Optical Fiber Sensing Using Quantum Dots
Abstract
:1. Introduction
2. Quantum dots: a brief overview
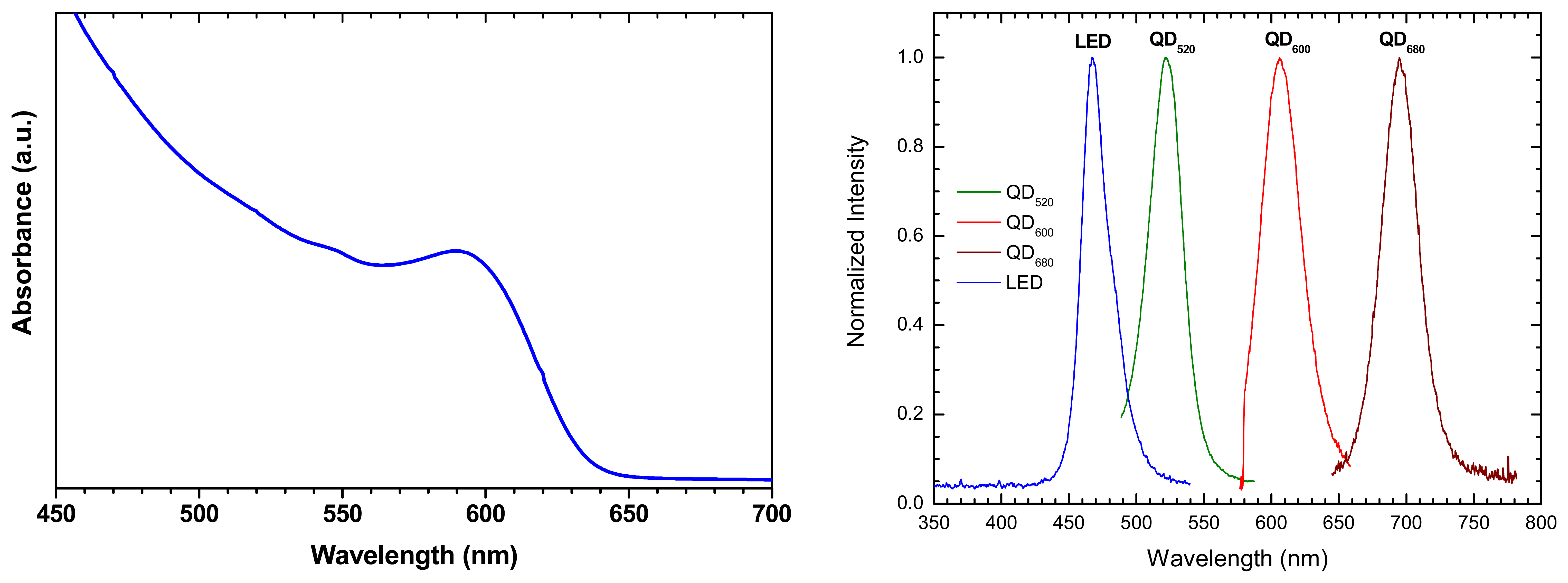
2.1. Quantum confinement and optical properties
2.1. Chemical synthesis
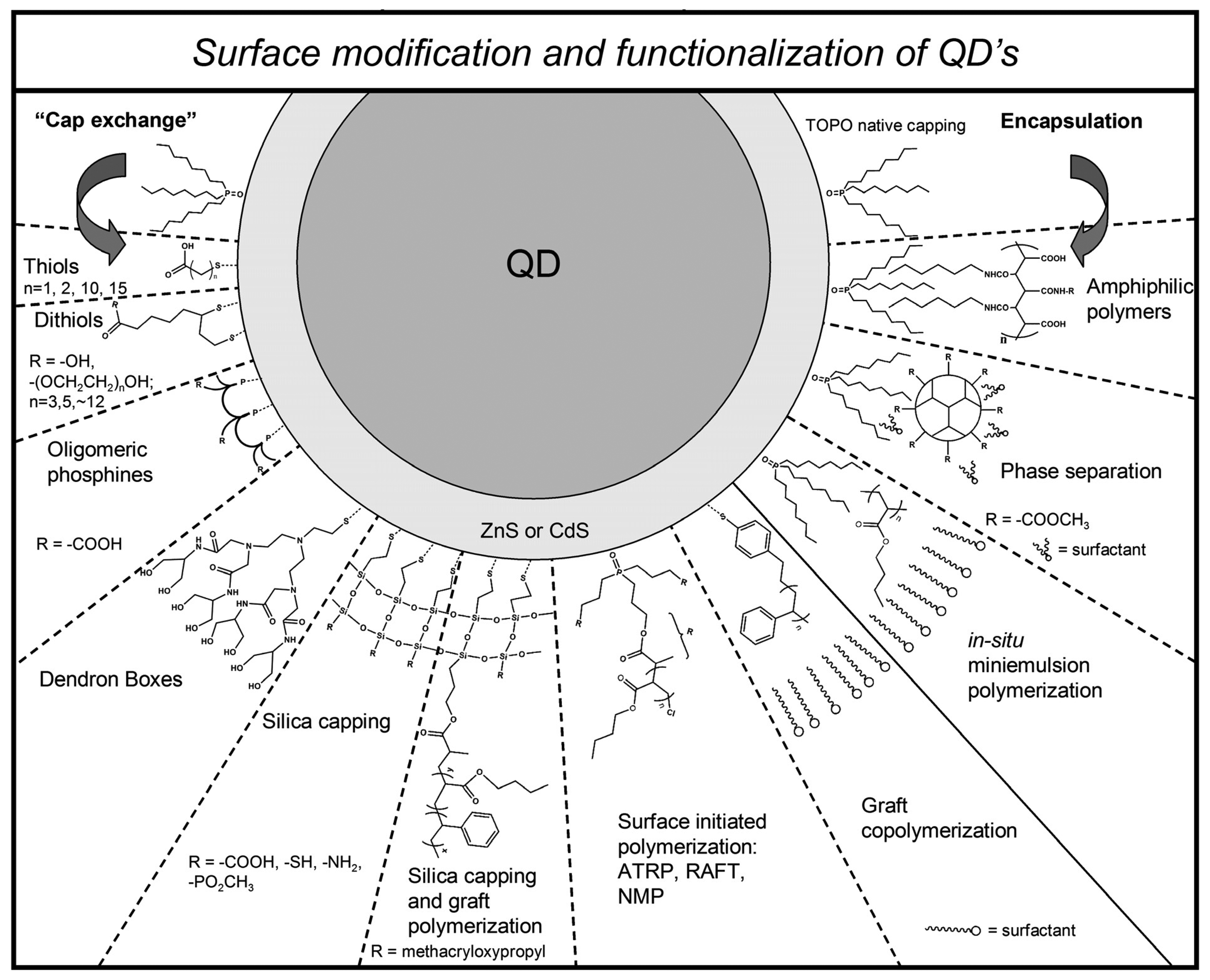
2.2 Surface modification and functionalization
3. Sensing mechanisms and applications
3.1. Physical sensors
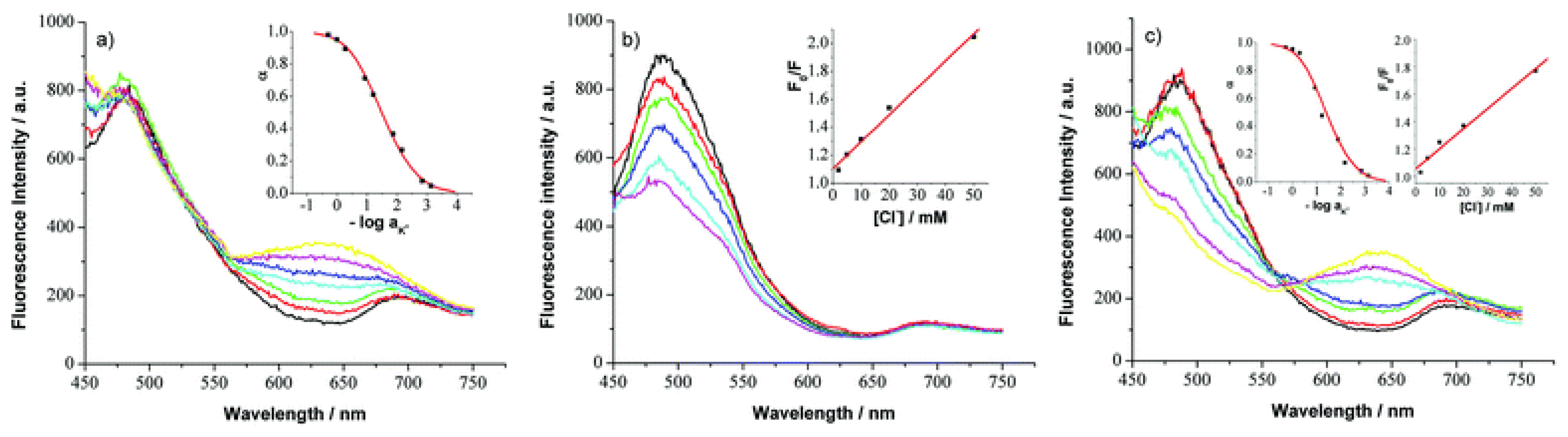
3.2. Chemical sensors
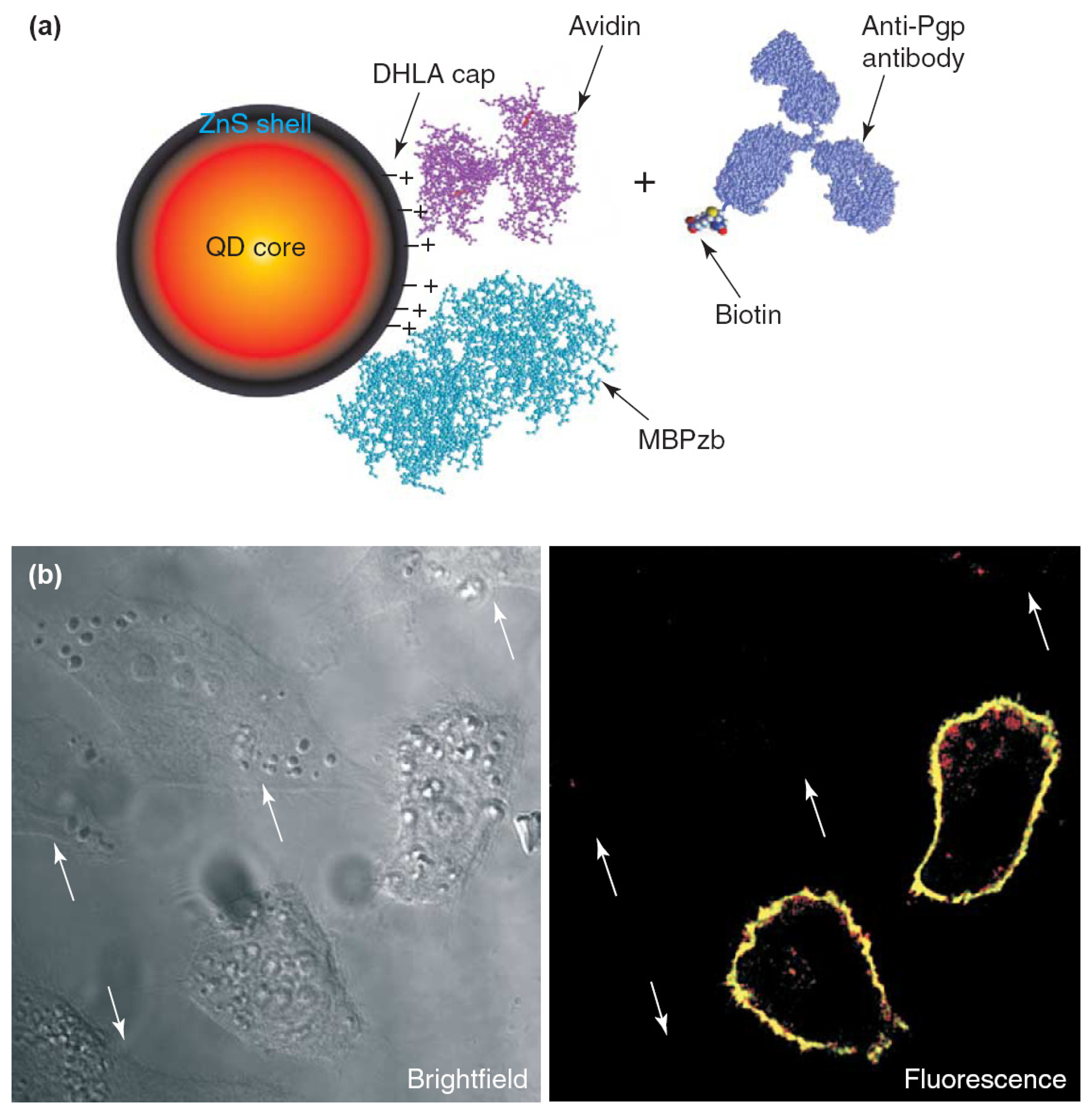
3.3. Biosensors
4. Optical fiber Sensing
4.1. QDs' immobilization at solid surfaces
4.2. Optical fiber sensing applications
4.3. Applications of QDs in planar structures
5. Concluding remarks
References and Notes
- Liang, S.; Pierce, D. T.; Amiot, C.; Zhao, X. Photoactive nanomaterials for sensing trace analytes in biological samples. Synthesis and Reactivity in Inorganic Metal-Organic and Nano-Metal Chemistry 2005, 35(9), 661–668. [Google Scholar]
- Ozkan, M. Quantum dots and other nanoparticles: what can they offer to drug discovery? Drug Discovery Today 2004, 9(24), 1065–1071. [Google Scholar]
- Willner, I.; Basnar, B.; Willner, B. Nanoparticle-enzyme hybrid systems for nanobiotechnology. Febs Journal 2007, 274(2), 302–309. [Google Scholar]
- Penn, S.G.; He, L.; Natan, M.J. Nanoparticles for bioanalysis. Current Opinion in Chemical Biology 2003, 7(5), 609–615. [Google Scholar]
- Kurner, J.M.; Klimant, I.; Krause, C.; Pringsheim, E.; Wolfbeis, O.S. A New Type of Phosphorescent Nanospheres for Use in Advanced Time-Resolved Multiplexed Bioassays. Analytical Biochemistry 2001, 297, 32–41. [Google Scholar]
- Teolato, P.; et al. Silica nanoparticles for fluorescence sensing of Zn-II: Exploring the covalent strategy. Chemistry-a European Journal 2007, 13(8), 2238–2245. [Google Scholar]
- Bruchez, M.B., Jr.; et al. Semiconductor nanocrystals as fluorescent biological labels. Science 1998, 281, 2013–2016. [Google Scholar]
- Mattoussi, H.; et al. Luminescent quantum dot-bioconjugates in immunoassays, FRET, biosensing, and imaging applications. JALA 2004, 9, 28–32. [Google Scholar]
- Zhang, Y.; et al. Using cadmium telluride quantum dots as a proton flux sensor and applying to detect H9 avian influenza virus. Analytical Biochemistry 2007, 364(2), 122–127. [Google Scholar]
- Trindade, T.; O'Brien, P.; Pickett, N.L. Nanocrystalline semiconductors: synthesis, properties, and perspectives. Chemistry of Materials 2001, 13(11), 3843–3858. [Google Scholar]
- Murphy, C.J. Optical sensing with quantum dots. Analytical Chemistry 2002, 74, 520A–526A. [Google Scholar]
- Jaiswal, J.K.; Simon, S.M. Potentials and pitfalls of fluorescent quantum dots for biological imaging. Trends in Cell Biology 2004, 14(9), 497–504. [Google Scholar]
- Riegler, J.; Nann, T. Application of luminescent nanocrystals as labels for biological molecules. Anal Bioanal Chem 2004, 379, 913–919. [Google Scholar]
- Costa-Fernandez, J.M.; Pereiro, R.; Sanz-Medel, A. The use of luminescent quantum dots for optical sensing. Trac-Trends in Analytical Chemistry 2006, 25(3), 207–218. [Google Scholar]
- Clapp, A.R.; Medintz, I.L.; Mattoussi, H. Forster resonance energy transfer investigations using quantum-dot fluorophores. Chemphyschem 2006, 7(1), 47–57. [Google Scholar]
- Wang, X.; Ruedas-Rama, M.J.; Hall, E.A.H. The Emerging Use of Quantum Dots in Analysis. Analytical Letters 2007, 40, 1497–1520. [Google Scholar]
- Somers, R.C.; Bawendi, M.G.; Nocera, D.G. CdSe nanocrystal based chem-/bio- sensors. Chem. Soc. Rev. 2007, 36, 579–591. [Google Scholar]
- Klimov, V.I. Nanocrystal quantum dots. Los Alamos Science 2003, 28, 214–220. [Google Scholar]
- Grundmann, M. The present status of quantum dot lasers. Physica E 2000, 5, 167–184. [Google Scholar]
- Skolnick, M.S.; Mowbray, D.J. Self-assembled semiconductor quantum dots: Fundamental physics and device applications. Annual Review of Materials Research 2004, 34, 181–218. [Google Scholar]
- Scherer, A.; Craighead, H.G. Fabrication of small laterally patterned multiple quantum-wells. Applied Physics Letters 1986, 49(19), 1284–1286. [Google Scholar]
- Fukui, T.; et al. GaAs tetrahedral quantum dot structures fabricated using selective area metalorganic chemical vapor-deposition. Applied Physics Letters 1991, 58(18), 2018–2020. [Google Scholar]
- Gaponenko, S.V. Optical properties of semiconductor nanocrystals; Cambridge Studies in Modern Optics; Knight, P.L., Miller, A., Eds.; Cambridge; Cambridge University Press; Vol. 23, 1998. [Google Scholar]
- Bawendi, M.G.; Steigerwald, M.L.; Brus, L.E. The quantum mechanics of larger semiconductor clusters (“quantum dots”). Annual Review of Physical Chemistry 1990, 41, 477–496. [Google Scholar]
- Hines, M.A.; Guyot-Sionnest, P. Synthesis and characterization of strongly luminescing ZnS-capped CdSe nanocrystals. J. Phys. Chem. 1996, 100, 468–471. [Google Scholar]
