Development of a Surface Plasmon Resonance n-dodecane Vapor Sensor
Abstract
:1. Introduction
2. Results and Discussion
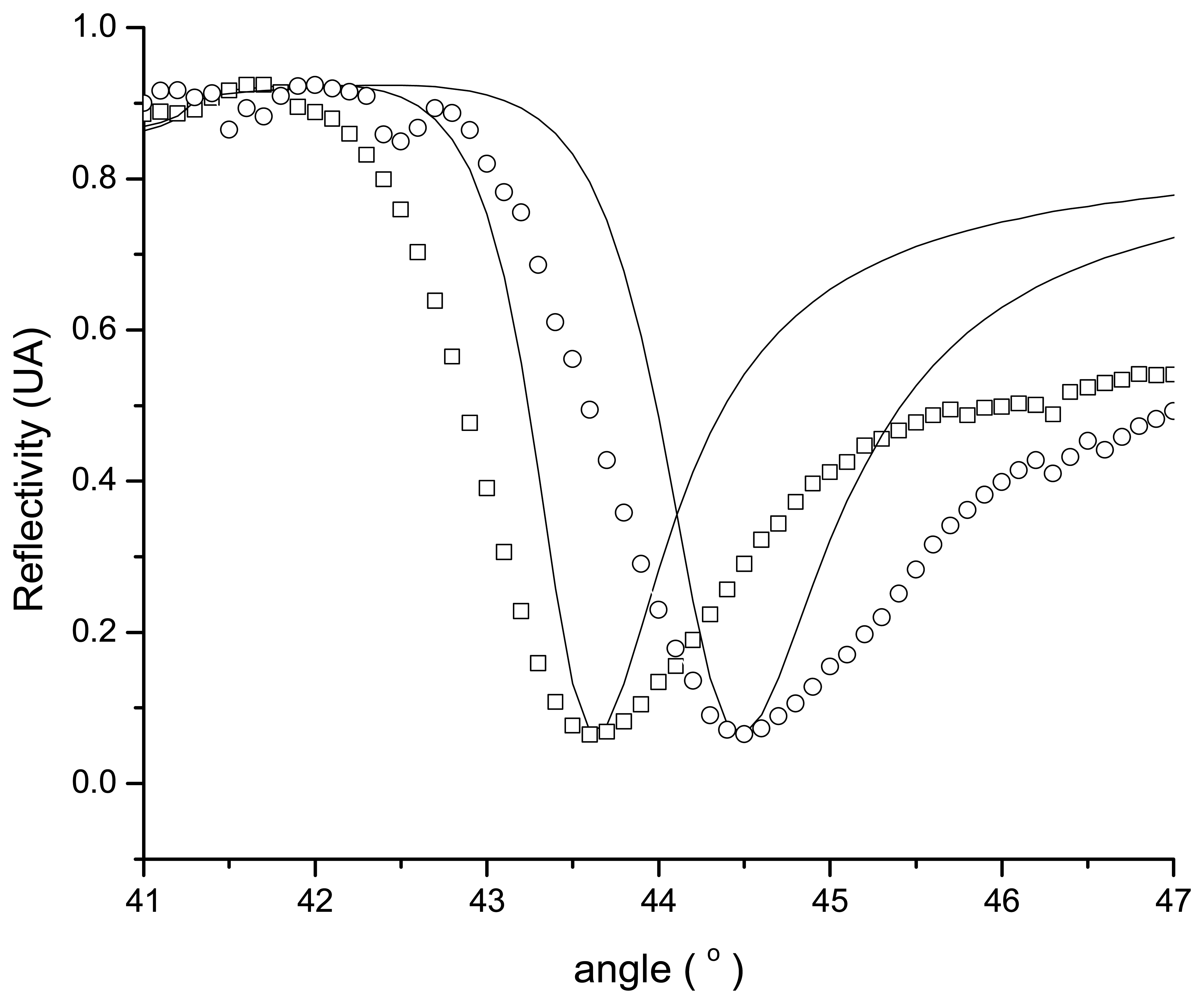
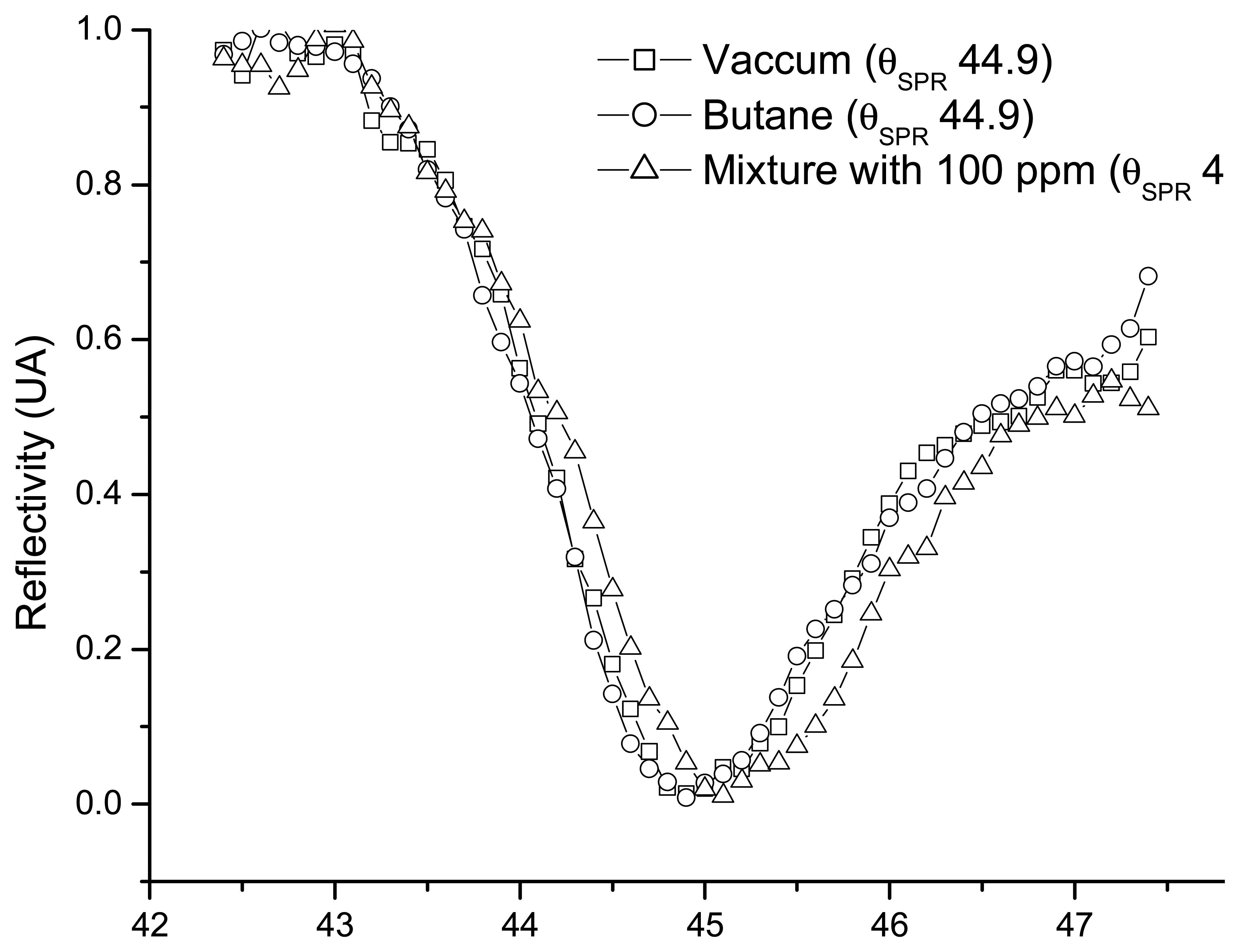
2.1. Sensitivity of the sensor
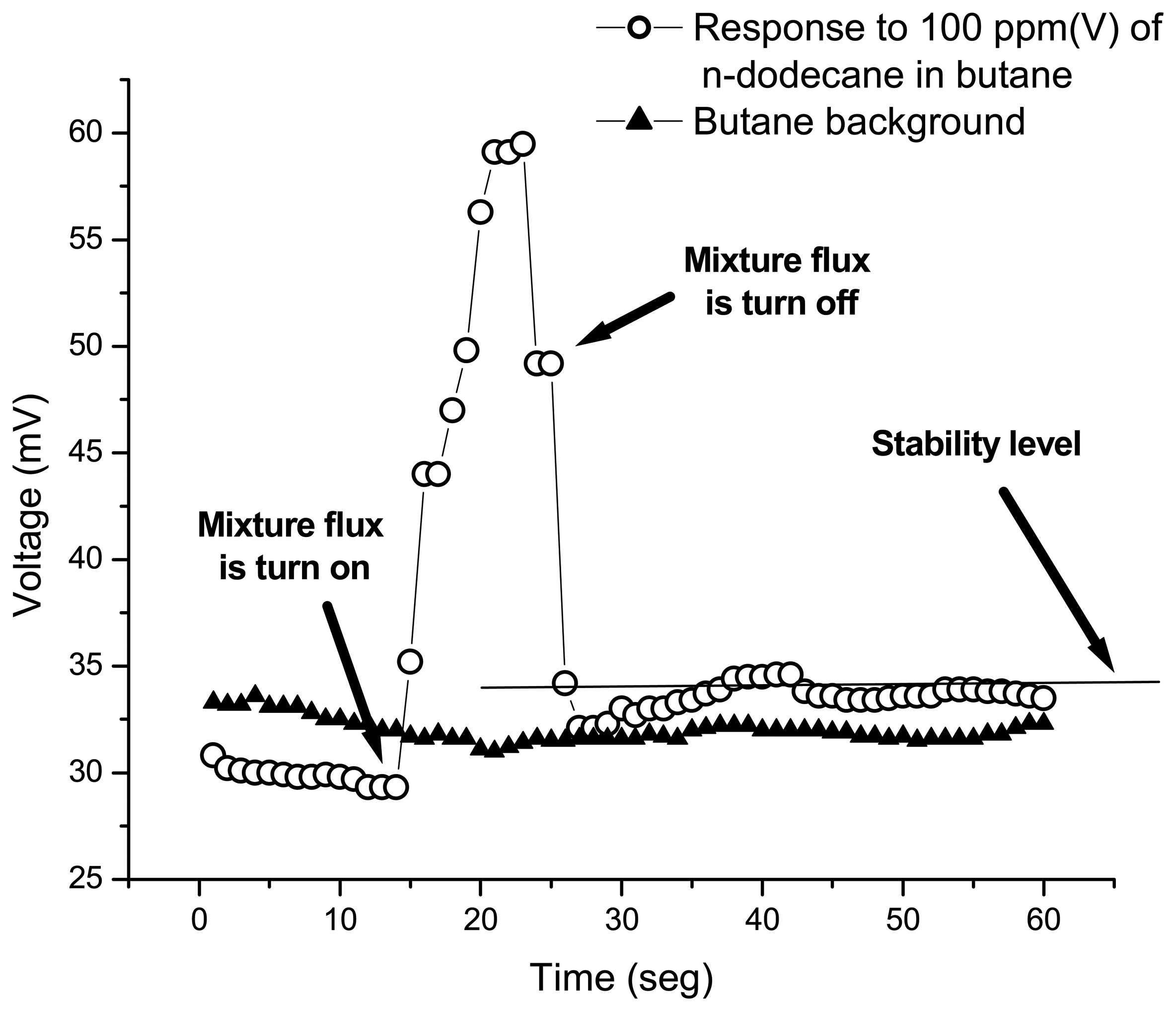
2.2. Response as a function of time
3. Experimental Section
3.1 Preparation of the sensing element
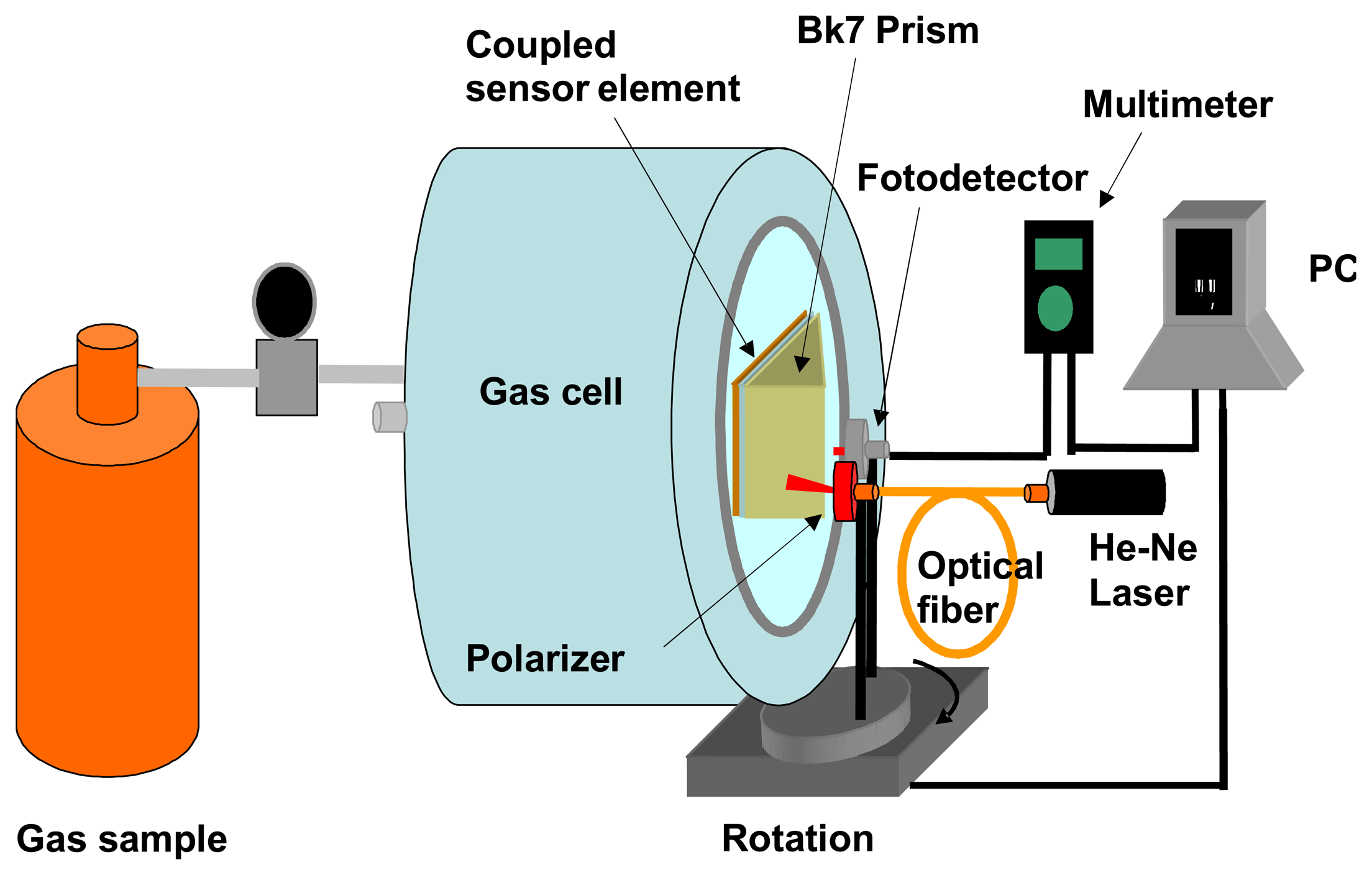
3.2 Detection gas experimental configuration
3.3 Preparation of the gas samples
4. Conclusions
Acknowledgments
References and Notes
- Homola, Jiri; Yee, Sinclair S.; Gauglitz, Gunter. Surface plasmons resonance sensors: review. Sens. Actuators B 1999, 54, 3–15. [Google Scholar]
- Muñoz Aguirre, N.; Passian, A.; Martínez Pérez, L.; López-Sandoval, E.; Vázquez-López, C.; Jiménez-Pérez, J. L.; Ferrell, T.L. The use of the surface plasmons resonance sensor in the study of the influence of “allotropic” cells on water. Sens. Actuators B 2004, 99, 149–155. [Google Scholar]
- Wong, C.I.; Ho, H.P.; Chan, K.S.; Wu, S. Y.; Lin, C.L. Application of spectral surface plasmon resonance to gas pressure sensing. Opt. Engineering 2005, 44(12). Art. No. 124403. [Google Scholar]
