Potentiometric Responses of Ion-Selective Electrodes Doped with Diureidocalix[4]arene towards Un-dissociated Benzoic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Membranes and Electrodes Preparation
2.3. EMF Measurements
3. Results and Discussion
3.1. Potential Response of ISEs Incorporating Diureidocalix[4]arene towards pH Changes
3.2. Potentiometric Responses of Diureidocalixarene-containing ISE towards Benzoic Acid
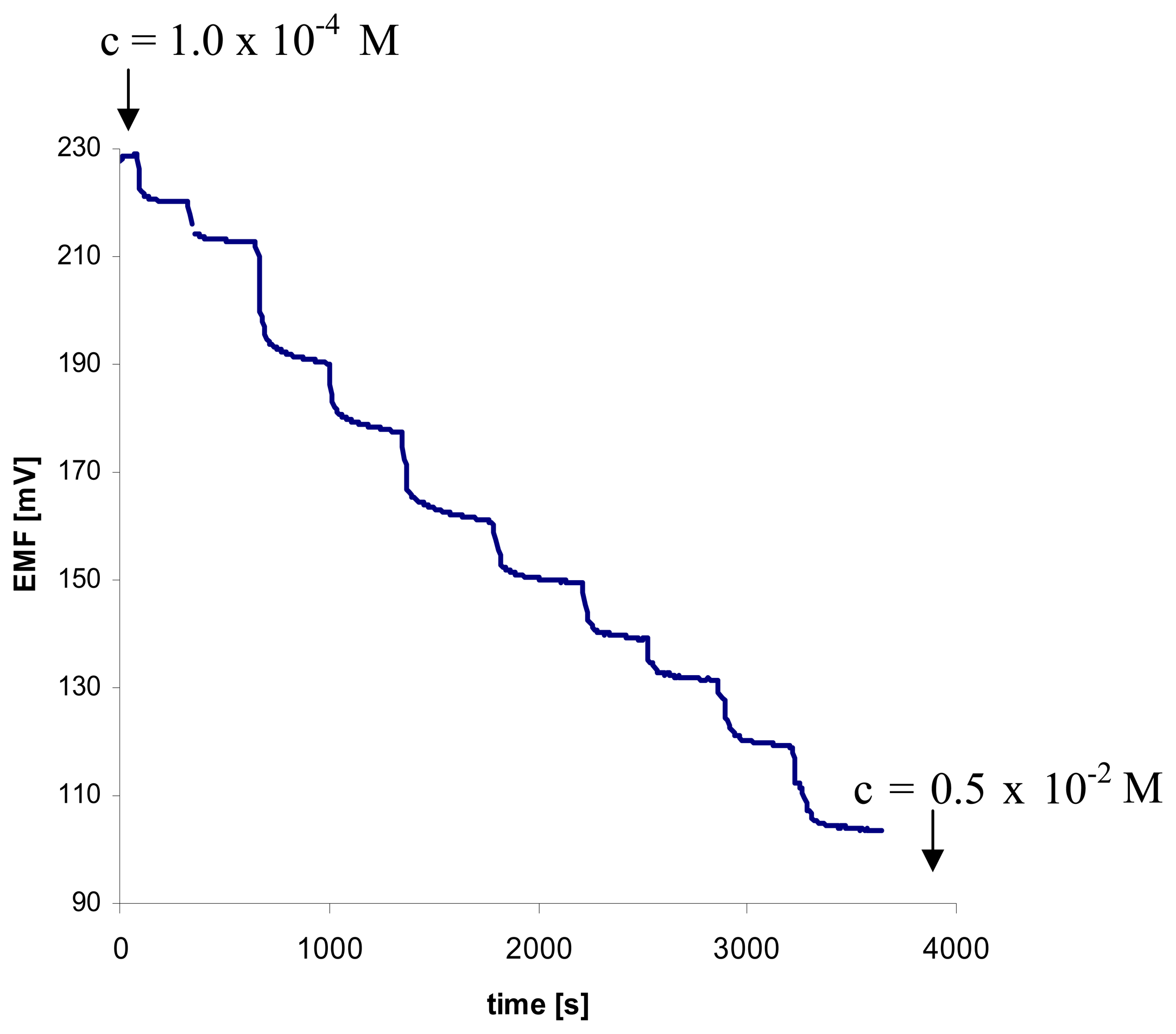
3.3. Time of Response of Diureidocalix[4]arene ISE towards Benzoic Acid
3.4. Potentiometric Selectivity of Electrode Incorporating Diureidocalix[4]arene
3.5. Determination of Benzoic Acid in Breverage
3.6. Recovery Test
3.7. Conclusions
Acknowledgments
References and Notes
- Ikeda, A.; Shinkai, S. Novel cavity design using calix[n]arene skeletons: toward molecular recognition and metal binding. Chem. Rev 1997, 97, 1713–1734. [Google Scholar]
- Ganjali, M.R.; Norouzi, P.; Rezapour, M.; Faridbod, F.; Pourjavid, M.R. Supramolecular based membrane sensors. Sensors 2006, 6, 1018–1086. [Google Scholar]
- Gutsche, C.D.; Iqgal, M.; Nam, K.S.; See, K.; Alam, I. Conformational and complexational characteristics of calixarenes. Pure and Appl. Chem. 1988, 60, 483–488. [Google Scholar]
- Chung, T.D.; Kim, H. Electrochemistry of calixarene and analytical applications. J. Incl. Phenom. Macro. 1998, 32, 179–193. [Google Scholar]
- van Dienst, E.; Bakker, W.I.I.; Engbersen, J.F.J.; Verboom, W.; Reinhoudt, D.N. Calixarenes, chemical chameleons. Pure and Appl. Chem. 1993, 65, 387–392. [Google Scholar]
- Stastny, V.; Lhotak, P.; Michlova, V.; Stibor, I.; Sykora, J. Novel biscalix[4]arene-based anion receptors. Tetrahedron 2002, 58, 7207–7211. [Google Scholar]
- Lhoták, P. Calixarene-based receptors for anion sensing. In Topics in Current Chemistry; 2005; Springer Verlag; Vol. 255, pp. 65–96. [Google Scholar]
- Beer, P.D.; Gale, P.A.; Hesek, D. A neutral upper to lower rim linked bis-calix[4]arene receptor that recognises anionic guest species. Tetrahedron Lett. 1995, 5, 767–770. [Google Scholar]