- Dabbousi, B.O.; et al. (CdSe)ZnS core-shell quantum dots: Synthesis and characterization of a size series of highly luminescent nanocrystallites. Journal of Physical Chemistry B 1997, 101(46), 9463–9475. [Google Scholar]
- Xie, R.; et al. Synthesis and Characterization of Highly Luminescent CdSe-Core CdS/Zn0.5Cd0.5S/ZnS Multishell Nanocrystals. J. Am. Chem. Soc. 2005, 127, 7480–7488. [Google Scholar]
- Korsunska, N.E.; et al. Reversible and non-reversible photo-enhanced luminescence in CdSe/ZnS quantum dots. Semiconductor Science and Technology 2005, 20(8), 876–881. [Google Scholar]
- Zhelev, Z.; et al. Enhancement of the photoluminescence of CdSe quantum dots during long-term UV-irradiation: privilege or fault in life science research? J Photochem. Photobiol. B 2004, 75(1-2), 99–105. [Google Scholar]
- Verma, P.; Irmer, G.; Monecke, J. Laser power dependence of the photoluminescence from CdSxSe1-x nanoparticles in glass. Journal of Physics-Condensed Matter 2000, 12(6), 1097–1110. [Google Scholar]
- Brus, L.E. A Simple-Model for the Ionization-Potential, Electron-Affinity, and Aqueous Redox Potentials of Small Semiconductor Crystallites. Journal of Chemical Physics 1983, 79(11), 5566–5571. [Google Scholar]
- Murray, C.B.; Kagan, C.R.; Bawendi, M.G. Synthesis and characterization of monodisperse nanocrystals and close-packed nanocrystal assemblies. Annual Review of Materials Science 2000, 30, 545–610. [Google Scholar]
- Murray, C.B.; Norris, D.J.; Bawendi, M.G. Synthesis and Characterization of Nearly Monodisperse Cde (E = S, Se, Te) Semiconductor Nanocrystallites. Journal of the American Chemical Society 1993, 115(19), 8706–8715. [Google Scholar]
- Cozzoli, P.D.; et al. Shape and phase control of colloidal ZnSe nanocrystals. Chemistry of Materials 2005, 17(6), 1296–1306. [Google Scholar]
- Trindade, T.; OBrien, P. A single source approach to the synthesis of CdSe nanocrystallites. Advanced Materials 1996, 8(2), 161–163. [Google Scholar]
- Trindade, T.; OBrien, P.; Zhang, X.M. Synthesis of CdS and CdSe nanocrystallites using a novel single-molecule precursors approach. Chemistry of Materials 1997, 9(2), 523–530. [Google Scholar]
- Nair, P.S.; et al. Cadmium ethylxanthate: A novel single-source precursor for the preparation of CdS nanoparticles. Journal of Materials Chemistry 2002, 12(9), 2722–2725. [Google Scholar]
- Malik, M.A.; O'Brien, P.; Revaprasadu, N. A simple route to the synthesis of core/shell nanoparticles of chalcogenides. Chemistry of Materials 2002, 14(5), 2004–2010. [Google Scholar]
- Murcia, M.J.; et al. Facile sonochemical synthesis of highly luminescent ZnS-shelled CdSe quantum dots. Chemistry of Materials 2006, 18(9), 2219–2225. [Google Scholar]
- Peng, Z.A.; Peng, X.G. Formation of high-quality CdTe, CdSe, and CdS nanocrystals using CdO as precursor. Journal of the American Chemical Society 2001, 123(1), 183–184. [Google Scholar]
- Peng, X.G. Green chemical approaches toward high-quality semiconductor nanocrystals. Chemistry-a European Journal 2002, 8(2), 335–339. [Google Scholar]
- Rogach, A.; et al. Colloidally prepared HgTe nanocrystals with strong room-temperature infrared luminescence. Advanced Materials 1999, 11(7), 552–555. [Google Scholar]
- Vossmeyer, T.; et al. Cds Nanoclusters - Synthesis, Characterization, Size-Dependent Oscillator Strength, Temperature Shift of the Excitonic-Transition Energy, and Reversible Absorbency Shift. Journal of Physical Chemistry 1994, 98(31), 7665–7673. [Google Scholar]
- Rogach, A.L.; et al. Synthesis and characterization of a size series of extremely small thiol-stabilized CdSe nanocrystals. Journal of Physical Chemistry B 1999, 103(16), 3065–3069. [Google Scholar]
- Rogach, A.L.; et al. Synthesis and characterization of thiol-stabilized CdTe nanocrystals. Berichte Der Bunsen-Gesellschaft-Physical Chemistry Chemical Physics 1996, 100(11), 1772–1778. [Google Scholar]
- Rogach, A.L.; et al. Synthesis, morphology and optical properties of thiol-stabilized CdTe nanoclusters in aqueous solution. Berichte Der Bunsen-Gesellschaft-Physical Chemistry Chemical Physics 1997, 101(11), 1668–1670. [Google Scholar]
- Gao, M.Y.; et al. Strongly photoluminescent CdTe nanocrystals by proper surface modification. Journal of Physical Chemistry B 1998, 102(43), 8360–8363. [Google Scholar]
- Green, M.; et al. A facile route to CdTe nanoparticles and their use in bio-labelling. Journal of Materials Chemistry 2007, 17(19), 1989–1994. [Google Scholar]
- Aldana, J.; Wang, Y.A.; Peng, X.G. Photochemical instability of CdSe nanocrystals coated by hydrophilic thiols. Journal of the American Chemical Society 2001, 123(36), 8844–8850. [Google Scholar]
- Liu, T.C.; et al. Temperature-dependent photoluminescence of water-soluble quantum dots for a bioprobe. Analytica Chimica Acta 2006, 559(1), 120–123. [Google Scholar]
