An Investigation of Glutathione-Platinum(II) Interactions by Means of the Flow Injection Analysis Using Glassy Carbon Electrode
Abstract
:1. Introduction
2. Experimental
2.1 Chemicals
2.2 Preparation of cisplatin solutions
2.3 Electrochemical measurements
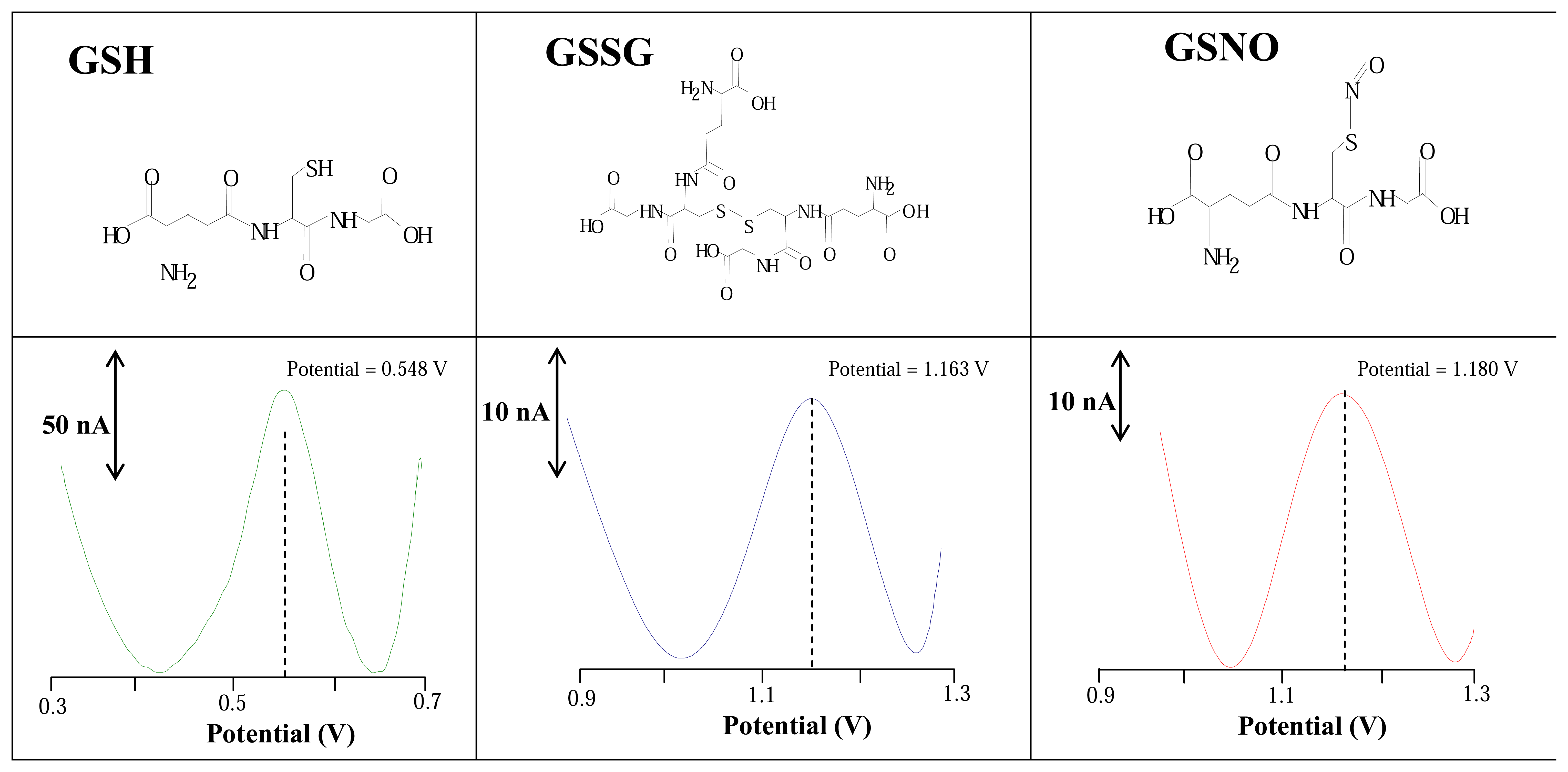
2.4 Square wave voltammetry measurements of GSH, GSSG and GSNO
2.5 Preparation of GS-Pt complexes
2.6 Flow electrochemical measurement
2.7 Spectroscopic analysis
2.8 Statistical analysis
3. Results and Discussion
3.1 Stationary electrochemical system
3.2 Electrochemical analysis of glutathiones in flow system
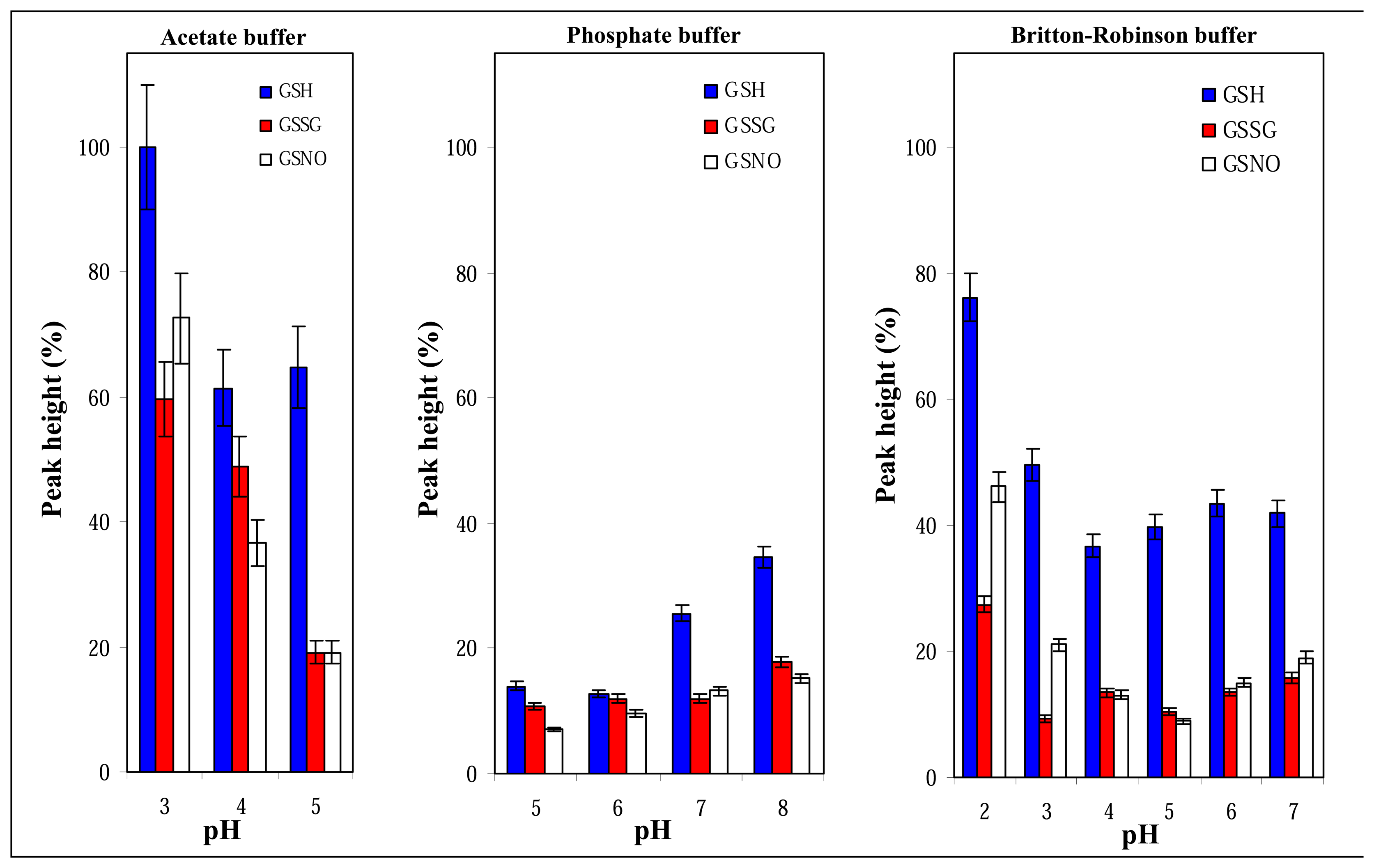
3.2.1 Mobile phase composition and its pH
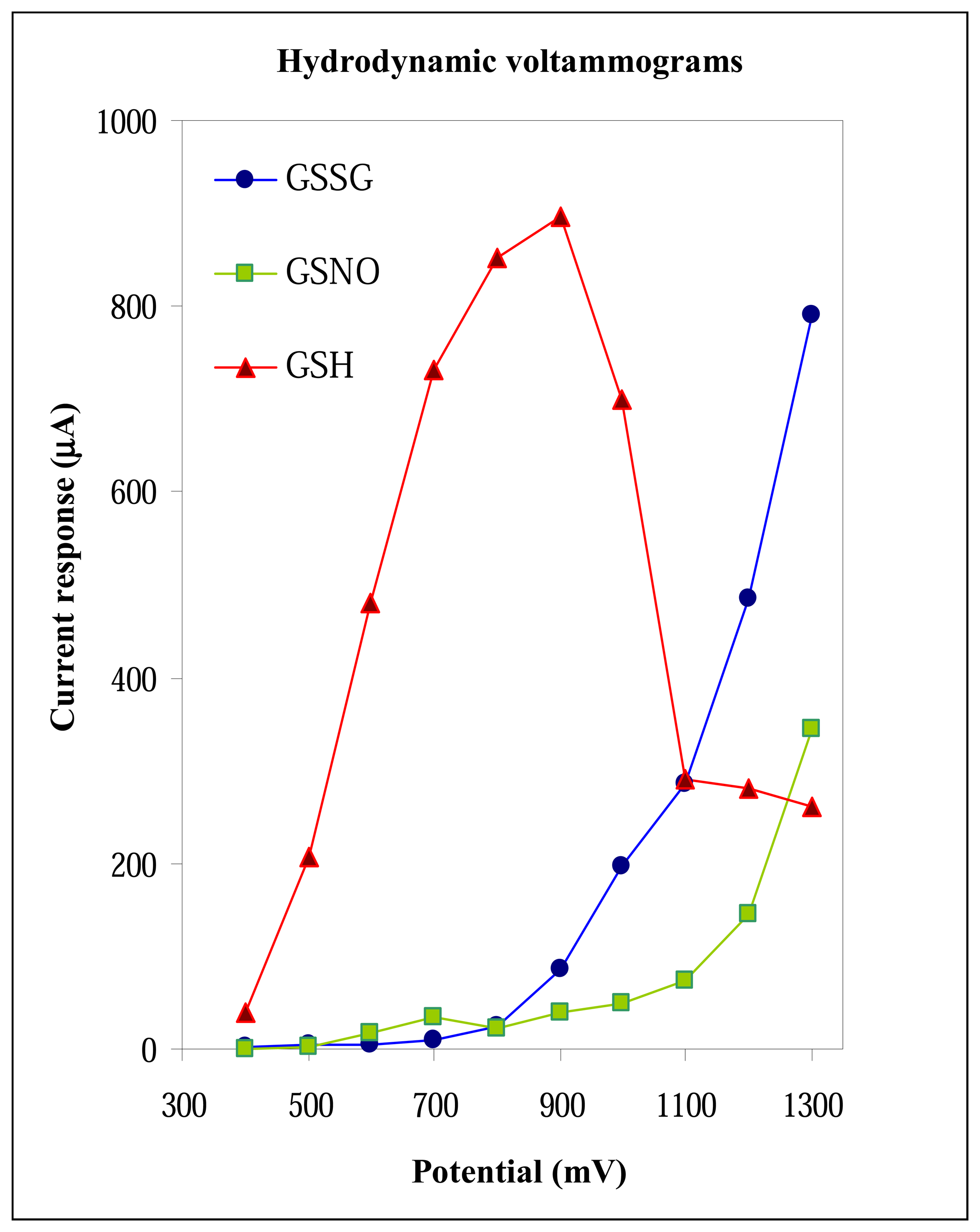
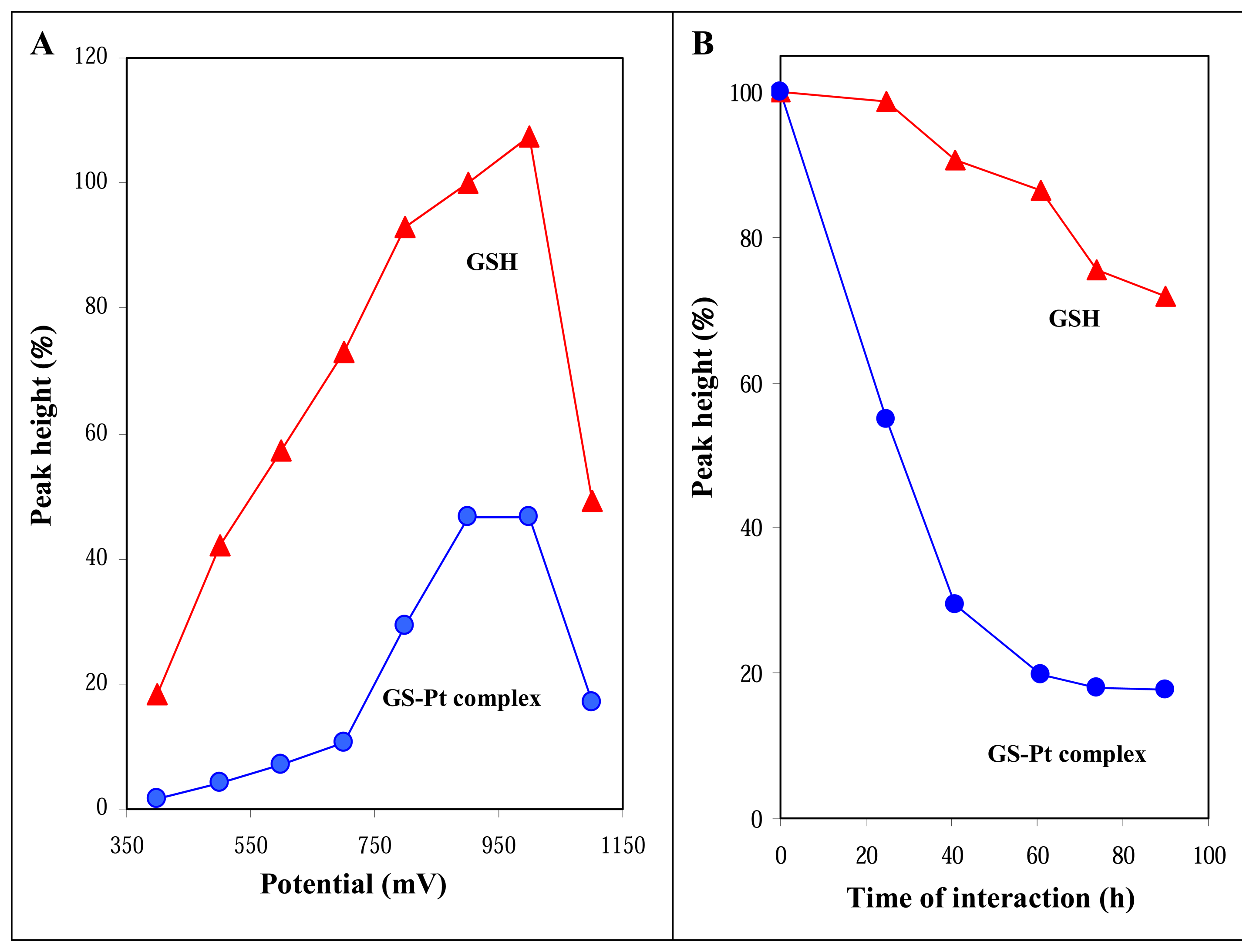
3.2.2 Applied potential on glassy carbon working electrode
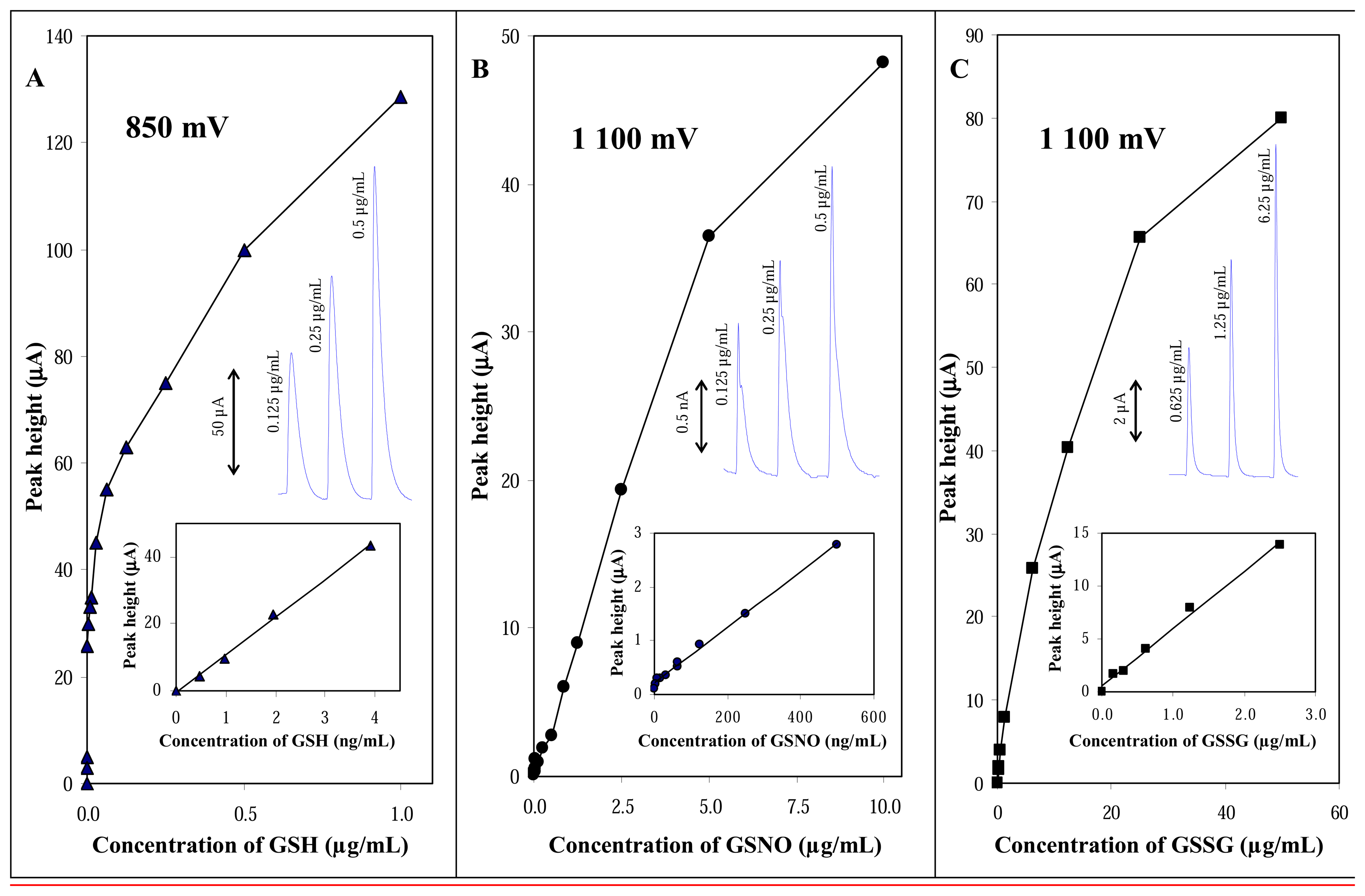
3.2.3 Calibration curves
3.3 Study of GSH-cisplatin interaction
Conclusion
Acknowledgments
References
- Hopkins, F.G. On an autoxidisable constituent of the cell. Biochem. J. 1921, 15, 286–305. [Google Scholar]
- Carelli, S.; Ceriotti, A.; Cabibbo, A.; Fassina, G.; Ruvo, M.; Sitia, R. Cysteine and glutathione secretion in response to protein disulfide bond formation in the ER. Science 1997, 277, 1681–1684. [Google Scholar]
- Locigno, R.; Castronovo, V. Reduced glutathione system: Role in cancer development, prevention and treatment (Review). Int. J. Oncol. 2001, 19, 221–236. [Google Scholar]
- Noctor, G.; Foyer, C.H. Ascorbate and glutathione: Keeping active oxygen under control. Annu. Rev. Plant Biol. 1998, 49, 249–279. [Google Scholar]
- Townsend, D.M.; Tew, K.D.; Tapiero, H. The importance of glutathione in human disease. Biomed. Pharmacother. 2003, 57, 145–155. [Google Scholar]
- Droge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar]
- Sen, C.K.; Packer, L. Antioxidant and redox regulation of gene transcription. Faseb J. 1996, 10, 709–720. [Google Scholar]
- Hayes, J.D.; Pulford, D.J. The glutathione S-Transferase supergene family: Regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit. Rev. Biochem. Mol. Biol. 1995, 30, 445–600. [Google Scholar]