- Budka, J.; Lhotak, P.; Michlova, V.; Stibor, I. Urea derivatives of calix[4]arene 1, 3-alternate: an anion receptor with profound negative allosteric effect. Tetrahedron Lett. 2001, 42, 1583–1586. [Google Scholar]
- Wibbertmann, A.; Kielhorm, J.; Koennecker, G.; Gelsdorsf, I.; Meller, C. Benzoic acid and sodium benzoate; Concise International Chemical Assessment; document no. 26; Geneva, 2000. [Google Scholar]
- Basan, H.; Dincer, Z.; Goger, N.G. Derivative UV spectrophotometric method for the simultaneous determination of ambroxol and preservatives in syrup. Chem. Anal. (Warsaw) 2005, 50, 465. [Google Scholar]
- Hamano, T.; Mitsuhashi, Y.; Aoki, N.; Semma, M.; Ito, Y. Enzymatic metod for the spetrophotometric determination oc benzoic acid in soy sauce and pickles. Analyst 1997, 122, 259–262. [Google Scholar]
- Dong, C.; Wang, W. Headspace solid-phase microextraction applied to the simultaneous determination of sorbic and benzoic acids on beverages. Anal. Chim. Acta 2006, 562, 23–29. [Google Scholar]
- Pan, Z.; Wang, L.; Mo, W.; Wang, C.; Hu, W.; Zhang, J. Determination of benzoic acid in soft drinks by gas chromatography with on-line pyrolytic methylation technique. Anal. Chim. Acta 2005, 545, 218–223. [Google Scholar]
- Tfouni, S. A V.; Toledo, M.C.F. Determination of benzoic and sorbic acids in Brazilian food. Food Control. 2002, 13, 117–123. [Google Scholar]
- Ferreira, I. M. P. L. V. O.; Mendes, E.; Brito, P.; Ferreira, M.A. Simultaneous determination of benzoic and sorbic acids in quince jam by HPLC. Food Research International 2000, 33, 113–117. [Google Scholar]
- Walker, J. C.; Zaugg, S. E.; Walker, E. B. Analysis of beverages by capillary electrophoresis. J. Chromatogr. A. 1997, 781, 481–485. [Google Scholar]
- Buck, R.P.; Lindner, E. Recomendations for nomenclature of ion-selective electrodes (IUPAC Recommendation 1994). Pure and Appl. Chem. 1994, 66, 2527–2536. [Google Scholar]
- Umezawa, Y.; Umezawa, K.; Sato, H. Selectivity coefficients for ion-selective electrodes: recommended methods for reporting KpotA,B values. Pure and Appl. Chem. 1995, 67, 507–510. [Google Scholar]
