A Sensitive Chemiluminescence Method for Determination of Hydroquinone and Catechol
Abstract
:1. Introduction
2. Experimental
2.1. Reagents
2.2. Apparatus
2.3. Procedures
3. Results and Discussions
3.1. The CL Characteristics of the Reaction System
3.2. Effect of β-CD and NaOH Concentrations
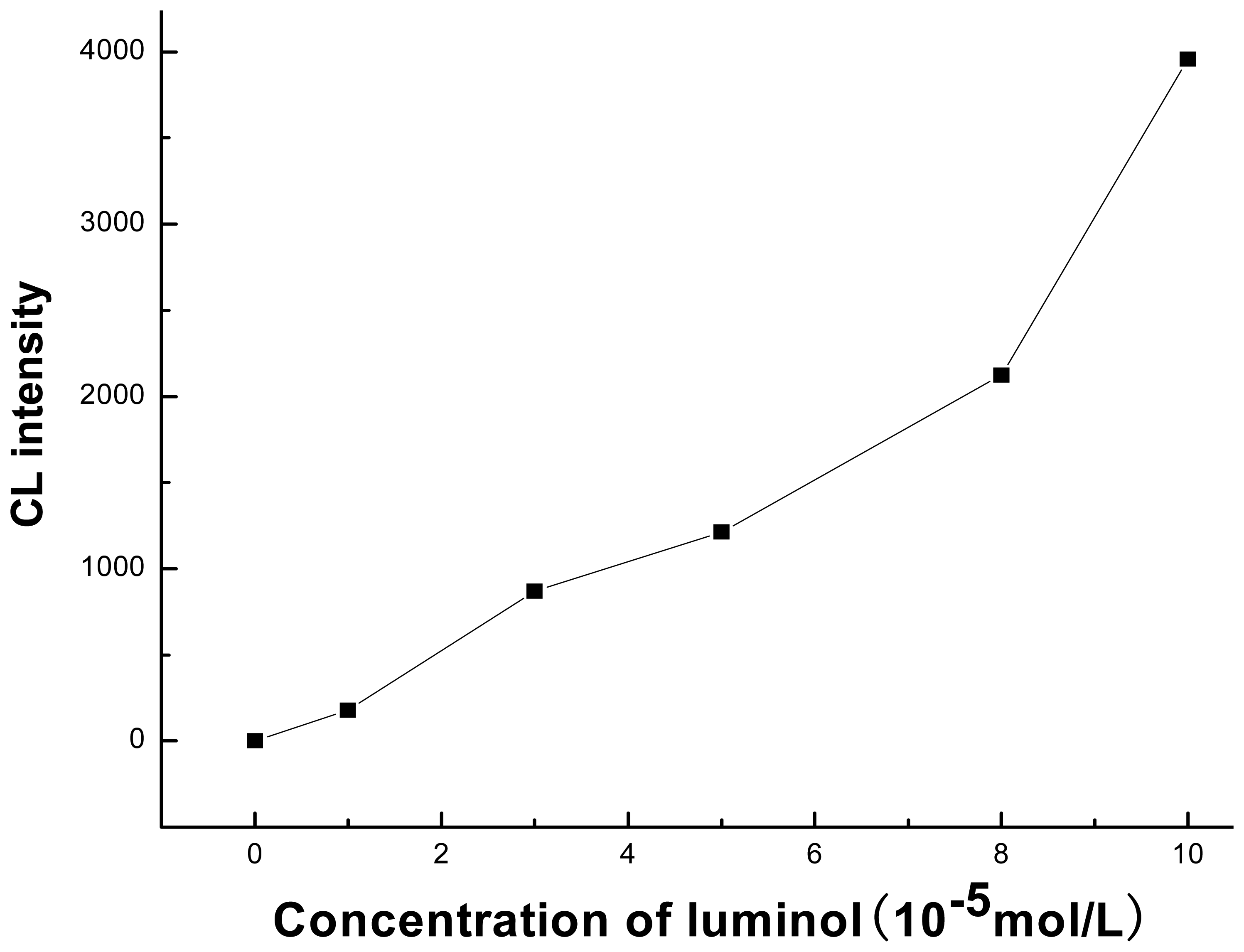
3.3. Effect of Luminol Concentration
3.4. Effect of KMnO4 Concentration
3.5. Calibration Curves and Detection Limits
3.6 Interference studies
3.7 Analytical applications
3.8 Possible CL mechanism of the reaction






4. Conclusions
Acknowledgments
References
- Rueda, M. E.; Sarabia, L. A.; Herrero, A.; Ortiz, M.C. Optimisation of a flow injection system with electrochemical detection using the desirability function Application to the determination of hydroquinone in cosmetics. Anal. Chim. Acta. 2003, 479, 173–184. [Google Scholar]
- Cui, H.; He, C. X.; Zhao, G. W. Determination of polyphenols by high-performance liquid chromatography with inhibited chemiluminescence detection. J. Chromatogr. A. 1999, 855, 171–179. [Google Scholar]
- Lavrent, K.; Julien, A.; Zofia, M.; Barry, D. Formation of Cyclopentadienyl Radical from the Gas-Phase Pyrolysis of Hydroquinone, Catechol, and Phenol. Environ. Sci. Technol. 2006, 40, 5071–5076. [Google Scholar]
- William, S. S.; Robert, M. M.; Maurice, E. S.; Ralph, E. W. Pyrolytic Studies on the Contribution of Tobacco Leaf Constituents to the Formation of Smoke Catechols. J. Agric. Food Chem. 1982, 30, 372–374. [Google Scholar]
- Steven, G. C.; Stephen, S. H.; T, T. C.; Dietrich, H. Roles of Tobacco Cellulose, Sugars, and Chlorogenic Acid as Precursors to Catechol in Cigarette Smoke. J. Agric. Food Chem. 1984, 32, 267–273. [Google Scholar]
- Nagaraja, P.; Vasantha, R.A.; Sunitha, K. R. A new sensitive and selective spectrophotometric method for the determination of catechol derivatives and its pharmaceutical preparations. J. Pharm. Biomed. Anal. 2001, 25, 417–424. [Google Scholar]
- Parham, H.; Pourreza, N.; Cheraghi, S. Kinetic-spectrophotometric determination of trace amounts of resorcinol based on its inhibitory effect on the formaldehyde catalyzed reaction between bromate and neutral red. Anal. Lett. 1999, 32, 1917–1926. [Google Scholar]
- Wang, L. H.; Kuo, Y. P. Simultaneous quantitative determination of resorcinol and 1-naphthol in haircolor products by high-performance liquid chromatography. Chromatographia 1999, 49, 208–211. [Google Scholar]
- Sun, Y. G.; Cui, H.; Li, Y. H.; Lin, X. Q. Determination of some catechol derivatives by a flow injection electrochemiluminescent inhibition method. Talanta. 2000, 53, 661–666. [Google Scholar]
- He, D. Y.; Zhang, Z. J.; He, C. Investigation on the interaction between dihydroxybenzene and Fe3+-H2O2-Rh6G system based on enhancing chemiluminescence. Luminescence 2006, 21, 15. [Google Scholar]
- Kamil, M.; Antonije, O.; Pavel, M.; Zbyněk, V. Flow-injection chemiluminescence determination of formaldehyde in water. Talanta. 2007, 71, 900–905. [Google Scholar]