- Liu, Y.S.; et al. pH-sensitive photoluminescence of CdSe/ZnSe/ZnS quantum dots in human ovarian cancer cells. Journal of Physical Chemistry C 2007, 111(7), 2872–2878. [Google Scholar]
- Huang, C.P.; et al. Plate-based biochemical assay using quantum dots as a fluorescent labeling agent. Sensors and Actuators B-Chemical 2005, 108(1-2), 713–720. [Google Scholar]
- Mattoussi, H.; et al. Self-assembly of CdSe-ZnS quantum dot bioconjugates using an engineered recombinant protein. Journal of the American Chemical Society 2000, 122(49), 12142–12150. [Google Scholar]
- Uyeda, H.T.; et al. Synthesis of compact multidentate ligands to prepare stable hydrophilic quantum dot fluorophores. Journal of the American Chemical Society 2005, 127(11), 3870–3878. [Google Scholar]
- Kim, S.; Bawendi, M.G. Oligomeric Ligands for luminescent and stable nanocrystal quantum dots. Journal of the American Chemical Society 2003, 125(48), 14652–14653. [Google Scholar]
- Guo, W.H.; et al. Luminescent CdSe/CdS core/shell nanocrystals in dendron boxes: Superior chemical, photochemical and thermal stability. Journal of the American Chemical Society 2003, 125(13), 3901–3909. [Google Scholar]
- Guo, W.Z.; et al. Conjugation chemistry and bioapplications of semiconductor box nanocrystals prepared via dendrimer bridging. Chemistry of Materials 2003, 15(16), 3125–3133. [Google Scholar]
- Gerion, D.; et al. Synthesis and properties of biocompatible water-soluble silica-coated CdSe/ZnS semiconductor quantum dots. Journal of Physical Chemistry B 2001, 105(37), 8861–8871. [Google Scholar]
- Iler, R.K. The Chemistry of Silica: Solubility, Polymerization, Colloid and Surface Properties and Biochemistry of Silica.; John Wiley & Sons: New York, 1979. [Google Scholar]
- Wu, X.Y.; et al. Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots. Nature Biotechnology 2003, 21(1), 41–46. [Google Scholar]
- Pellegrino, T.; et al. Hydrophobic nanocrystals coated with an amphiphilic polymer shell: A general route to water soluble nanocrystals. Nano Letters 2004, 4(4), 703–707. [Google Scholar]
- Gao, X.H.; et al. In vivo cancer targeting and imaging with semiconductor quantum dots. Nature Biotechnology 2004, 22(8), 969–976. [Google Scholar]
- Dubertret, B.; et al. In vivo imaging of quantum dots encapsulated in phospholipid micelles. Science 2002, 298(5599), 1759–1762. [Google Scholar]
- Mattheakis, L.C.; et al. Optical coding of mammalian cells using semiconductor quantum dots. Analytical Biochemistry 2004, 327(2), 200–208. [Google Scholar]
- Ballou, B.; et al. Noninvasive imaging of quantum dots in mice. Bioconjugate Chemistry 2004, 15(1), 79–86. [Google Scholar]
- Jin, T.; et al. Calixarene-coated water-soluble CdSe-ZnS semiconductor quantum dots that are highly fluorescent and stable in aqueous solution. Chemical Communications 2005, 22, 2829–2831. [Google Scholar]
- Jin, T.; et al. Amphiphilic p-sulfonatocalix[4]arene-coated CdSe/ZnS quantum dots for the optical detection of the neurotransmitter acetylcholine. Chemical Communications 2005, 34, 4300–4302. [Google Scholar]
- Liu, J.A.; et al. Use of ester-terminated polyamidoamine dendrimers for stabilizing quantum dots in aqueous solutions. Small 2006, 2(8-9), 999–1002. [Google Scholar]
- Erdem, B.; et al. Encapsulation of inorganic particles via miniemulsion polymerization. III. Characterization of encapsulation. Journal of Polymer Science Part a-Polymer Chemistry 2000, 38(24), 4441–4450. [Google Scholar]
- Erdem, B.; et al. Encapsulation of inorganic particles via miniemulsion polymerization. II. Preparation and characterization of styrene miniemulsion droplets containing TiO2 particles. Journal of Polymer Science Part a-Polymer Chemistry 2000, 38(24), 4431–4440. [Google Scholar]
- Erdem, B.; et al. Encapsulation of inorganic particles via miniemulsion polymerization. I. Dispersion of titanium dioxide particles in organic media using OLOA 370 as stabilizer. Journal of Polymer Science Part a-Polymer Chemistry 2000, 38(24), 4419–4430. [Google Scholar]
- Tiarks, F.; Landfester, K.; Antonietti, M. Silica nanoparticles as surfactants and fillers for latexes made by miniemulsion polymerization. Langmuir 2001, 17(19), 5775–5780. [Google Scholar]
- Tiarks, F.; Landfester, K.; Anonietti, M. Encapsulation of carbon black by miniemulsion polymerization. Macromolecular Chemistry and Physics 2001, 202(1), 51–60. [Google Scholar]
- Esteves, A.C.C.; et al. Polymer encapsulation of CdE (E = S, Se) quantum dot ensembles via in-situ radical polymerization in miniemulsion. Journal of Nanoscience and Nanotechnology 2005, 5(5), 766–771. [Google Scholar]
- Joumaa, N.; et al. Synthesis of quantum dot-tagged submicrometer polystyrene particles by miniemulsion polymerization. Langmuir 2006, 22(4), 1810–1816. [Google Scholar]
- Martins, M.A.; et al. Biofunctionalized ferromagnetic CoPt3/polymer nanocomposites. Nanotechnology 2007, 18(21), 5609–5615. [Google Scholar]