- Tohda, K.; Dragoe, D.; Shibata, M.; Umezawa, Y. Studies on the matched potential method for determining the selectivity coefficients of ion-selective electrodes based on neutral ionophores: experimental and theoretical verification. Analytical Sciences 2001, 17, 733–743. [Google Scholar]
- www.hort.purdue.edu, www.fineli.fi
- Bader, M. A systematic approach to standard addition methods in instrumental analysis. J. Chem. Educ. 1980, 57, 703–706. [Google Scholar]
- Christian, G.D. Analytical chemistry; J. Wiley and Sons, 1994; pp. 370–379. [Google Scholar]
- Amemiya, S.; Buhlmann, P.; Pretsch, E.; Rusterholz, B.; Umezawa, Y. Cationic or anionic sites? Selectivity optimization of ion-selective electrodes based on charged ionophores. Anal. Chem. 2000, 72, 1618–1631. [Google Scholar]
- Buhlmann, P.; Yajima, S.; Tohda, K.; Umezawa, K.; Nishizawa, S.; Umezawa, Y. Studies on the phase boundaries and the significance of ionic sites of liquid membrane ion-selective electrodes. Electroanalysis. 1995, 7, 811–816. [Google Scholar]
- Buhlmann, P.; Yajima, S.; Tohda, K.; Umezawa, Y. EMF response of neutral-carrier based ion-selective field efect transistors with membranes free of ionic sites. Electrochim. Acta. 1995, 40, 3021–3027. [Google Scholar]
- Bakker, E.; Buhlmann, P.; Pretsch, E. The phase-boundary potential model. Talanta. 2004, 62, 843–860. [Google Scholar]
- Radecki, J.; Radecka, H.; Piotrowski, T.; Depraetere, S.; Dehaen, W.; Plavec, J. Interface host-guest interaction between calix[4]pyrrole and neutral derivatives of phenol as the base for their potentiometric discrimination. Electroanalysis 2004, 16, 2073–2081. [Google Scholar]
- Kral, V.; Sessler, J.L.; Shishkanova, T.V.; Gale, P.A.; Volf, R. Molecular recognition at an organic-aqueous interface: heterocalixarenes as anion bindings agents in liquid polymeric membrane ion-selective electrodes. J. Am. Chem. Soc. 1999, 121, 8771–8775. [Google Scholar]
- Bulgariu, L.; Radecka, H.; Pietraszkiewicz, M.; Pietraszkiewicz, O. Potentiometric response of liquid membrane electrode incorporated with macrocyclic polyamine towards benzoate. Anal. Let. 2003, 36, 1325–1334. [Google Scholar]
