly the cellulose disk was introduced into a mercury ion solution, using either an acetonitrile/water mixture (7:3) or pure acetonitrile, and after drying it introduced into the solution of the bis(ferrocene)azine
1 sensor. For the initial impregnation of the paper disks with the mercury ions it is also possible to use only water for the deposition of Hg(II) on the cellulose but then the sensitivity decreases considerably due to the poor solubility of the bis(ferrocene) azine in the stagnant water inside the cellulose fibers giving a poor homogeneity of the colour change. It is worth mentioning that the reverse procedure for the impregnation process, consisting firstly in soaking the cellulose with an organic solution of the detecting molecule and then with mercury ion solutions, does not provide positive results. This may be attributed to the better interaction of the mercury ions with the cellulose. In fact, it has been recently found that the cellulose is, on average, negatively charged,
15 so the interaction between the mercury cation and the surface is driven by very strong electroatractive forces that may change with the media.
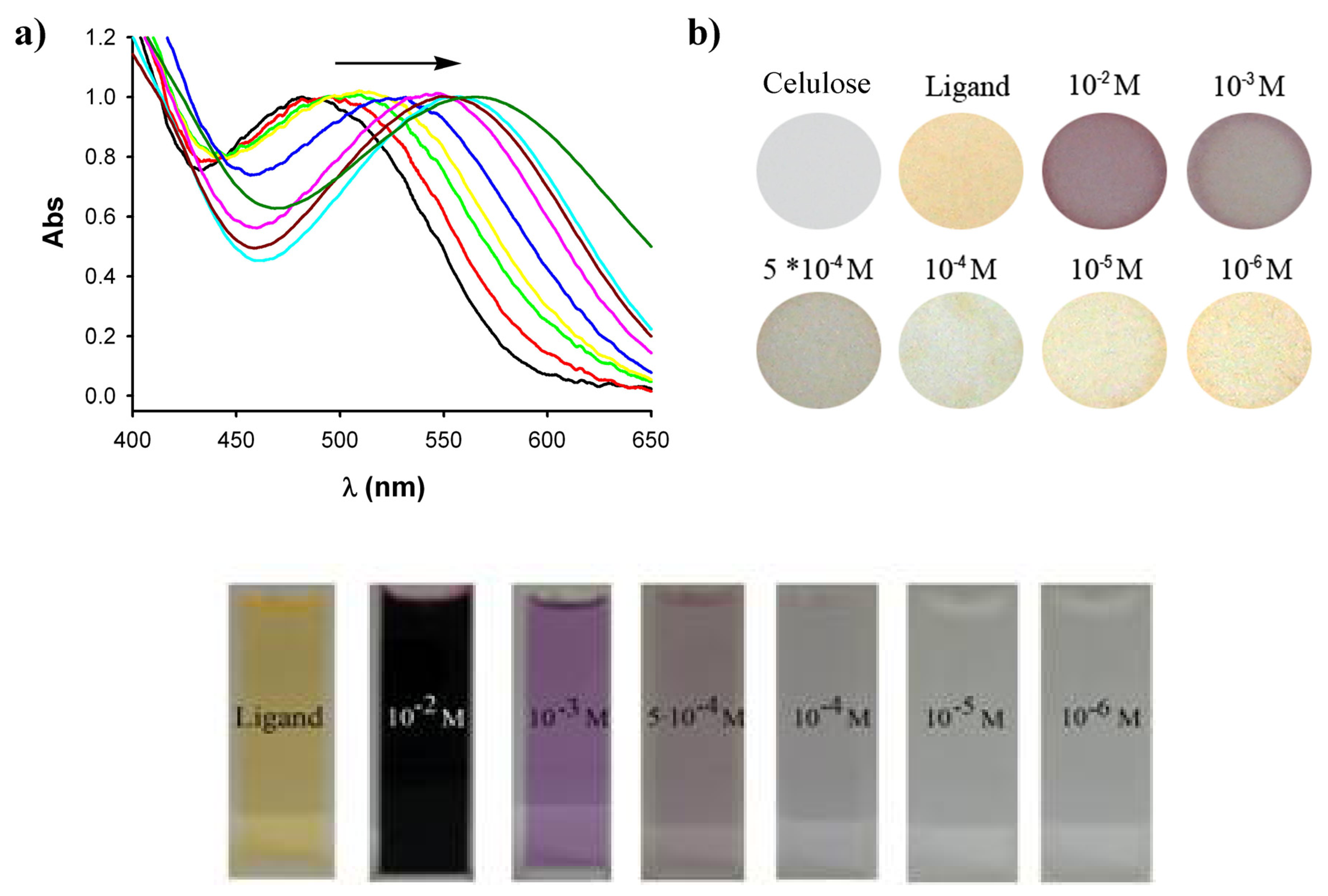
The quantitative detection measurements of the cellulose probes were made using an UV-Vis spectrophotometer with an integrating sphere expansion pack, which allowed us to determine the absorption spectra of our solid substrates by light reflectance. An untreated cellulose paper was set as reference sample during the measurements. The measurements were performed from 400 to 700 nm. The resulting spectra were normalized showing that a neat interconversion between the uncomplexed and the mercury complexed species derived from 1 occurs on the surface of the indicator by the increasing amount of mercury ions present in the analyzed solutions.