- Cao, W.; Yang, J. H.; Sun, C. X.; Zhang, Z. J.; Gao, Q. F. Flow-injection-chemiluminescence method for the determination of penicillin G potassium. Luminescence. 2005, 20, 238–242. [Google Scholar]
- Lv, Y.; Zhao, R.; Zhu, Z. L.; Xu, X.S.; Zhang, X. R. A novel chemiluminescence method for the determination of orciprenaline based on ferricyanide-rhodamine 6G. Luminescence. 2005, 20, 298–302. [Google Scholar]
- Yao, H.; Sun, Y. Y.; Lin, X. H.; Cheng, J. H.; Huang, L. Y. Flow-injection chemiluminescence determination of catecholamines based on their enhancing effects on the luminol–potassium periodate system. Luminescence. 2006, 21, 112–117. [Google Scholar]
- Cui, H.; Xie, C. G.; Lai, C. Z. A novel chemiluminescent method for determination of phloroglucinol. Luminescence. 2003, 18, 318–323. [Google Scholar]
- Liu, E. B.; Xue, B. C. Flow injection determination of adenine at trace level based on luminol– K2Cr2O7 chemiluminescence in a micellar medium. J. Pharm. Biomed. Anal. 2006, 41, 649–653. [Google Scholar]
- Du, J. X.; Li, Y. H.; Lu, J. R. Flow injection chemiluminescence determination of polyhydroxy phenols using luminol–ferricyanide/ferrocyanide system. Talanta 2001, 55, 1055–1058. [Google Scholar]
- Lu, Y.; Zhang, Z. J.; He, D. Y.; Hu, Y. F. Flow injection chemiluminescence determination of polyhydroxy phenols in the presence of Rhodamine B as a sensitiser. Chem. Anal. 2003, 48, 959–966. [Google Scholar]
- Song, Z. H.; Wang, L. Resorcinol chemosensor based on detection of chemiluminescence with immobilized reagents. Microchem. J. 2001, 68, 47–52. [Google Scholar]
- Du, J. X.; Liu, W. X.; Lu, J. R. Investigation on the chemiluminescence behavior of alkaline earth metal ions Mg2+, Ca2+, Sr2+ and Ba2+ in luminol-permanganate reaction. Acta Chimica Sinica. 2004, 14, 1323–1326. [Google Scholar]
- Li, Y. H.; Niu, W. F.; Lu, J. R. Sensitive determination of phenothiazines in pharmaceutical preparation and biological fluid by flow injection chemiluminescence method using luminol– KMnO4 system. Talanta 2007, 71, 1124–1129. [Google Scholar]
- Emil, H. W.; Oliver, Z.; Heinz, H. K.; John, H. M. H. Chemiluminescence of Luminol and Related Hydrazides : The Light Emission Step. J. Am. Chem.Soc. 1964, 86, 941–942. [Google Scholar]
- Cao, Z. J.; Choiwan, L.; Lu, J. Z. A general chemiluminescence method for the determination of surfactants based on its quenching effect on the luminol-NaIO4-cyclodextrin reaction. Analyst. 2004, 129, 1262–1266. [Google Scholar]






© 2007 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Zhao, L.; Lv, B.; Yuan, H.; Zhou, Z.; Xiao, D. A Sensitive Chemiluminescence Method for Determination of Hydroquinone and Catechol. Sensors 2007, 7, 578-588. https://doi.org/10.3390/s7040578
Zhao L, Lv B, Yuan H, Zhou Z, Xiao D. A Sensitive Chemiluminescence Method for Determination of Hydroquinone and Catechol. Sensors. 2007; 7(4):578-588. https://doi.org/10.3390/s7040578
Chicago/Turabian StyleZhao, Lijun, Baoqiang Lv, Hongyan Yuan, Zaide Zhou, and Dan Xiao. 2007. "A Sensitive Chemiluminescence Method for Determination of Hydroquinone and Catechol" Sensors 7, no. 4: 578-588. https://doi.org/10.3390/s7040578