- Piotrowski, T.; Szymańska, I.; Radecka, H.; Radecki, J.; Pietraszkiewicz, M.; Pietraszkiewicz, O.; Wojciechowski, K. Influence of substituents in nitrogen atoms in macrocyclic polyamnine incorporated into poly(winyl chloride) matrix liquid membrane on its potentiometric response to neutral phenols. Electroanalysis 2000, 12, 1397–1402. [Google Scholar]
- Nechita, M.T.; Lotrean, S.; Radecki, J.; Radecka, H.; Depreatere, S.; Dehaen, W. Potentiometric response of liquid membrane electrodes incorporated with new calix[4]pyrrole and pyrrole derivatives towards neutral nitrophenol. Pol. J. Food Nutr. Sci. 2003, 12, 81–87. [Google Scholar]
- Piotrowski, T.; Radecka, H.; Radecki, J.; Depreatere, S.; Dehaen, W. Potentiometric response of calix[4]pyrrole liquid membrane electrode towards neutral nitrophenols. Electroanalysis 2001, 13, 342–346. [Google Scholar]
- Radecki, J.; Stenka, I.; Dolusic, E.; Dehaen, W.; Plavec, J. Potentiometric discrimination of neutral forms of nitrophenol isomers by liquid membrane electrodes incorporated with corroles. Comb. Chem. High Throughput Screen. 2004, 7, 375–381. [Google Scholar]
- Radecki, J.; Dehaen, W. Nitrogen-containing macrocycles as host molecules for the recognition of undissociated phenol derivatives: mechanizm of potentiometric signal generatin. Comb. Chem. High Throughput Screen. 2006, 9, 399–406. [Google Scholar]
- Pezza, L.; Santini, A.O.; Pezza, H.R.; Melios, C.B.; Ferreira, V.J.F.; Nasser, A.L.M. Benzoate ion determination in beverages by using a potentiometric sensor immobilized in a graphite matrix. Anal. Chim. Acta 2001, 433, 281–288. [Google Scholar]
- Regulation of the Minister of Health of the 27thDecember 2000 on the list of permissible levels of the food additives and foreign substances added to food products and stimulants as well as contaminamts that are likely to occur in food products and stimulants. Official Journal of Laws No 9, item 72 of 2001
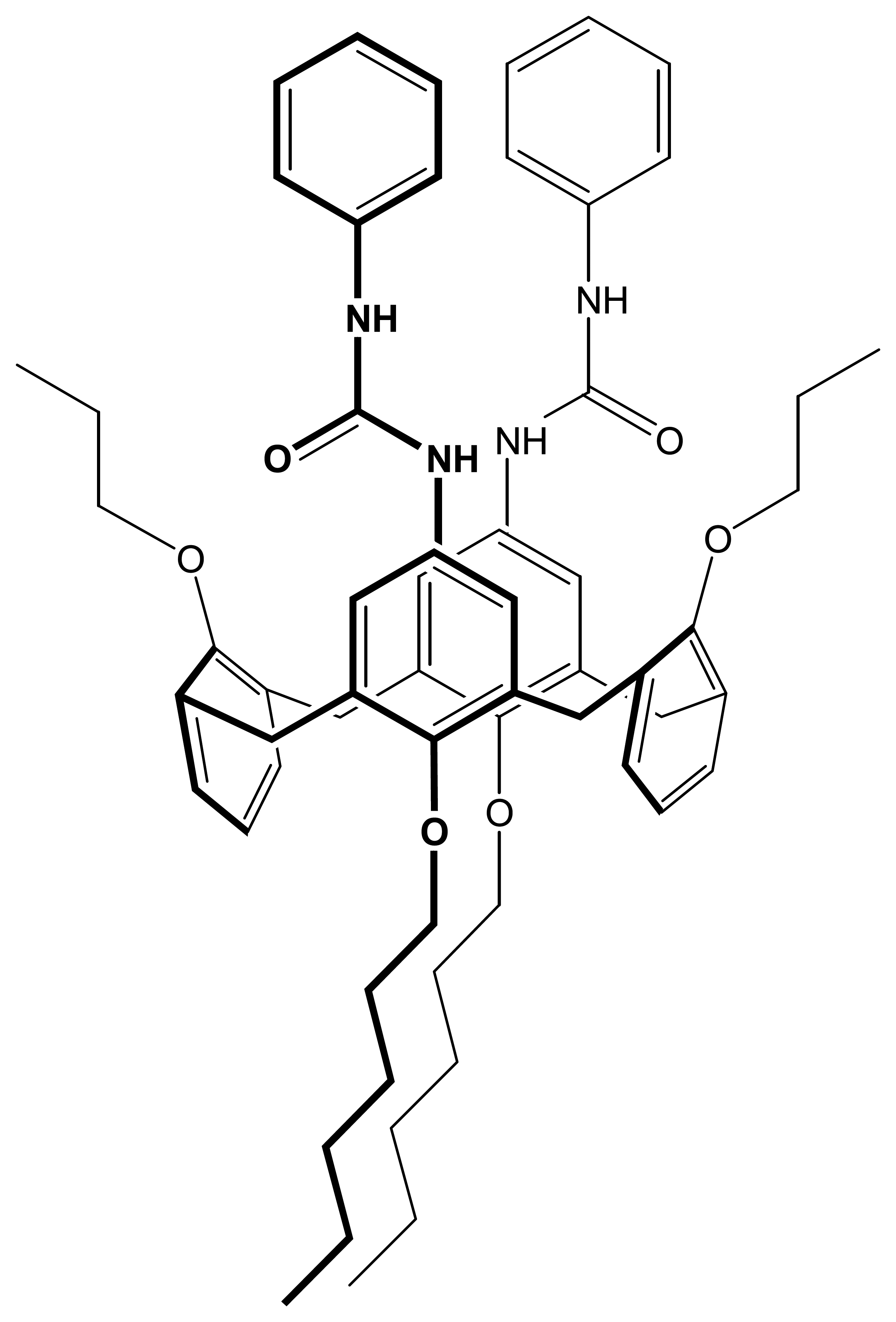
 ligand;
ligand;
 ligand + 50% TDDMACl;
ligand + 50% TDDMACl;
 ligand + 50% K-TpClPB (pH was changed from 9.5 to 2.5); (●) ligand; (▴) ligand + 50% TDDMACl; (▪) ligand + 50% K-TpClPB (pH was changed from 2.5 to 9.5;. Measuring conditions – see part 2.3 .