- Fleischhaker, F.; Zentel, R. Photonic crystals from core-shell colloids with incorporated highly fluorescent quantum dots. Chemistry of Materials 2005, 17(6), 1346–1351. [Google Scholar]
- Peres, M.; et al. A green-emitting CdSe/poly(butyl acrylate) nanocomposite. Nanotechnology 2005, 16(9), 1969–1973. [Google Scholar]
- Zhu, M.Q.; et al. Surface modification and functionalization of semiconductor quantum dots through reactive coating of silanes in toluene. Journal of Materials Chemistry 2007, 17(8), 800–805. [Google Scholar]
- Ohno, K.; et al. Fabrication of ordered arrays of gold nanoparticles coated with high-density polymer brushes. Angewandte Chemie-International Edition 2003, 42(24), 2751–2754. [Google Scholar]
- Bao, Z.Y.; Bruening, M.L.; Baker, G.L. Rapid growth of polymer brushes from immobilized initiators. Journal of the American Chemical Society 2006, 128(28), 9056–9060. [Google Scholar]
- Ohno, K.; et al. Synthesis of monodisperse silica particles coated with well-defined, high-density polymer brushes by surface-initiated atom transfer radical polymerization. Macromolecules 2005, 38(6), 2137–2142. [Google Scholar]
- Zhao, H.Y.; Kang, X.L.; Liu, L. Comb-coil polymer brushes on the surface of silica nanoparticles. Macromolecules 2005, 38(26), 10619–10622. [Google Scholar]
- Marutani, E.; et al. Surface-initiated atom transfer radical polymerization of methyl methacrylate on magnetite nanoparticles. Polymer 2004, 45(7), 2231–2235. [Google Scholar]
- Skaff, H.; Emrick, T. Reversible addition fragmentation chain transfer (RAFT) polymerization from unprotected cadmium selenide nanoparticles. Angewandte Chemie-International Edition 2004, 43(40), 5383–5386. [Google Scholar]
- Sill, K.; Emrick, T. Nitroxide-mediated radical polymerization from CdSe nanoparticles. Chemistry of Materials 2004, 16(7), 1240–1243. [Google Scholar]
- Esteves, A.C.C.; et al. Polymer grafting from Cds quantum dots via AGET ATRP in miniemulsion. Small 2007, 3(7), 1230–1236. [Google Scholar]
- Wu, Y.-h.; Arai, K.; Yao, T. Temperature dependence of the photoluminescence of ZnSe/ZnS quantum-dot structures. Physical Review B 1996, 53(16), R 10 485–R 10 488. [Google Scholar]
- Labeau, O.; Tamarat, P.; Lounis, B. Temperature dependence of the luminescence lifetime of single CdSe/ZnS quantum dots. Physical Review Letters 2003, 90(25), 7404–7407. [Google Scholar]
- Biju, V.; et al. Temperature-sensitive photoluminescence of CdSe quantum dot clusters. Journal of Physical Chemistry B 2005, 109(29), 13899–13905. [Google Scholar]
- Walker, G.W.; et al. Quantum-dot optical temperature probes. Applied Physics Letters 2003, 83(17), 3555–3557. [Google Scholar]
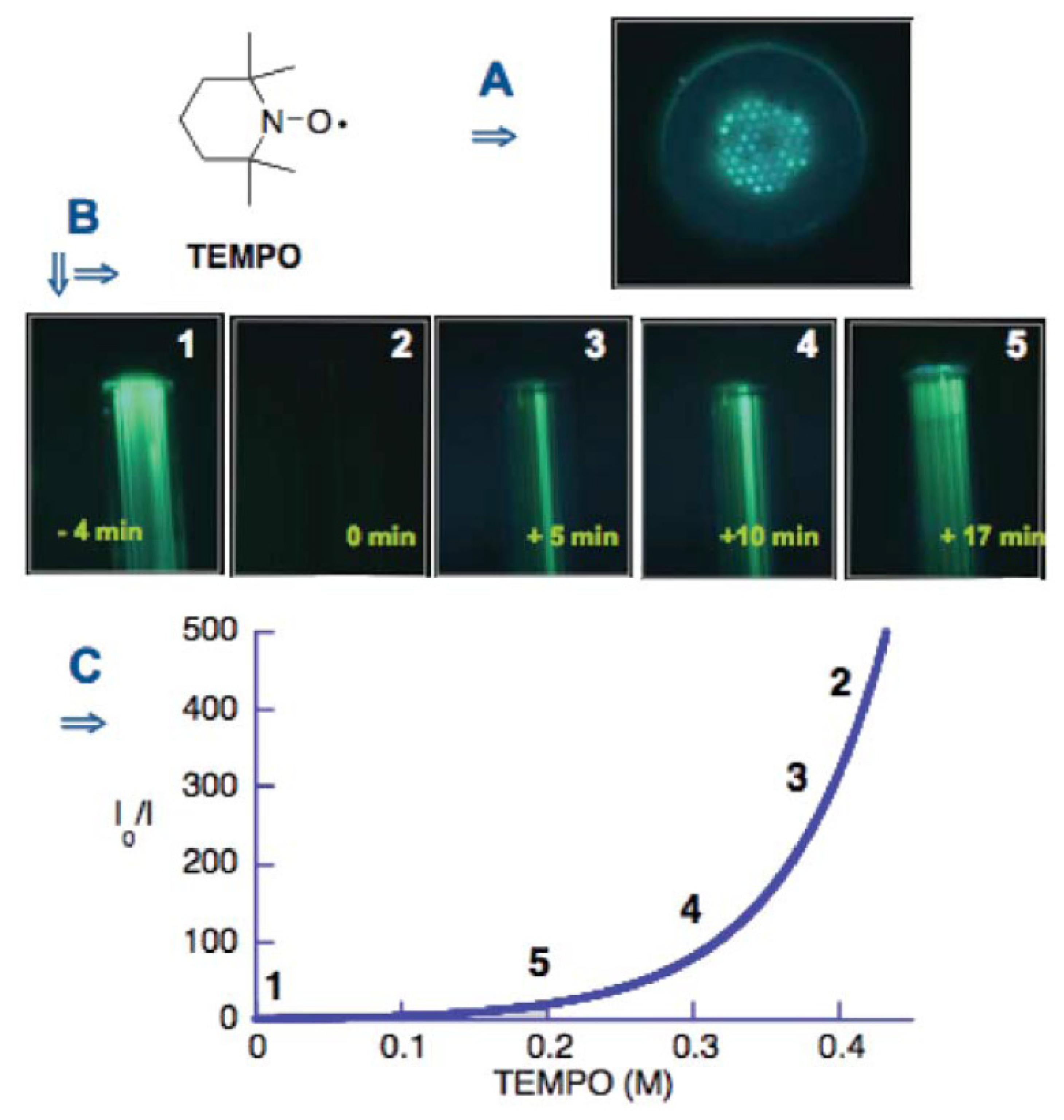
- Chen, Y.; Rosenzweig, Z. Luminescent CdS quantum dots as selective ion probes. Analytical Chemistry 2002, 74(19), 5132–5138. [Google Scholar]
- Xie, H.-Y.; et al. Luminescent CdSe-ZnS quantum dots as selective Cu2+ probe. Spectrochimica Acta Part A 2004, 60, 2527–2530. [Google Scholar]
- Jin, W.J.; et al. Surface-modified CdSe quantum dots as luminescent probes for cyanide determination. Analytica Chimica Acta 2004, 522, 1–8. [Google Scholar]
- Shi, G.H.; et al. Fluorescence quenching of CdSe quantum dots by nitroaromatic explosives and their relative compounds. Spectrochimica Acta Part A 2007. [Google Scholar]
- Albert, K.e.J. Field-Deployable Sniffer for 2,4-Dinitrotoluene Detection. Environ. Sci. Technol. 2001, 35, 3193–3200. [Google Scholar]
- Nazzal, A.Y.; et al. Photoactivated CdSe Nanocrystals as Nanosensors for Gases. Nano Lett. 2003, 3(6), 819–822. [Google Scholar]
- Potyrailo, R.A.; Leach, A.M. Selective gas nanosensors with multisize CdSe nanocrystal/polymer composite films and dynamic pattern recognition. Applied Physics Letters 2006, 88, 134110. [Google Scholar]
- Tomasulo, M.; et al. pH-Sensitive Ligand for Luminescent Quantum Dots. Langmuir 2006, 22, 10284–10290. [Google Scholar]
- Snee, P.T.; et al. A Ratiometric CdSe/ZnS Nanocrystal pH Sensor. J. Am. Chem. Soc. 2006, 128, 13320–13321. [Google Scholar]
- Ruedas-Rama, M.J.; Wang, X.; Hall, E.A.H. A multi-ion particle sensor. Chem. Commun. 2007, 1544–1546. [Google Scholar]
- Xu, C.; Bakker, E. Multicolor Quantum Dot Encoding for Polymeric Particle-Based Optical Ion Sensors. Anal. Chem. 2007, 79, 3716–3723. [Google Scholar]
- Lin, C.I.; et al. Molecularly imprinted polymeric film on semiconductor nanoparticles: analyte detection by quantum dot photoluminescence. Journal of Chromatography A 2004, 1027, 259–262. [Google Scholar]
- Alivisatos, P. The use of nanocrystals in biological detection. Nature Biotechnology 2004, 22(1), 47–52. [Google Scholar]
- Evident Technologies. Available from: www.evidenttech.com cited 2007.
- Quantum Dot Corporation. Available from: www.qdots.com cited 2007.