- Yegorova, S.; Yegorov, O.; Lou, M.F. Thioredoxin induced antioxidant gene expressions in human lens epithelial cells. Exp. Eye Res. 2006, 83, 783–792. [Google Scholar]
- Sehlotho, N.; Griveau, S.; Nyokong, T.; Bedioui, F. Cobalt phthalocyanine molecular electrode for the electrochemical investigation of the release of glutathione upon copper-catalyzed decomposition of S-nitrosoglutathione. Electroanalysis 2007, 19, 103–106. [Google Scholar]
- Musameh, M.; Moezzi, N.; Schauman, L.M.; Meyerhoff, M.E. Glutathione peroxidase-based amperometric biosensor for the detection of S-nitrosothiols. Electroanalysis 2006, 18, 2043–2048. [Google Scholar]
- Balendiran, G.K.; Dabur, R.; Fraser, D. The role of glutathione in cancer. Cell Biochem. Function 2004, 22, 343–352. [Google Scholar]
- Clancy, R.M.; Levartovsky, D.; Leszczynskapiziak, J.; Yegudin, J.; Abramson, S.B. Nitric-oxide reacts with intracellular glutathione and activates the hexose-monophosphate shunt in human neutrophils - Evidence for S-nitrosoglutathione as a bioactive intermediary. P. Natl. Acad. Sci. USA 1994, 91, 3680–3684. [Google Scholar]
- Vitecek, J.; Petrlova, J.; Petrek, J.; Adam, V.; Potesil, D.; Havel, L.; Mikelova, R.; Trnkova, L.; Kizek, R. Electrochemical study of S-nitrosoglutathione and nitric oxide by carbon fibre NO sensor and cyclic voltammetry - possible way of monitoring of nitric oxide. Electrochim. Acta 2006, 51, 5087–5094. [Google Scholar]
- Meister, A. Glutathione Metabolism and Its Selective Modification. J. Biol. Chem. 1988, 263, 17205–17208. [Google Scholar]
- Brabec, V.; Kasparkova, J. Molecular aspects of resistance to antitumor platinum drugs. Drug Resist. Update 2002, 5, 147–161. [Google Scholar]
- Kasparkova, J.; Novakova, O.; Vrana, O.; Intini, F.; Natile, G.; Brabec, V. Molecular aspects of antitumor effects of a new platinum(IV) drug. Mol. Pharmacol. 2006, 70, 1708–1719. [Google Scholar]
- Ramos-Lima, F.J.; Vrana, O.; Quiroga, A.G.; Navarro-Ranninger, C.N.; Halamikova, A.; Rybnickova, H.; Hejmalova, L.; Brabec, V. Structural characterization, DNA interactions, and cytotoxicity of new transplatin analogues containing one aliphatic and one planar heterocyclic amine ligand. J. Med. Chem. 2006, 49, 2640–2651. [Google Scholar]