The increasing concentration of mercury ions in the tested solution caused the apparent shift of the band initially appearing at 485 nm and shifted up to 565 nm (
Figure 2). The band at 485 nm corresponds to the UV-Vis absorption spectrum of the cellulose indicator paper coated only with the free ligand
1 while the band at 565 nm corresponds to the UV-Vis absorption spectrum of the completely complexed ligand
1·Hg
2+. For the intermediate mercury concentrations, when the free ligand
1 deposited on the paper is not completely complexed, there is the simultaneous presence of both bands at 485 nm and 565 nm with different intensities depending on the amount of mercury ions in the tested solution. The large bathochromic shift of 80 nm observed for this band is responsible of the spectacular color change from orange (neutral azine
1) to deep purple (complexed azine
1·Hg
2+) suffered by the cellulose indicator paper (
Figure 2). This impressive color change as well as the high reproducibility and the large persistence (days) of the coloration of the cellulose probes permit a “naked-eye” detection of Hg
2+ ions similar to the well known
pH paper indicator, as shown in
Figure 2. Taking into account that the colour of
1·Hg
2+ complex in solution is not very persistent, since it is less intensive and disappears after about 20 min, these results clearly demonstrate the higher stability and sensitivity of the complex in solid state than in solution (
Figure 2). The spectra obtained in
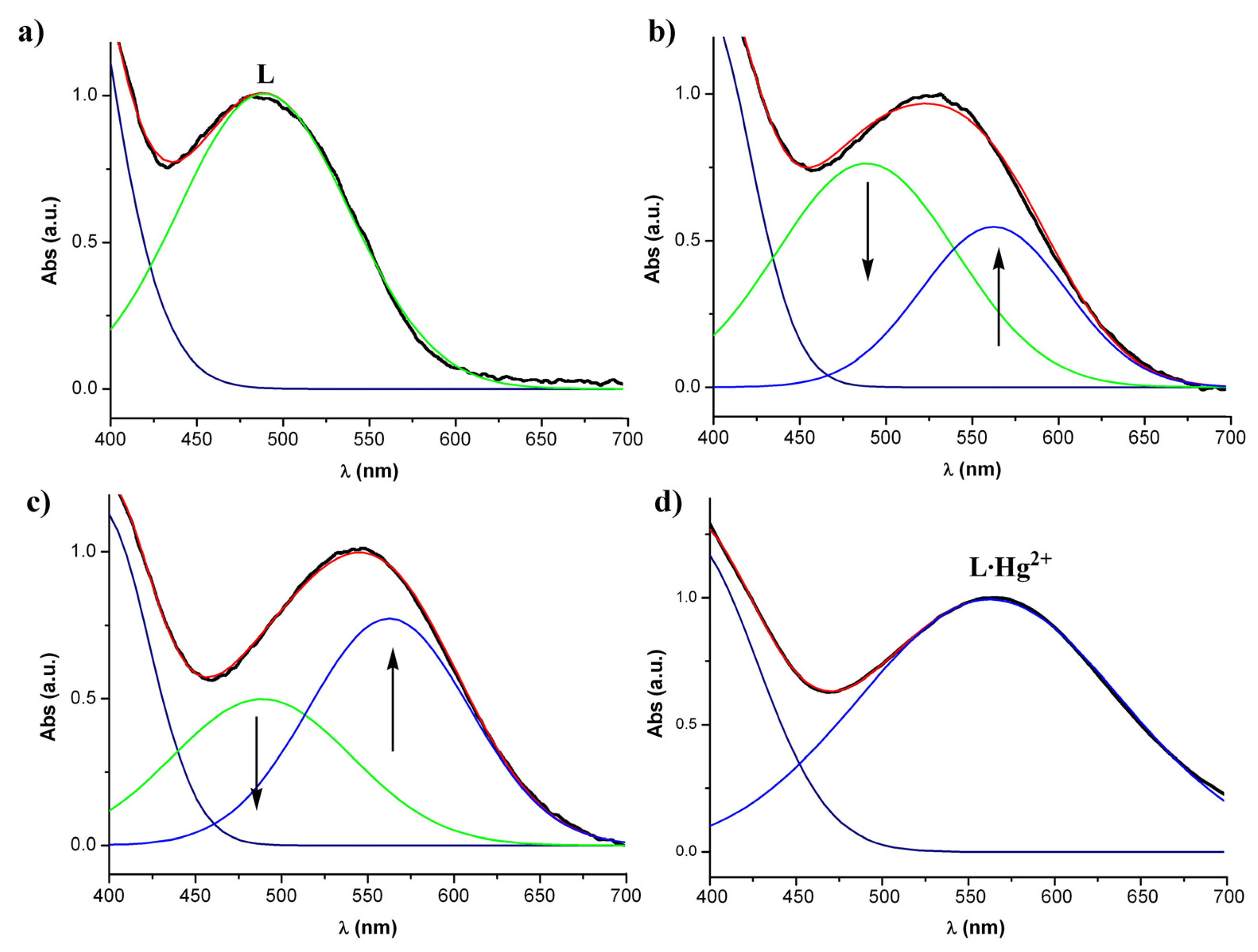
Figure 3 can be quantified by their deconvolutions into two different Gaussian bands (
Figure 3); one at 485 nm that corresponds to the absorption maximum for the free ligand
1 and another band corresponding to the mercury complex
1·Hg
2+ at 565 nm.