- Chan, W.C.W.; Nie, S. Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science 1998, 281, 2016–2018. [Google Scholar]
- Sark, W.G.J.H.M.v.; et al. Blueing, bleaching, and blinking of single CdSe/ZnS quantum dots. Chemphyschem 2002, 3(10), 871–879. [Google Scholar]
- Jaiswal, J.K.; et al. Long-term multiple color imaging of live cells using quantum dot bioconjugates. Nature Biotechnology 2003, 21(1), 47–51. [Google Scholar]
- Gao, X.; et al. In vivo molecular and cellular imaging with quantum dots. Current Opinion in Biotechnology 2005, 16, 63–72. [Google Scholar]
- Smith, A.M.; et al. Multicolor quantum dots for molecular diagnostics of cancer. Exopert Rev. Mol. Diagn. 2006. [Google Scholar]
- Smith, J.D.; et al. The use of quantum dots for analysis of chick CAM vasculature. Microvascular Research 2007, 73, 75–83. [Google Scholar]
- Murphy, C.; Coffer, J. Quantum dots: a primer. Appl. Spectrosc. 2002, 56(1), 16A–27A. [Google Scholar]
- Liang, J.; et al. Study on DNA damage induced by CdSe quantum dots using nucleic acid molecular “light switches” as probe. Talanta 2007, 71, 1675–1678. [Google Scholar]
- Guo, G.; et al. Probing the cytotoxicity of CdSe quantum dots with surface modification. Materials Letters 2007, 61, 1641–1644. [Google Scholar]
- Mattoussi, H.; et al. Self-assembly of CdSe-ZnS quantum dot bioconjugates using an engineered recombinant protein. Journal of the American Chemical Society 2000, 122(49), 12142–12150. [Google Scholar]
- Clapp, A.R.; et al. Fluorescence resonance energy transfer between quantum dot donors and dye-labeled protein acceptors. Journal of the American Chemical Society 2004, 126(1), 301–310. [Google Scholar]
- Liu, Z.D.; et al. Light scattering sensing detection of pathogens based on the molecular recognition of immunoglobulin with cell wall-associated protein A. Analytica Chimica Acta 2007, 599, 279–286. [Google Scholar]
- Yu, Y.; et al. Synthesis of functionalized CdTe/CdS QDs for spectrofluorimetric detection of BSA. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2007. [Google Scholar] [CrossRef]
- Stsiapura, V.; et al. Functionalized nanocrystal-tagged fluorescent polymer beads: synthesis, physicochemical characterization and immunolableing application. Analytical Biochhemistry 2004, 334, 257–265. [Google Scholar]
- Wang, H.-Q.; et al. Multicolor encoding of polystyrene microbeads with CdSe-ZnS quantum dots and its application in immunoassay. Jornal of Colloid and Interface Science 2007. [Google Scholar] [CrossRef]
- Cordes, D.B.; Gamsey, S.; Singaram, B. Fluorescent Quantum Dots with Boronic Acid Substituted Viologens To Sense Glucose in Aqueous Solution. Angew. Chem. Int. Ed. 2006, 45, 3829–3832. [Google Scholar]
- Huang, C.-P.; et al. A new approach for quantitative determination of glucose by using CdSe/ZnS quantum dots. Sens. Actuators B: Chem 2007. [Google Scholar] [CrossRef]
- Holst, G.; Mizaikoff, B. Fiber Optic Sensors for Environmental Applications. In Handbook of Optical Fibre Sensing Technology; John Wiley & Sons, LTD., 2002; pp. 729–755. [Google Scholar]
- Grattan, K.T.V.; Sun, T. Fiber optic sensor technology: an overview. Sensors and Actuators a-Physical 2000, 82(1-3), 40–61. [Google Scholar]
- Wolfbeis, O.S. Fiber Optic Chemical Sensors and Biosensors. Anal. Chem. 2002, 72, 81R–89R. [Google Scholar]