- Loe, D.W.; Almquist, K.C.; Deeley, R.G.; Cole, S.P.C. Multidrug resistance protein (MRP)-mediated transport of leukotriene C-4 and chemotherapeutic agents in membrane vesicles -Demonstration of glutathione-dependent vincristine transport. J. Biol. Chem. 1996, 271, 9675–9682. [Google Scholar]
- Estrela, J.M.; Ortega, A.; Obrador, E. Glutathione in cancer biology and therapy. Crit. Rev. Clin. Lab. Sci. 2006, 43, 143–181. [Google Scholar]
- Kauvar, L.M.; Morgan, A.S.; Sanderson, P.E.; Henner, W.D. Glutathione based approaches to improving cancer treatment. Chem.-Biol. Interact. 1998, 112, 225–238. [Google Scholar]
- Clynes, M.; Heenan, M.; Hall, K. Human Cell-Lines as Models for Multidrug-Resistance in Solid Tumors. Cytotechnology 1993, 12, 231–256. [Google Scholar]
- Mitchell, J.B.; Glatstein, E. Radiation Oncology - Past Achievements and Ongoing Controversies. Cancer Res. 1991, 51, S5065–S5073. [Google Scholar]
- Riddick, D.S.; Lee, C.; Ramji, S.; Chinje, E.C.; Cowen, R.L.; Williams, K.J.; Patterson, A.V.; Stratford, I.J.; Morrow, C.S.; Townsend, A.J.; Jounaidi, Y.; Chen, C.S.; Su, T.; Lu, H.; Schwartz, P.S.; Waxman, D.J. Cancer chemotherapy and drug metabolism. Drug Metab. Dispos. 2005, 33, 1083–1096. [Google Scholar]
- Raymond, E.; Faivre, S.; Chaney, S.; Woynarowski, J.; Cvitkovic, E. Cellular and molecular pharmacology of oxaliplatin. Mol. Cancer Ther. 2002, 1, 227–235. [Google Scholar]
- Raymond, E.; Chaney, S.G.; Taamma, A.; Cvitkovic, E. Oxaliplatin: A review of preclinical and clinical studies. Ann. Oncol. 1998, 9, 1053–1071. [Google Scholar]
- Fuertes, M.A.; Castilla, J.; Alonso, C.; Perez, J.M. Cisplatin biochemical mechanism of action: From cytotoxicity to induction of cell death through interconnections between apoptotic and necrotic pathways. Curr. Med. Chem. 2003, 10, 257–266. [Google Scholar]
- Ong, S.T.; Vogelzang, N.J. Chemotherapy in malignant pleural mesothelioma: A review. J. Clin. Oncol. 1996, 14, 1007–1017. [Google Scholar]
- Weiss, R.B.; Christian, M.C. New Cisplatin Analogs in Development - a Review. Drugs 1993, 46, 360–377. [Google Scholar]
- Pivonkova, H.; Brazdova, M.; Kasparkova, J.; Brabec, V.; Fojta, M. Recognition of cisplatin-damaged DNA by p53 protein: Critical role of the p53 C-terminal domain. Biochem. Biophys. Res. Commun. 2006, 339, 477–484. [Google Scholar]