With this treatment a perfect image over the decreasing of the free ligand band and the increasing of the
1·Hg
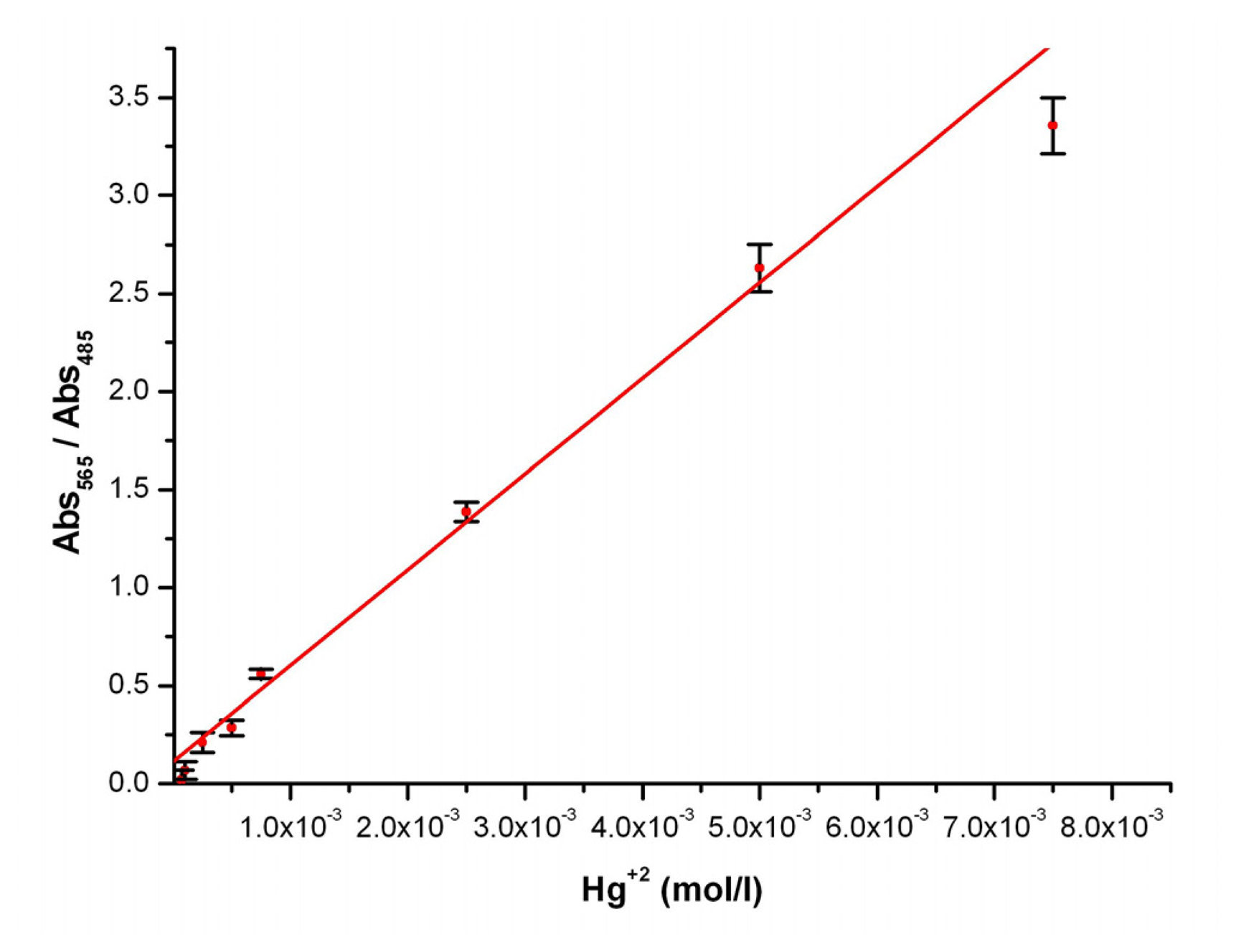
2+complex band upon increasing amounts of mercury ions is observed. Thus, measuring the area below the complex curve at each mercury concentration it has been possible to calibrate the cellulose-based optical indicator (
Figure 4) with a high reproducibility. The detection limit of the presented mercury indicator papers is calculated by interpolation from the graph reaching a concentration limit down to tens of ppm.