- Wolfbeis, O.S. Fiber Optic Chemical Sensors and Biosensors. Anal. Chem. 2004, 76, 3269–3284. [Google Scholar]
- Butler, T.M.; MacCraith, B.D.; McDonagh, C. Leaching in sol–gel-derived silica films for optical pH sensing. Journal of Non-Crystalline Solids 1998, 224, 249–258. [Google Scholar]
- Hartmann, P.; Leiner, M.J.P.; Kohlbacher, P. Photobleaching of ruthenium complex in polymers used for oxygen optodes and its inhibition by singlet oxygen quenchers. Sensors and Actuators B 1998, 51, 196–202. [Google Scholar]
- Costa, V.C.; Shen, Y.; Bray, K.L. Luminescence properties of nanocrystalline CdS and CdS:Mn2+ doped silica-type glasses. Journal of Non-Crystalline Solids 2002, 304, 217–223. [Google Scholar]
- Litran, R.; et al. Confinement of CdS Nanocrystals in a Sonogel Matrix. Journal of Sol-Gel Science and Technology 1997, 8, 275–283. [Google Scholar]
- Wang, Y.; et al. Optical responses of ZnSe quantum dots in silica gel glasses. Journal of Crystal Growth 2004, 268, 580–584. [Google Scholar]
- Arachchige, I.U.; Brock, S.L. Sol-Gel Methods for the Assembly of Metal Chalcogenide Quantum Dots. Acc. Chem. Res. 2007, 40, 801–809. [Google Scholar]
- Reisfeld, R.; Saraidarov, T. Innovative materials based on sol-gel technology. Optical Materials 2006, 28, 64–70. [Google Scholar]
- Bullen, C.; et al. Incorporation of a highly luminescent semiconductor quantum dot in ZrO2-SiO2 hybrid sol-gel glass film. J. Mater. Chem. 2004, 14, 1112–1116. [Google Scholar]
- Ferreira, P.M.S.; et al. Langmuir-Blodgett manipulation of capped cadmium sulfide quantum dots. Thin Solid Films 2001, 389, 272–277. [Google Scholar]
- Crisp, M.T.; Kotov, N.A. Preparation of Nanoparticle Coatings on Surfaces of Complex Geometry. Nano Lett. 2003, 3(2), 174–177. [Google Scholar]
- Barmenkov, Y.O.; Starodumov, A.N.; Lipovskii, A.A. Temperature fiber sensor based on semiconductor nanocrystallite-doped phosphate glasses. Applied Physics Letters 1998, 73, 4. [Google Scholar]
- Jorge, P.A.S.; et al. Luminescence-based optical fiber chemical sensors. Fiber and Integrated Optics 2005, 24(3-4), 201–225. [Google Scholar]
- Jorge, P.A.S.; et al. Quantum dots as self-referenced optical fibre temperature probes for luminescent chemical sensors. Measurement Science & Technology 2006, 17(5), 1032–1038. [Google Scholar]
- Benrashid, R.; Velasco, P. High performance sol-gel spin-on glass materials; Waveguide Solutions: Charlotte, NC, 2005; p. p. 27. [Google Scholar]
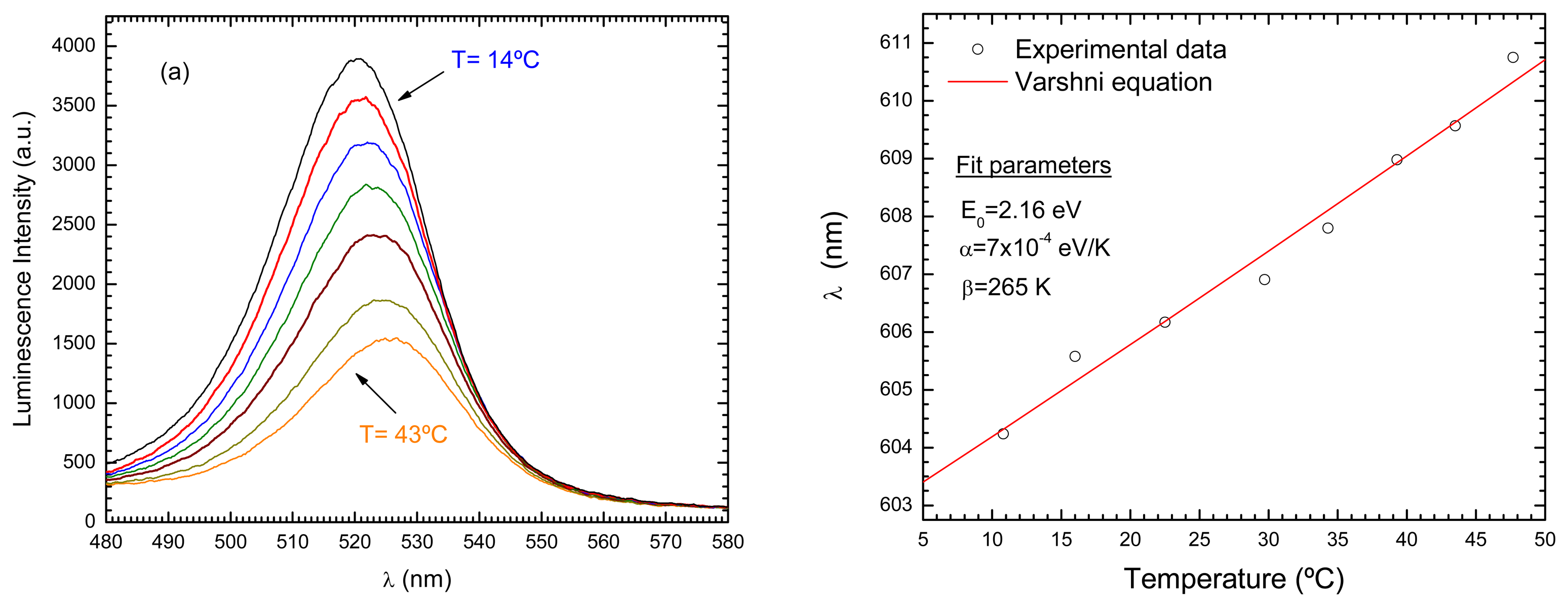
- Odonnell, K.P.; Chen, X. Temperature-dependence of semiconductor band-gaps. Applied Physics Letters 1991, 58(25), 2924–2926. [Google Scholar]
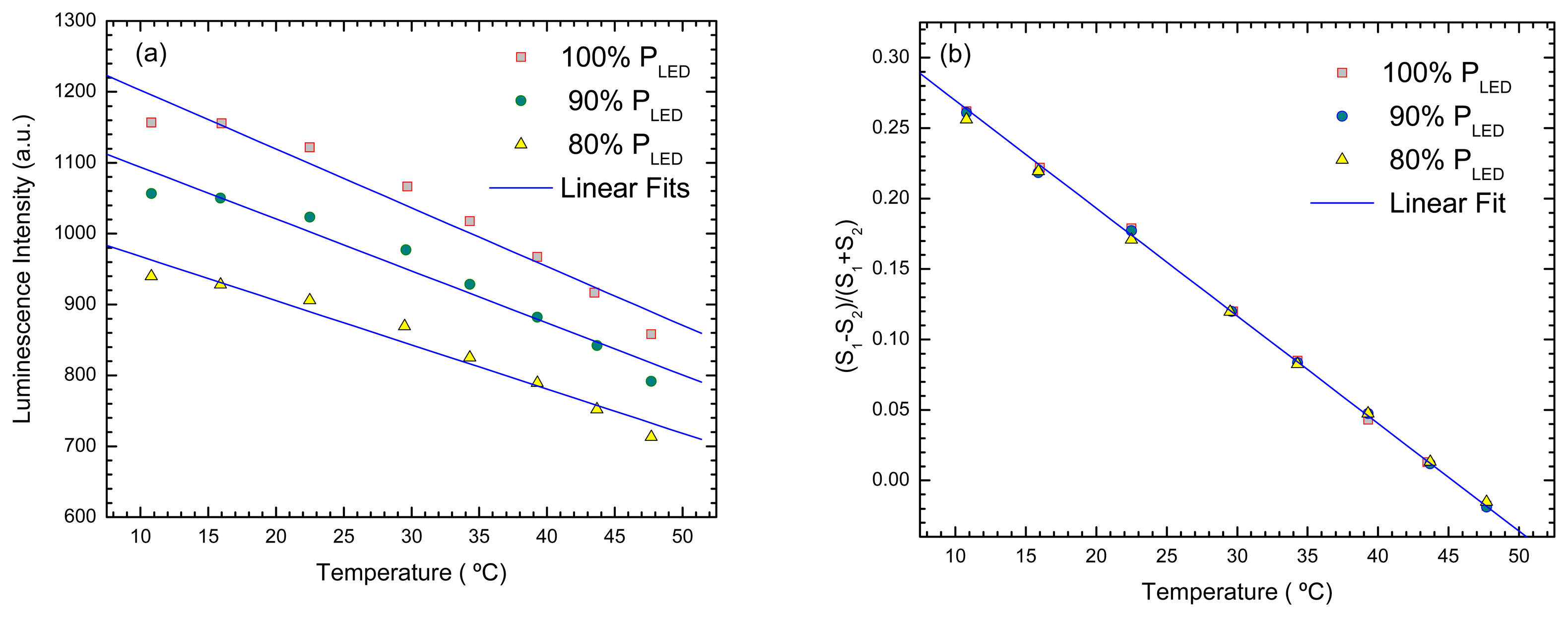
- Valerini, D.; et al. Temperature dependence of the photoluminescence properties of colloidal CdSe/ZnS core/shell quantum dots embedded in a polystyrene matrix. Phys. Rev. B 2005, 71, 235409. [Google Scholar]
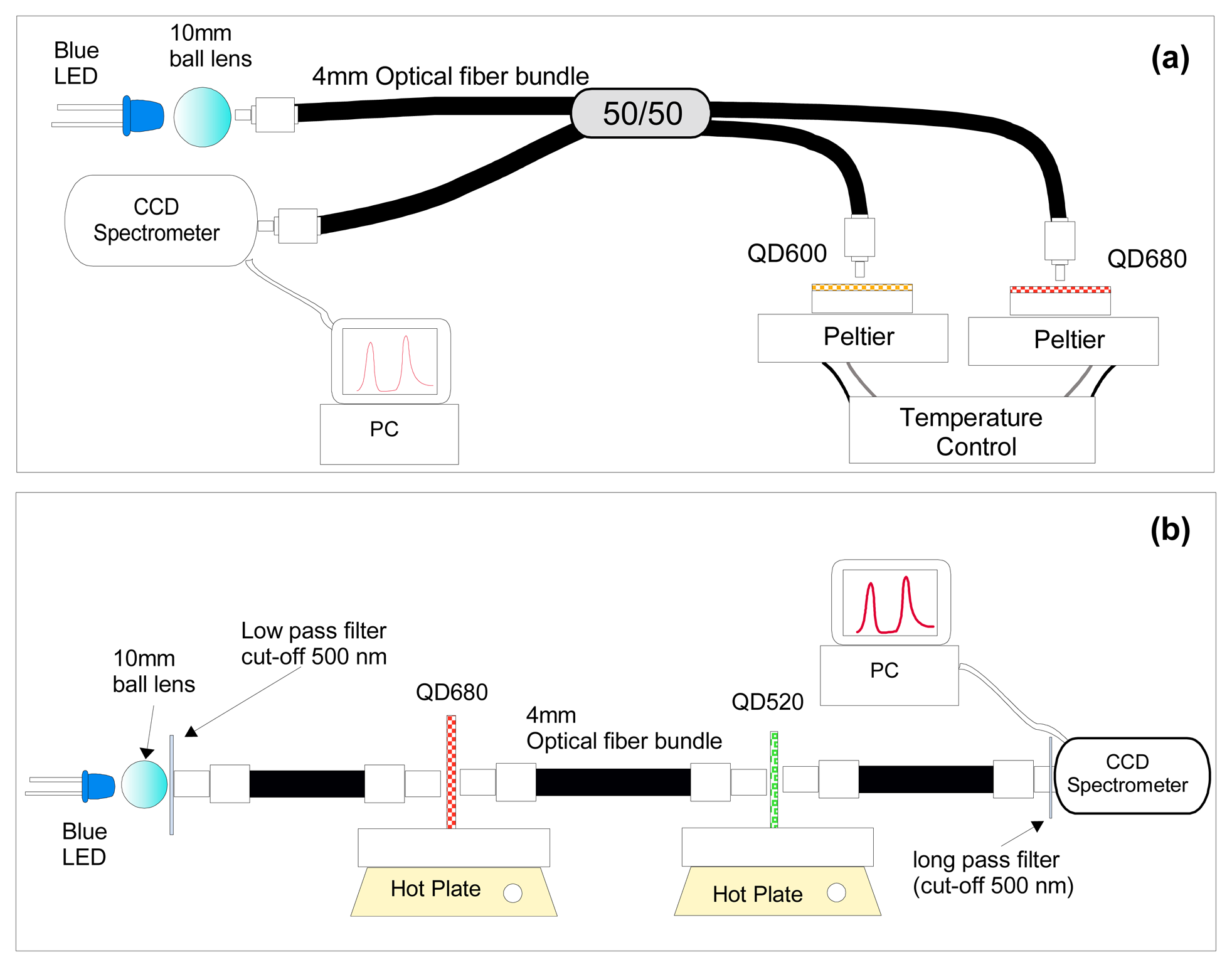
- deBastida, G.; et al. Quantum dots based optical fiber temperature sensors fabricated by Layer-by-Layer. IEEE Sensors Journal 2006. [Google Scholar]
- Bravo, J.; et al. Fiber Optic temperature sensor depositing quantum dots inside hollow core fibers using layer by layer technique. In EWOFS 2007.; Napoli; SPIE; Volume 6619, pp. 661919–1. 2007. [Google Scholar]
- Ruan, H.; et al. Self assembled optical detectors for optical fiber sensors. In EWOFS 2007.; Napoli; SPIE; Volume 6619, pp. 66192W–1. 2007. [Google Scholar]