- Quinn, J. G.; O'Neil, S.; Doyle, A.; McAtamney, C.; Diamond, D.; MacCraith, B. D.; O'Kennedy, R. Development and application of Surface Plasmon Resonance-Based Biosensors for the Detection of Cell-Ligand Interactions. Analytical Biochemistry 2000, 281, 135–143. [Google Scholar]
- Akimoto, T.; Sasaki, S.; Ikebukuro, K.; Karube, I. Effect of incident angle of light on sensitivity and detection limit for layers of antibody with surface plasmon resonance spectroscopy. Biosens. Bioelectron. 2000, 15, 355–362. [Google Scholar]
- Peng, W.; Banerji, S.; Kim, Y. C.; Booksh, K.S. Investigation of dual-channel fiber-optic surface plasmon resonance sensing for biological applications. Optics Letters 2005, 30(22), 2988–2990. [Google Scholar]
- Maciak, E.; Opilski, Z.; Pustelny, T.; Bednorz, M. An optical detection NH3 gas by means of a WO3 thin films based on SPR technique. J. Physique IV 2005, 129, 131–136. [Google Scholar]
- Feresenbet, E.B.; Busi, M.; Ugozzoli, F.; Dalcanale, E.; Shenoy, D. K. Influence of cavity depth on the response of SPR sensors coated with self-assembled monolayers of cavitands. Sens. Lett. 2004, 2(3-4), 186–193. [Google Scholar]