- Kasparkova, J.; Pospisilova, S.; Brabec, V. Different recognition of DNA modified by antitumor cisplatin and its clinically ineffective trans isomer by tumor suppressor protein p53. J. Biol. Chem. 2001, 276, 16064–16069. [Google Scholar]
- Rosenberg, B.; Van Camp, L.; Krigas, T. Inhibition of cell division in Escherichia coli by electrolysis products from a platinum electrode. Nature 1965, 205, 698–699. [Google Scholar]
- Rietz, B.; Krarup-Hansen, A.; Rorth, M. Determination of platinum by radiochemical neutron activation analysis in neural tissues from rats, monkeys and patients treated with cisplatin. Anal. Chim. Acta 2001, 426, 119–126. [Google Scholar]
- Stergiou, V.A.; Thomas, L.V.; Adams, M.R. Interactions of nisin with glutathione in a model protein system and meat. J. Food Prot. 2006, 69, 951–956. [Google Scholar]
- Kikugawa, K.; Kawa, N.; Miyazawa, A.; Shindo, K.; Kato, T. Interaction of nitric oxide with glutathione or cysteine generates reactive oxygen species causing DNA single strand breaks. Biol. Pharm. Bull. 2005, 28, 998–1003. [Google Scholar]
- Spuches, A.M.; Kruszyna, H.G.; Rich, A.M.; Wilcox, D.E. Thermodynamics of the As(III)-thiol interaction: Arsenite and monomethylarsenite complexes with glutathione, dihydrolipoic acid, and other thiol ligands. Inorg. Chem. 2005, 44, 2964–2972. [Google Scholar]
- Pietrini, F.; Iannelli, M.A.; Pasqualini, S.; Massacci, A. Interaction of cadmium with glutathione and photosynthesis in developing leaves and chloroplasts of Phragmites australis (Cav.) Trin. ex steudel. Plant Physiol. 2003, 133, 829–837. [Google Scholar]
- Petrlova, J.; Potesil, D.; Zehnalek, J.; Sures, B.; Adam, V.; Trnkova, L.; Kizek, R. Cisplatin electrochemical biosensor. Electrochim. Acta 2006, 51, 5169–5173. [Google Scholar]
- Krizkova, S.; Adam, V.; Petrlova, J.; Zitka, O.; Stejskal, K.; Zehnalek, J.; Sures, B.; Trnkova, L.; Beklova, M.; Kizek, R. A suggestion of electrochemical biosensor for study of platinum(II)-DNA interactions. Electroanalysis 2007, 19, 331–338. [Google Scholar]