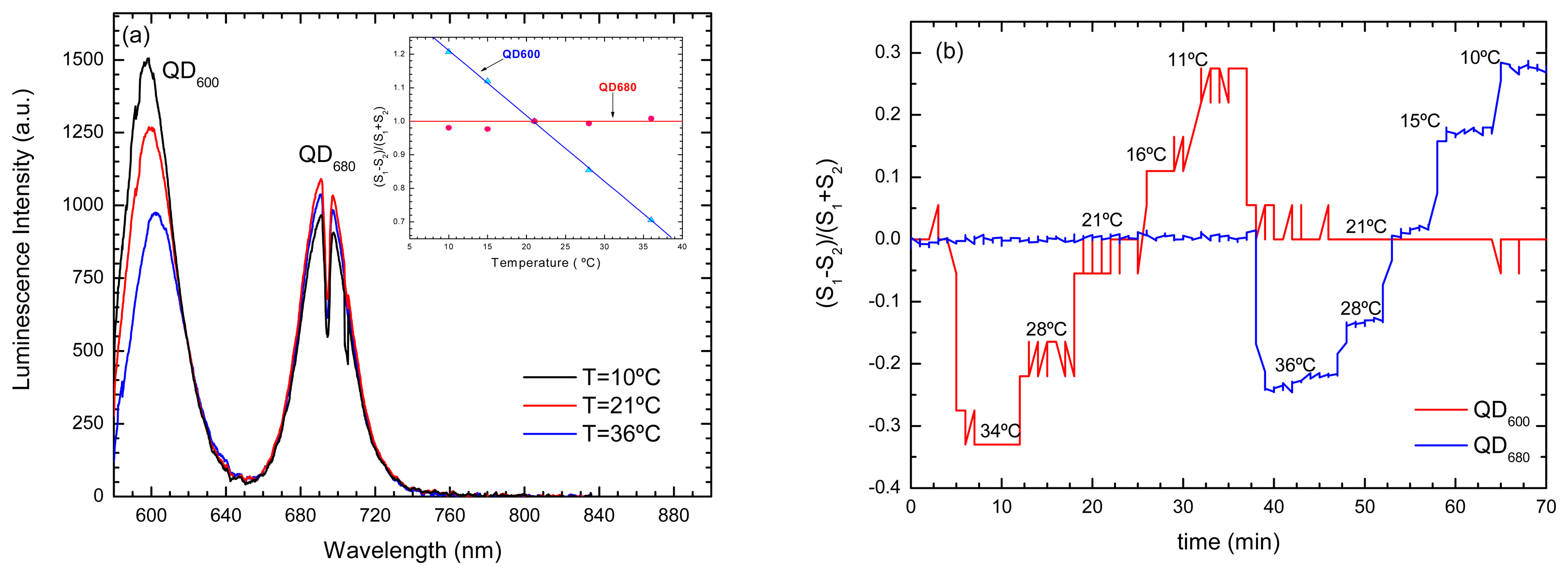
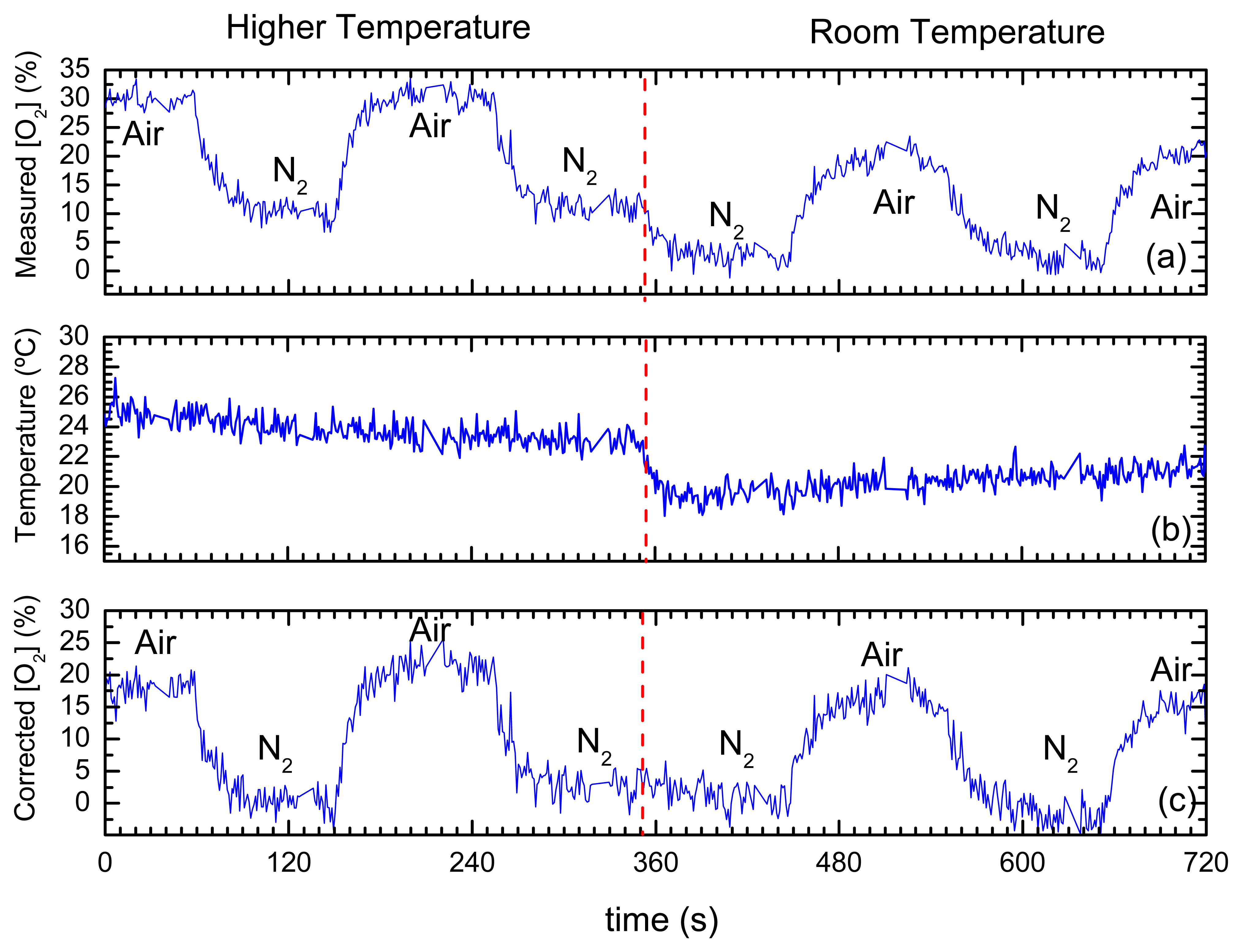
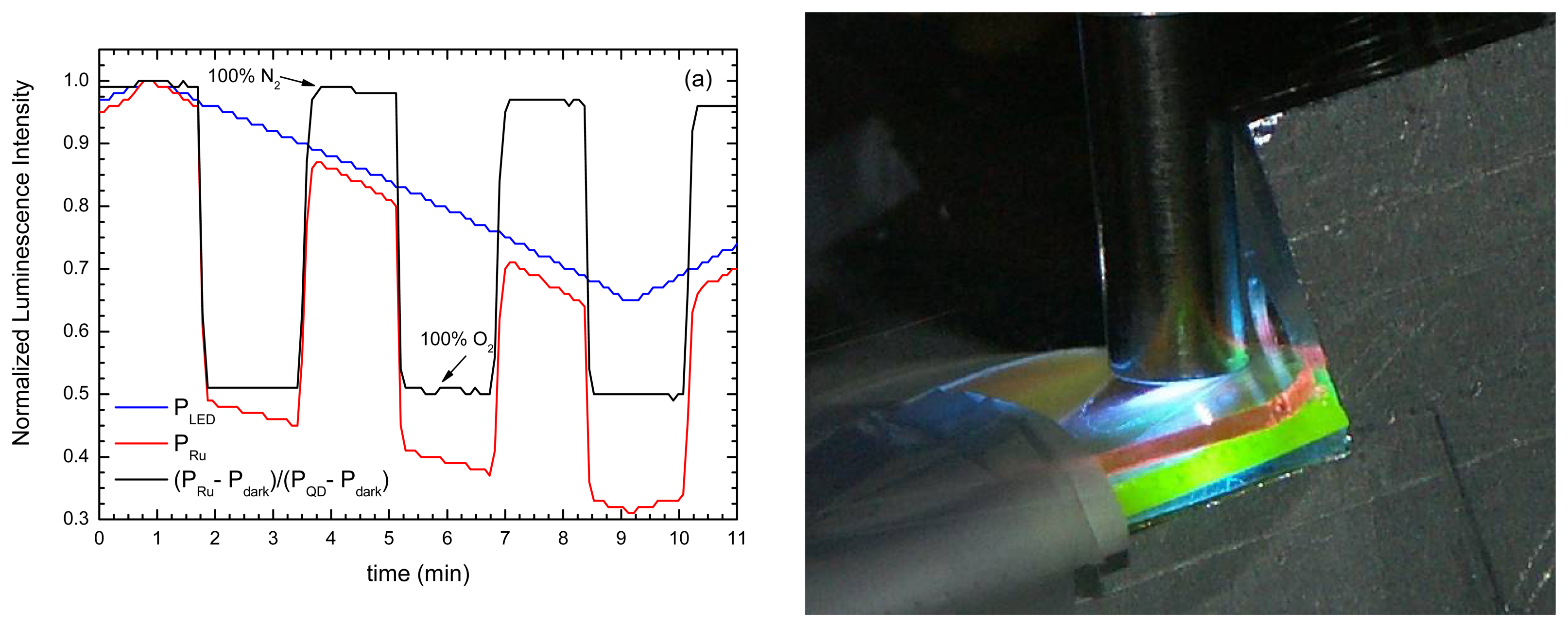
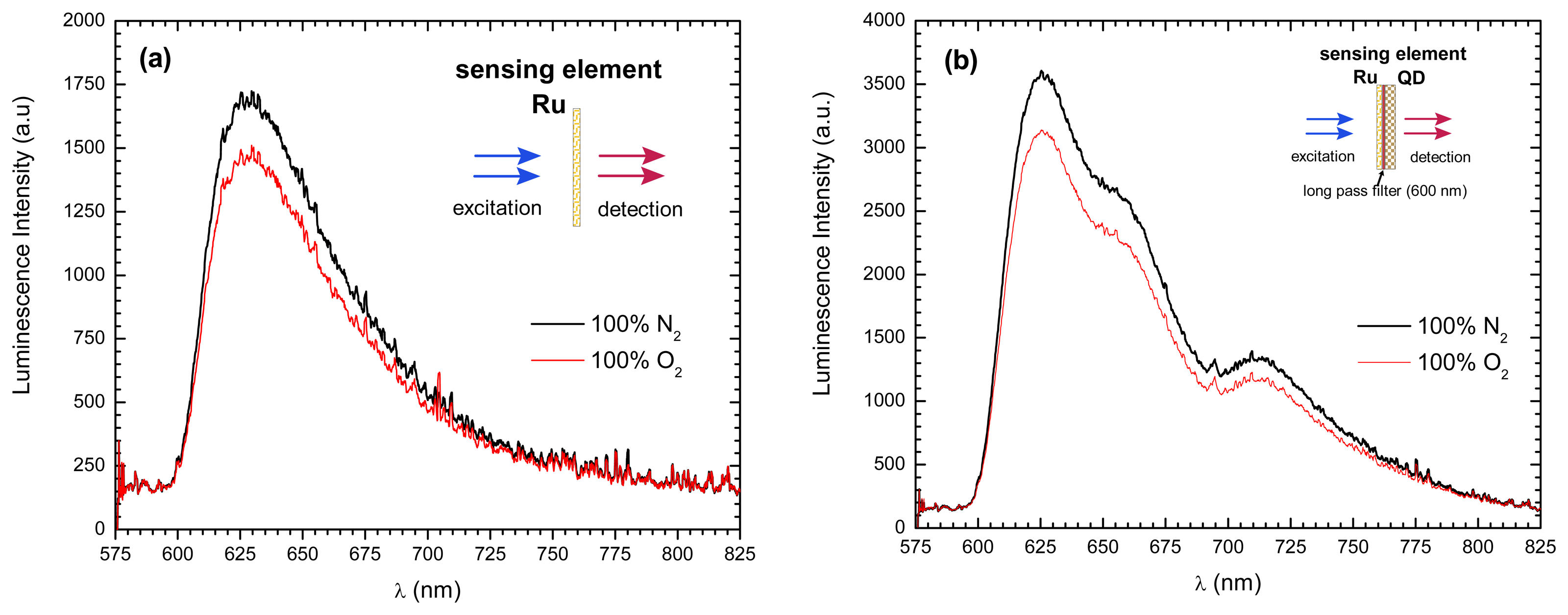
- Jorge, P.A.S.; et al. Simultaneous determination of oxygen and temperature using quantum dots and a ruthenium complex. In EWOFS 2007.; Napoli; SPIE; Volume 6619, p. 66191Y. 2007. [Google Scholar]
- Jorge, P.A.S.; et al. Applications of quantum dots in optical fiber luminescent oxygen sensors. Applied Optics 2006, 45(16), 3760–3767. [Google Scholar]
- Aoyagi, S.; Kudo, M. Development of fluorescence change-based, reagent-less optic immunosensor. Biosensors and Bioelectronics 2005, 20, 1680–1684. [Google Scholar]
- Meissner, K.E.; Holton, C.; Spillmann, W.B., Jr. Optical characterization of quantum dots entrained in microstructured optical fibers. Physica E 2005, 26, 377–381. [Google Scholar]
- Finlayson, C.E. Comment on “Optical characterization of quantum dots entrained in microstructured optical fibers” Physica E 26 (2005) 377-381. Physica E 2006, 31, 107–108. [Google Scholar]
- Meissner, K.E.; Holton, C.; Spillmann, W.B., Jr. Response to comment on “Optical characterization of quantum dots entrained in microstructured optical fibers”. Physica E 2006, 31, 109–110. [Google Scholar]
- Yu, H.C.Y.; et al. Quantum dot and silica nanoparticle doped polymer optical fibers. Optics Express 2007, 15(16), 9989–9994. [Google Scholar]
- Galian, R.E.; Laferriere, M.; Scaiano, J.C. Doping of photonic crystal fibers with fluorescent probes: possible functional materials for optrode sensors. J. Mater. Chem. 2006, 16, 1697–1701. [Google Scholar]
- Rindorf, L.; et al. Towards biochip using microstructured optical fiber sensors. Anal. Bioanal. Chem. 2006, 385, 1370–1375. [Google Scholar]
- Hassani, A.; Skorobogatiy, M. Design of the Microstructured Optical Fiber-based Surface Plasmon Resonance sensors with enhanced microfluidics. Optics Express 2006, 14(24), 11616. [Google Scholar]
- Craighead, H. Future lab-on-a-chip technologies for interrogating individual molecules. Nature 2006, 442(7101), 387–393. [Google Scholar]
- Dittrich, P.S.; Manz, A. Lab-on-a-chip: microfluidics in drug discovery. Nature Reviews Drug Discovery 2006, 5(3), 210–218. [Google Scholar]
- Loonberg, M.; Carlsson, J. Lab-on-a-chip technology for determination of protein isoform profiles. Journal of Chromatography A 2006, 1127(1-2), 175–182. [Google Scholar]
- Riegger, L.; et al. Read-out concepts for multiplexed bead-based fluorescence immunoassays on centrifugal microfluidic platforms. Sensors and Actuators A 2006, 126, 455–462. [Google Scholar]
- Vossmeyer, T.; et al. Combinatorial approaches toward patterning nanocrystals. Journal of Applied Physics 1998, 84(7), 3664–3670. [Google Scholar]
- Meldrun, A.; et al. Micropixelated Luminescent nanocrystal arrays synthesized by ion implantation. Advanced Materials 2004, 16(1), 31–34. [Google Scholar]
- Chen, C.-C.; et al. Self-Assembly of Monolayers of Cadmium Selenide Nanocrystals with Dual Color Emission. Langmuir 1999, 15, 6845–6850. [Google Scholar]
- Bertino, M.F.; et al. Patterning porous matrices and planar substrates with quantum dots. J Sol-Gel Sci Techn 2006, 39, 299–306. [Google Scholar]
- Pompa, P.P.; et al. Fluorescence enhancement in colloidal semiconductor nanocrystals by metallic nanopatterns. Sensors and Actuators B 2007, 126, 187–192. [Google Scholar]