ligand + 50% K-TpClPB (pH was changed from 9.5 to 2.5); (●) ligand; (▴) ligand + 50% TDDMACl; (▪) ligand + 50% K-TpClPB (pH was changed from 2.5 to 9.5;. Measuring conditions – see part 2.3 .
 ligand;
ligand;
 ligand + 50% TDDMACl;
ligand + 50% TDDMACl;
 ligand + 50% K-TpClPB (pH was changed from 9.5 to 2.5); (●) ligand; (▴) ligand + 50% TDDMACl; (▪) ligand + 50% K-TpClPB (pH was changed from 2.5 to 9.5;. Measuring conditions – see part 2.3 .
ligand + 50% K-TpClPB (pH was changed from 9.5 to 2.5); (●) ligand; (▴) ligand + 50% TDDMACl; (▪) ligand + 50% K-TpClPB (pH was changed from 2.5 to 9.5;. Measuring conditions – see part 2.3 .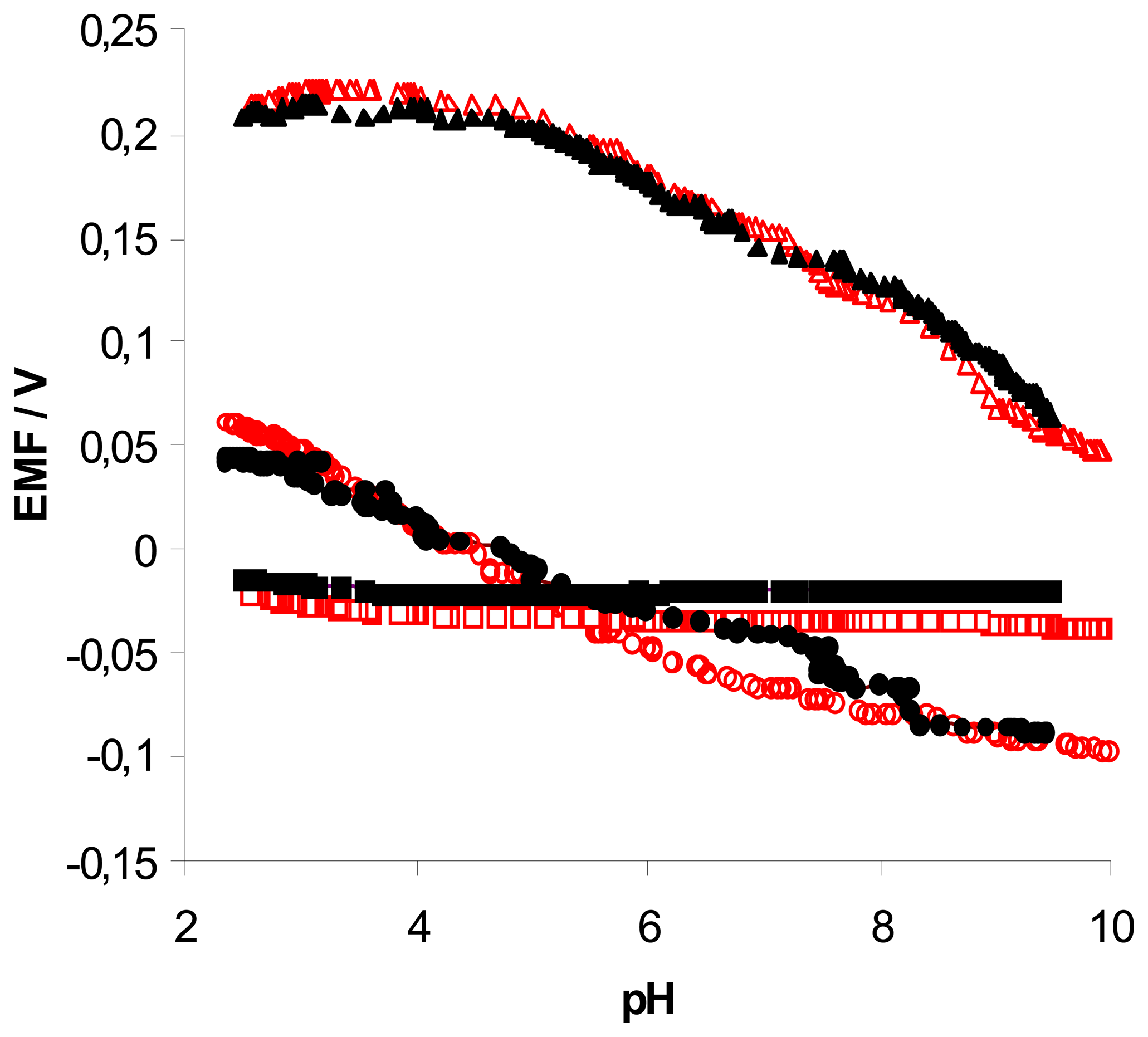
 and ligand + 50% TDDMACl
and ligand + 50% TDDMACl
 . Measurements were taken in 1.0 × 10-2 M NaHSO4 pH 3.0 (n = 4; 0,31 < SD < 15,46).
. Measurements were taken in 1.0 × 10-2 M NaHSO4 pH 3.0 (n = 4; 0,31 < SD < 15,46).
 and ligand + 50% TDDMACl
and ligand + 50% TDDMACl
 . Measurements were taken in 1.0 × 10-2 M NaHSO4 pH 3.0 (n = 4; 0,31 < SD < 15,46).
. Measurements were taken in 1.0 × 10-2 M NaHSO4 pH 3.0 (n = 4; 0,31 < SD < 15,46).
| Interferent, j | ΔEMF = -2 mV |