- Kizek, R.; Vacek, J.; Trnkova, L.; Jelen, F. Cyclic voltammetric study of the redox system of glutathione using the disulfide bond reductant tris(2-carboxyethyl)phosphine. Bioelectrochemistry 2004, 63, 19–24. [Google Scholar]
- Dolezel, P.; Kuban, V. Mass spectrometric study of platinum complexes based on cisplatin. Chem. Pap.-Chem. Zvesti 2002, 56, 236–240. [Google Scholar]
- Adam, V.; Zehnalek, J.; Petrlova, J.; Potesil, D.; Sures, B.; Trnkova, L.; Jelen, F.; Vitecek, J.; Kizek, R. Phytochelatin modified electrode surface as a sensitive heavy-metal ion biosensor. Sensors 2005, 5, 70–84. [Google Scholar]
- Kizek, R.; Masarik, M.; Kramer, K.J.; Potesil, D.; Bailey, M.; Howard, J.A.; Klejdus, B.; Mikelova, R.; Adam, V.; Trnkova, L.; Jelen, F. An analysis of avidin, biotin and their interaction at attomole levels by voltammetric and chromatographic techniques. Anal. Bioanal. Chem. 2005, 381, 1167–1178. [Google Scholar]
- Masarik, M.; Kizek, R.; Kramer, K.J.; Billova, S.; Brazdova, M.; Vacek, J.; Bailey, M.; Jelen, F.; Howard, J.A. Application of avidin-biotin technology and adsorptive transfer stripping square-wave voltammetry for detection of DNA hybridization and avidin in transgenic avidin maize. Anal. Chem. 2003, 75, 2663–2669. [Google Scholar]
- Petrlova, J.; Masarik, M.; Potesil, D.; Adam, V.; Trnkova, L.; Kizek, R. Zeptomole detection of streptavidin using carbon paste electrode and square wave voltammetry. Electroanalysis 2007, 19, 1177–1182. [Google Scholar]
- Bromba, M.U.A.; Ziegler, H. Application hints for Savitzky-Golay digital smoothing filtres. Anal. Chem. 1981, 53, 1583–1586. [Google Scholar]
- Kizek, R.; Vacek, J.; Trnkova, L.; Klejdus, B.; Havel, L. Application of catalytic reactions on a mercury electrode for metallothionein electrochemical detection. Chem. Listy 2004, 98, 166–173. [Google Scholar]
- Ishikawa, T.; Aliosman, F. Glutathione-Associated Cis-Diamminedichloroplatinum(Ii) Metabolism and Atp-Dependent Efflux from Leukemia-Cells - Molecular Characterization of Glutathione-Platinum Complex and Its Biological Significance. J. Biol. Chem. 1993, 268, 20116–20125. [Google Scholar]
- Arner, E.S.J.; Nakamura, H.; Sasada, T.; Yodoi, J.; Holmgren, A.; Spyrou, G. Analysis of the inhibition of mammalian thioredoxin, thioredoxin reductase, and glutaredoxin by cis-diamminedichloroplatinum (II) and its major metabolite, the glutathione-platinum complex. Free Radic. Biol. Med. 2001, 31, 1170–1178. [Google Scholar]