- Fernández, C.D.; Manera, M. G.; Spadavecchia, J.; Maggioni, G.; Quaranta, A.; Mattei, G.; Bazzan, M.; Cattaruzza, E.; Bonafini, M.; Negro, E.; Vomiero, A.; Carturan, S.; Scian, C.; Della, G. M.; Rella, R.; Vasanelli, L. Study of the gas optical sensing properties of Au-polyimide nanocomposite films prepared by ion implantation. Sens. Actuators B 2005, 111, 225–229. [Google Scholar]
- Kim, Y. C.; Banerji, S.; Masson, J. F.; Peng, W.; Booksh, K. S. Fiber-optic surface plasmon resonance for vapor phase analyses. Analyst 2005, 130(6), 838–843. [Google Scholar]
- Samoylov, A. V.; Mirsky, V. M.; Hao, Q.; Swart, C.; Shirshov, Y.M.; Wolfbeis, O. S. Nanometer-thick SPR sensor for gaseous HCl. Sens. Actuators B 2005, 106, 369–372. [Google Scholar]
- Manera, M. G.; Leo, G.; Curri, M. L.; Cozzoli, P. D.; Rella, R.; Siciliano, P.; Agostiano, A.; Vasanelli, L. Investigation on alcohol vapours/TiO2nanocrystal thin films interaction by SPR technique for sensing application. Sens. Actuators B 2004, 100(1-2), 75–80. [Google Scholar]
- Conoci, S.; Palumbo, M.; Pignataro, B.; Rella, R.; Valli, L.; Vasapollo, G. Optical recognition of organic vapours through ultrathin calix[4]pyrrole films. Colloids Surf. A 2002, 198, 869–873. [Google Scholar]