|---|---|
| Chloride | -0,42 |
| Ascorbate | ≪0 |
| Phosphate | -1,22 |
| Acetate | ≪0 |
| Citrate | ≪0 |
| Glucose | ≪0 |
| Concentration known (mM) | Concentration found (mM) | Recovery (%) |
|---|---|---|
| 0.479 | 0.516 ± 0.042 | 107.7 ± 8.7 |
| 0.741 | 0.760 ± 0.031 | 102.6 ± 4.1 |
| 1.000 | 1.016 ± 0.033 | 101.6 ± 3.3 |
| 1.380 | 1.391 ± 0.028 | 100.8 ± 2.1 |
© 2007 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Krajewska, A.; Lhotak, P.; Radecka, H. Potentiometric Responses of Ion-Selective Electrodes Doped with Diureidocalix[4]arene towards Un-dissociated Benzoic Acid. Sensors 2007, 7, 1655-1666. https://doi.org/10.3390/s7081655
Krajewska A, Lhotak P, Radecka H. Potentiometric Responses of Ion-Selective Electrodes Doped with Diureidocalix[4]arene towards Un-dissociated Benzoic Acid. Sensors. 2007; 7(8):1655-1666. https://doi.org/10.3390/s7081655
Chicago/Turabian StyleKrajewska, Agnieszka, Pavel Lhotak, and Hanna Radecka. 2007. "Potentiometric Responses of Ion-Selective Electrodes Doped with Diureidocalix[4]arene towards Un-dissociated Benzoic Acid" Sensors 7, no. 8: 1655-1666. https://doi.org/10.3390/s7081655