- Adam, V.; Petrlova, J.; Potesil, D.; Zehnalek, J.; Sures, B.; Trnkova, L.; Jelen, F.; Kizek, R. Study of metallothionein modified electrode surface behavior in the presence of heavy metal ions-biosensor. Electroanalysis 2005, 17, 1649–1657. [Google Scholar]
- Long, G.L.; Winefordner, J.D. Limit of Detection. Anal. Chem. 1983, 55, A712, -&. [Google Scholar]
- Cole, S.P.C.; Sparks, K.E.; Fraser, K.; Loe, D.W.; Grant, C.E.; Wilson, G.M.; Deeley, R.G. Pharmacological Characterization of Multidrug-Resistant Mrp-Transfected Human Tumor-Cells. Cancer Res. 1994, 54, 5902–5910. [Google Scholar]
- Prusa, R.; Svoboda, M.; Blastik, O.; Adam, V.; Zitka, O.; Beklova, M.; Eckschlager, T.; Kizek, R. Increase in content of metallothionein as marker of resistence to cisplatin treatment. Clin. Chem. 2006, 52, A174–A175. [Google Scholar]
- Prusa, R.; Petrlova, J.; Kukacka, J.; Adam, V.; Sures, B.; Beklova, M.; Kizek, R. Study of interaction of glutathiones and metallothionein with cytostatics. Clin. Chem. 2006, 52, A175–A175. [Google Scholar]
- Petrlova, J.; Potesil, D.; Mikelova, R.; Blastik, O.; Adam, V.; Trnkova, L.; Jelen, F.; Prusa, R.; Kukacka, J.; Kizek, R. Attomole voltammetric determination of metallothionein. Electrochim. Acta 2006, 51, 5112–5119. [Google Scholar]
- Prusa, R.; Blastik, O.; Potesil, D.; Trnkova, L.; Zehnalek, J.; Adam, V.; Petrlova, J.; Jelen, F.; Kizek, R. Analytic method for determination of metallothioneins as tumor markers. Clin. Chem. 2005, 51, A56–A56. [Google Scholar]
- Prusa, R.; Kizek, R.; Trnkova, L.; Vacek, J.; Zehnalek, J. Study of relationship between metallothionein and heavy metals by CPSA method. Clin. Chem. 2004, 50, A28–A29. [Google Scholar]
- Ciardiello, F.; Caputo, R.; Bianco, R.; Damiano, V.; Pomatico, G.; De Placido, S.; Bianco, A.R.; Tortora, G. Antitumor effect and potentiation of cytotoxic drugs activity in human cancer cells by ZD-1839 (Iressa), an epidermal growth factor receptor-selective tyrosine kinase inhibitor. Clin. Cancer Res. 2000, 6, 2053–2063. [Google Scholar]