- Sapsford, K.E.; et al. Surface-Immobilized Self-Assembled Protein-Based Quantum Dot Nanoassemblies. Langmuir 2004, 20, 7720–7728. [Google Scholar]
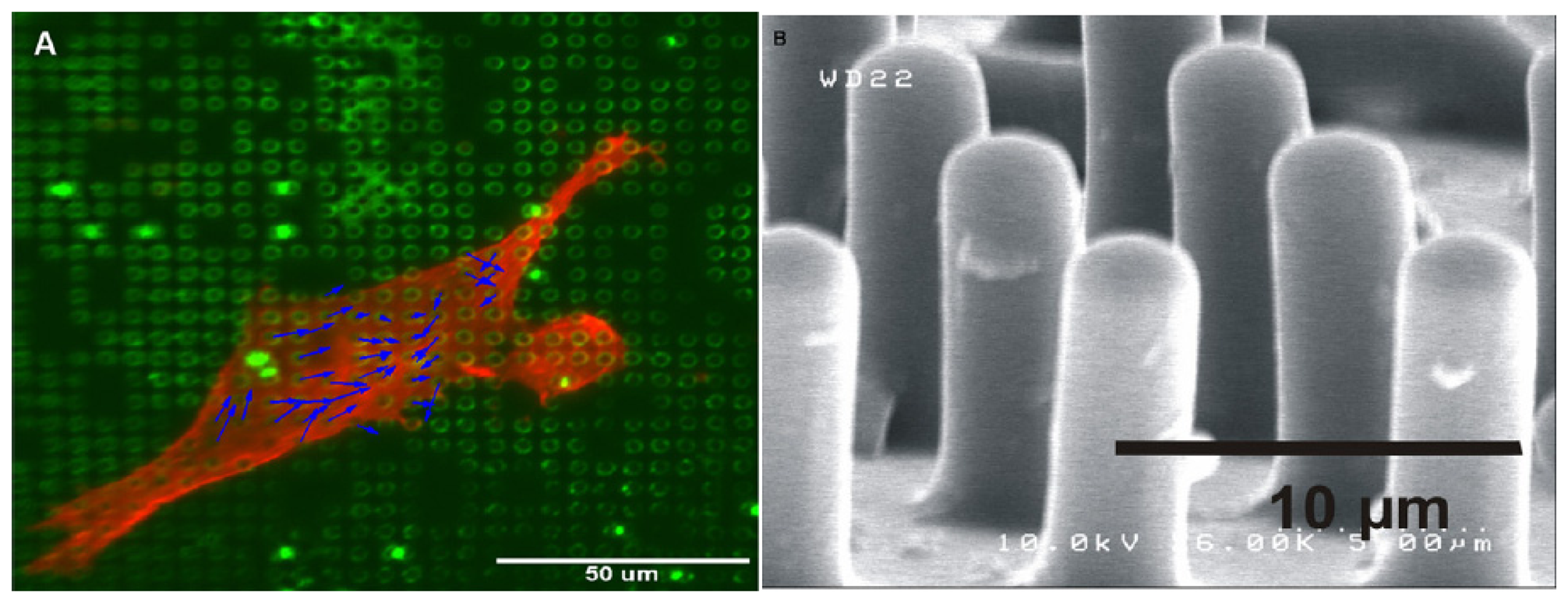
- Addae-Mensah, K.A.; et al. A flexible, quantum dot-labeled cantilever post array for studying cellular microforces. Sensors and Actuators A 2007, 136, 385–397. [Google Scholar]


| QD-coating | Matrix | measurand | Mechanism | Features | platform | REF |
|---|---|---|---|---|---|---|
| CdSe | SiO2Sol-gel, PLMA | Temperature | luminescence quenching and bandgap shift; | 100-315 K, 0.1 nm/K | glass slides | [91] |
| CdSe | PMMA | Methanol and toluene | luminescence quenching and enhancement | Selectivity, PCA | glass slides | [98] |
| CdSe-ZnS | Acrylic (nanospheres) | K+, Cl- | FRET with chromophores and luminophores | Dual sensing | latex microspheres | [101] |
| CdSe-CdS | PVC/DOS | Na+ | Ion exchange and energy transfer | Selectivity | microspheres | [102] |
| CdSe-ZnS | MIP | Caffeine | luminescence quenching; | Selectivity | grinded polymer | [103] |
| CdSe-ZnS | polystyrene | IgG | affinity binding with labelled goat antihuman IgG | QDs used for color coding | polymer microbeads | [121] |
| QD-coating | Matrix | Measurand | Mechanism | Features | Fiber platform | probe | REF |
|---|---|---|---|---|---|---|---|
| CdSe | Phosphate glass | Temperature | bandgap shift - absorption | 0-150°C, 0.12 nm/K | multimode | extrinsic | [138] |
| CdSe | Schott glass filter | Temperature | bandgap shift - absorption | Simultaneous detection O2 and temperature | multimode | extrinsic | [139] |
| CdSe-ZnS-TOPO; CdTe-ZnS | Sol-gel | Temperature | luminescence quenching and bandgap shift; | Self referenced Multiplexed | fiber bundle | extrinsic | [140] |
| CdTe- PDDA | LbL | Temperature | luminescence quenching; | 30-100°C, 0,2 nm/°C | Multimode/ tapered | intrinsic | [144] |
| CdTe- PDDA | LbL | Temperature | luminescence quenching; | 30-100C, 0,2 nm/°C | hollow core | intrinsic | [145] |
| CdSe-ZnS-TOPO + Ru(dpp) | Sol-gel | O2 | ratiometric detection | QD as intensity reference | fiber bundle | extrinsic | [148] |
| CdSe-ZnS-TOPO + Ru(dpp) | Sol-gel | O2 & temperature | luminescence quenching and bandgap shift; | Simultaneous detection O2 and temperature | multimode | extrinsic | [147] |
| CdTe-ZnS +Ru(dpp) | Sol-gel | O2 | QD excited by O2 sensing dye. | O2 sensitivity at higher λ | fiber bundle | extrinsic | [148] |
| CdSe | solution in PCF holes | Oxidative species | luminescence quenching | partially reversible | PCF | intrinsic | [154] |
| Qdot655-ProteinA | Glass plate | IgG | FRET quenching | fast reagent less imunodetection | fiber bundle | extrinsic | [149] |
© 2007 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Jorge, P.; Martins, M.A.; Trindade, T.; Santos, J.L.; Farahi, F. Optical Fiber Sensing Using Quantum Dots. Sensors 2007, 7, 3489-3534. https://doi.org/10.3390/s7123489
Jorge P, Martins MA, Trindade T, Santos JL, Farahi F. Optical Fiber Sensing Using Quantum Dots. Sensors. 2007; 7(12):3489-3534. https://doi.org/10.3390/s7123489
Chicago/Turabian StyleJorge, Pedro, Manuel António Martins, Tito Trindade, José Luís Santos, and Faramarz Farahi. 2007. "Optical Fiber Sensing Using Quantum Dots" Sensors 7, no. 12: 3489-3534. https://doi.org/10.3390/s7123489