Further experiments with other divalent cations, like Mg2+, Ca2+, Ni2+, Zn2+, Cd2+, Pb2+, and Cu2+, in CH3CN/H2O mixtures have also been performed showing a lack of any optical change of the indicator papers. This result demonstrates a high selectivity toward mercury ions of the reported cellulose-based optical indicator.
In summary, the developing of a new selective and highly sensitive heterogeneous indicator of mercury ions in acetonitrile-water solutions by the combined use of a cellulose substrate and a molecule with excellent detecting characteristics is presented. Depending on the amount of mercury ions in contact with the cellulose indicator papers a gradual color change is produced, being possible to determine by naked eye its concentration, with a detection limit of tens of ppm. Additionally, and no less important, this protocol takes advantage of surpassing the drawback of the low solubility in water of most of the reported Hg2+ chemosensors, allowing them to be used for detecting Hg2+ cations in water solutions. We are now working in order to decrease the detection limit down to ppb which is the limit acceptable for the EPA (United States Environmental Protection Agency) in potable water.
3. Experimental Section
The probes were fabricated with ALBET, 142 mm Ø, pore size 0.20μm regenerated cellulose mixted ester paper. 1H and 13C NMR spectra were recorded at 400 MHz on a Brucker AC400 spectrometer. The EI mass spectrum was recorded on a Fisons AUTOSPEC 500 VG spectrometer. Microanalyses was performed on a Carlo Erba 1108 instrument. The UV-Vis measurements were followed using a Shimadzu UV-2101 PC equipped with a reflectance absorption accessory (integrating sphere) and a Hitachi S-570 scanning electron microscope was used to perform SEM imaging of the samples. The camera used to take the pictures of the membranes was a Canon Power Shot A530 and Microsoft Paint software was used as a imaging treatment program.
3.1. Preparation of 1,4-diferrocenyl-2,3-diaza-1,3-butadiene
To a solution of formylferrocene (0.250 g, 1.17 mmol) in ethanol (50 ml) hydrazine hydrate (28.3 ml, 0. 585 mmol) was dropped and the resulting solution was stirred at room temperature for 10 h. After cooling, the precipitated solid was filtered, air-dried and recrystallized from ethanol to give 1 as orange crystals in 70% yield. M. p. > 260 °C (decomposes). 1H NMR (CDCl3): δ= 4.24 (10 H, s), 4.45 ( 4 H, st), 4.70 ( 4 H, st), 8.48 ( 2 H, s). 13C NMR (CDCl3): δ 68.6 (4xCH, Fc), 69.3 (10xCH, Fc), 70.9 (4xCH, Fc), 78.0 (2xq, Fc), 161.2 (2xCH=N). EIMS, m/z (%): 424 (M+, 67), 211 (74), 184 (55), 121 (100). Anal Calc for C22H20Fe2N2: C, 62.31; H, 4.75; N, 6.61. Found: C, 62.55; H, 4.51; N, 6.88.
3.2. Preparation of the mercury ion indicator paper
Regenerated cellulose mixed ester paper with 0,2 μm pore size have been chosen as our fiber substrate due to its high surface porosity which gives a high surface coverage area to interact with the sensing molecule 1, for the preparation of a mercury ion indicator paper. Preparation of the probes was carried out in several steps. Firstly the 40mm Ø disks of paper were introduced for 20s into acetonitrile solutions of different concentrations of Hg(ClO4)2 (from 10-6M to 10-2M). Then the paper was left to air dry for 40s and once dried, carefully introduced for 2s and immediately removed from a toluene solution of 1 (10-3 M) and then homogeneously dried. Indeed, it is also possible to use acetonitrile/water mixtures (7:3, v/v) of Hg(ClO4)2 which is worth mentioning due to the fact that the possibility to use water environments for selective Hg+2 metal ion detection is one of the major drawbacks of most of the reported chemosensors.