- Rose, P.G.; Bundy, B.N.; Watkins, E.B.; Thigpen, J.T.; Deppe, G.; Maiman, M.A.; Clarke-Pearson, D.L.; Insalaco, S. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N. Engl. J. Med. 1999, 340, 1144–1153. [Google Scholar]
- Eisenhauer, E.A.; Huinink, W.W.T.; Swenerton, K.D.; Gianni, L.; Myles, J.; Vanderburg, M.E.L.; Kerr, I.; Vermorken, J.B.; Buser, K.; Colombo, N.; Bacon, M.; Santabarbara, P.; Onetto, N.; Winograd, B.; Canetta, R. European-Canadian Randomized Trial of Paclitaxel in Relapsed Ovarian-Cancer - High-Dose Versus Low-Dose and Long Versus Short Infusion. J. Clin. Oncol. 1994, 12, 2654–2666. [Google Scholar]
- Babula, P.; Huska, D.; Hanustiak, P.; Baloun, J.; Krizkova, S.; Adam, V.; Hubalek, J.; Havel, L.; Zemlicka, M.; Horna, A.; Beklova, M.; Kizek, R. Flow injection analysis coupled with carbon electrodes as the tool for analysis of naphthoquinones with respect to their content and functions in biological samples. Sensors 2006, 11, 1466–1482. [Google Scholar]
- Prasek, J.; Adamek, M.; Hubalek, J.; Adam, V.; Trnkova, L.; Kizek, R. New hydrodynamic electrochemical arrangement for cadmium ions detection using thick-film chemical sensor electrodes. Sensors 2006, 11, 1498–1512. [Google Scholar]
- Supalkova, V.; Petrek, J.; Havel, L.; Krizkova, S.; Petrlova, J.; Adam, V.; Potesil, D.; Babula, P.; Beklova, M.; Horna, A.; Kizek, R. Electrochemical sensors for detection of acetylsalicylic acid. Sensors 2006, 11, 1483–1497. [Google Scholar]
- Supalkova, V.; Huska, D.; Diopan, V.; Hanustiak, P.; Zitka, O.; Stejskal, K.; Baloun, J.; Pikula, J.; Havel, L.; Zehnalek, J.; Adam, V.; Trnkova, L.; Beklova, M.; Kizek, R. Electroanalysis of plant thiols. Sensors 2007, 7, 932–959. [Google Scholar]
- Supalkova, V.; Petrek, J.; Baloun, J.; Adam, V.; Bartusek, K.; Trnkova, L.; Beklova, M.; Diopan, V.; Havel, L.; Kizek, R. Multi-instrumental investigation of affecting of early somatic embryos of Spruce by cadmium(II) and lead(II) ions. Sensors 2007, 7, 743–759. [Google Scholar]
- Trnkova, L.; Jelen, F.; Petrlova, J.; Adam, V.; Potesil, D.; Kizek, R. Elimination voltammetry with linear scan as a new detection method for DNA sensors. Sensors 2005, 5, 448–464. [Google Scholar]