- Abdelghani, A.; Renault, N. SPR fibre sensor sensitized by fluoroxiloxane polymers. Sens. Actuators B 2001, 74(1-3), 117–123. [Google Scholar]
- Rella, R.; Rizzo, A.; Licciulli, A.; Siciliano, P.; Troisi, L.; Valli, L. Tests in controlled atmosphere on new optical gas sensing layers based on TiO2/metalphthalocyanines hybrid system. Materials Sci. Engineering C 2002, 22(2), 439–443. [Google Scholar]
- Sugimoto, I.; Nakamura, M.; Ogawa, S.; Seyama, M. Petroleum pollution sensing at ppt level using quartz crystal resonators sputtered with porous polyethylene under photo-excitation. Sens. Actuators B 2000, 64, 216–223. [Google Scholar]
- Rodríguez Juárez, M.; Muñoz Aguirre, N.; Martínez Pérez, L.; Garibay-Febles, V.; Lozada-Cassou, M.; Becerril, M.; Zelaya Angel, O. Optical characterization of polyethylene and cobalt phthalocyanine ultra-thin films by means of ATR technique at the Surface Plasmons Resonance. Phys. Stat. Solidi a 2006, 203(10), 2506–2512. [Google Scholar]
- Muñoz Aguirre, N.; Buenrostro, E.; López, S.; Garibay-Febles, V.; Lozada-Cassou, M. Dispositivo de Resonancia de Plasmones Superficiales para la detección de gases. Submitted to Mexican Office Patent 2007 (Instituto Mexicano de la Propiedad Intelectual-IMPI), submission number MX/a/2007/005838. date 16th may 2007.
- Ascencióndel Romero Martínez, Estudio delequilibrio entre fases líquido-vapor y líquido-líquido-vapor de sistemas binarios y ternarios formados por disolvente polar + hidrocarburo. In Philosophical Thesis Dissertation; 2004; Universidad Nacional Autónoma de México-Facultad de Química (UNAM): México D.F.

© 2007 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Aguirre, N.M.; Pérez, L.M.; Colín, J.A.; Buenrostro-Gonzalez, E. Development of a Surface Plasmon Resonance n-dodecane Vapor Sensor. Sensors 2007, 7, 1954-1961. https://doi.org/10.3390/s7091954
Aguirre NM, Pérez LM, Colín JA, Buenrostro-Gonzalez E. Development of a Surface Plasmon Resonance n-dodecane Vapor Sensor. Sensors. 2007; 7(9):1954-1961. https://doi.org/10.3390/s7091954
Chicago/Turabian StyleAguirre, Narcizo Muñoz, Lilia Martínez Pérez, Jose Alfredo Colín, and Eduardo Buenrostro-Gonzalez. 2007. "Development of a Surface Plasmon Resonance n-dodecane Vapor Sensor" Sensors 7, no. 9: 1954-1961. https://doi.org/10.3390/s7091954