- Adam, V.; Krizkova, S.; Zitka, O.; Trnkova, L.; Petrlova, J.; Beklova, M.; Kizek, R. A determination of apo-metallothionein using adsorptive transfer stripping technique in connection with differential pulse voltammetry. Electroanalysis 2007, 19, 339–347. [Google Scholar]
- Beklova, M.; Krizkova, S.; Supalkova, V.; Mikelova, R.; Adam, V.; Pikula, J.; Kizek, R. Determination of bromadiolone in pheasants and foxes by differential pulse voltammetry. Int. J. Environ. Anal. Chem. 2007, 87, 459–469. [Google Scholar]
- Huska, D.; Zitka, O.; Adam, V.; Beklova, M.; Krizkova, S.; Zeman, L.; Horna, A.; Havel, L.; Zehnalek, J.; Kizek, R. A sensor for investigating the interaction between biologically important heavy metals and glutathione. Czech J. Anim. Sci. 2007, 52, 37–43. [Google Scholar]
- Krizkova, S.; Zitka, O.; Adam, V.; Beklova, M.; Horna, A.; Svobodova, Z.; Sures, B.; Trnkova, L.; Zeman, L.; Kizek, R. Possibilities of electrochemical techniques in metallothionein and lead detection in fish tissues. Czech J. Anim. Sci. 2007, 52, 143–148. [Google Scholar]
- Zitka, O.; Stejskal, K.; Kleckerova, A.; Adam, V.; Beklova, M.; Horna, A.; Havel, L.; Kizek, R. Utilizing of electrochemical techniques for detection of biological samples. Chem. Listy 2007, 101, 225–231. [Google Scholar]
© 2007 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Zitka, O.; Huska, D.; Krizkova, S.; Adam, V.; Chavis, G.J.; Trnkova, L.; Horna, A.; Hubalek, J.; Kizek, R. An Investigation of Glutathione-Platinum(II) Interactions by Means of the Flow Injection Analysis Using Glassy Carbon Electrode. Sensors 2007, 7, 1256-1270. https://doi.org/10.3390/s7071256
Zitka O, Huska D, Krizkova S, Adam V, Chavis GJ, Trnkova L, Horna A, Hubalek J, Kizek R. An Investigation of Glutathione-Platinum(II) Interactions by Means of the Flow Injection Analysis Using Glassy Carbon Electrode. Sensors. 2007; 7(7):1256-1270. https://doi.org/10.3390/s7071256
Chicago/Turabian StyleZitka, Ondrej, Dalibor Huska, Sona Krizkova, Vojtech Adam, Grace J. Chavis, Libuse Trnkova, Ales Horna, Jaromir Hubalek, and Rene Kizek. 2007. "An Investigation of Glutathione-Platinum(II) Interactions by Means of the Flow Injection Analysis Using Glassy Carbon Electrode" Sensors 7, no. 7: 1256-1270. https://doi.org/10.3390/s7071256



