Experimental Study on the Effects of Alumina Abrasive Particle Behavior in MR Polishing for MEMS Applications
Abstract
:1. Introduction
2. Characteristics of MR Fluids
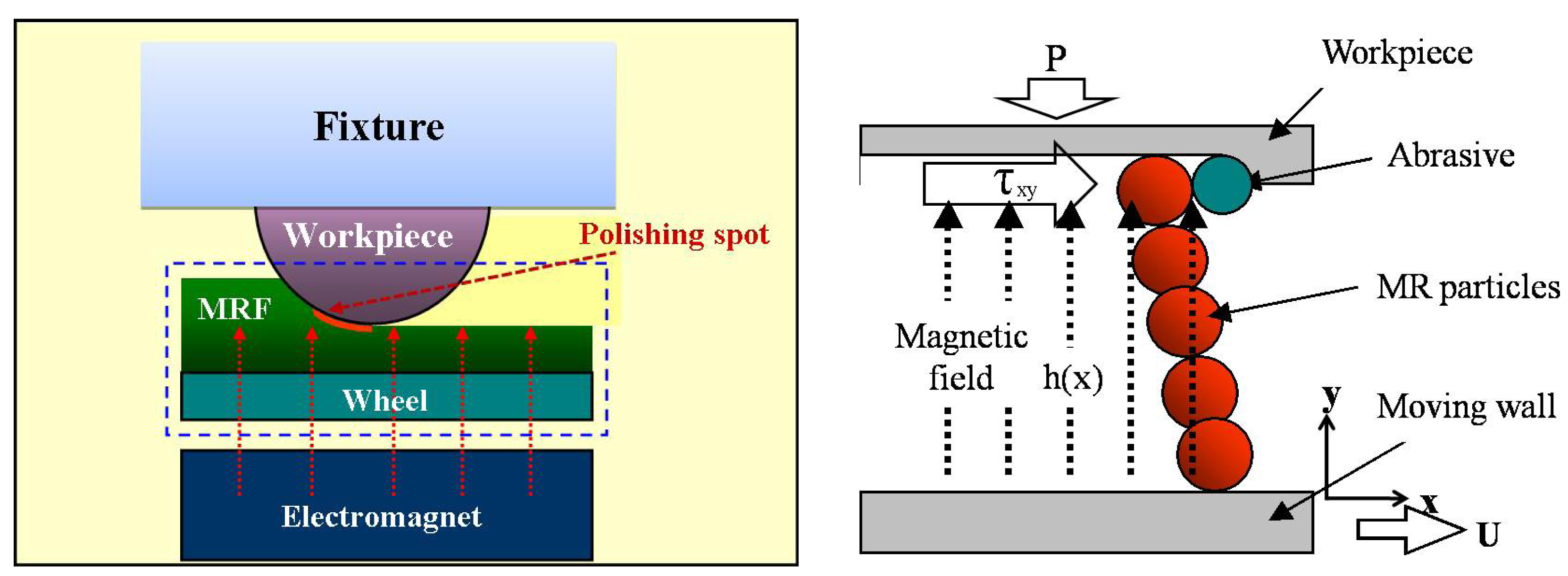
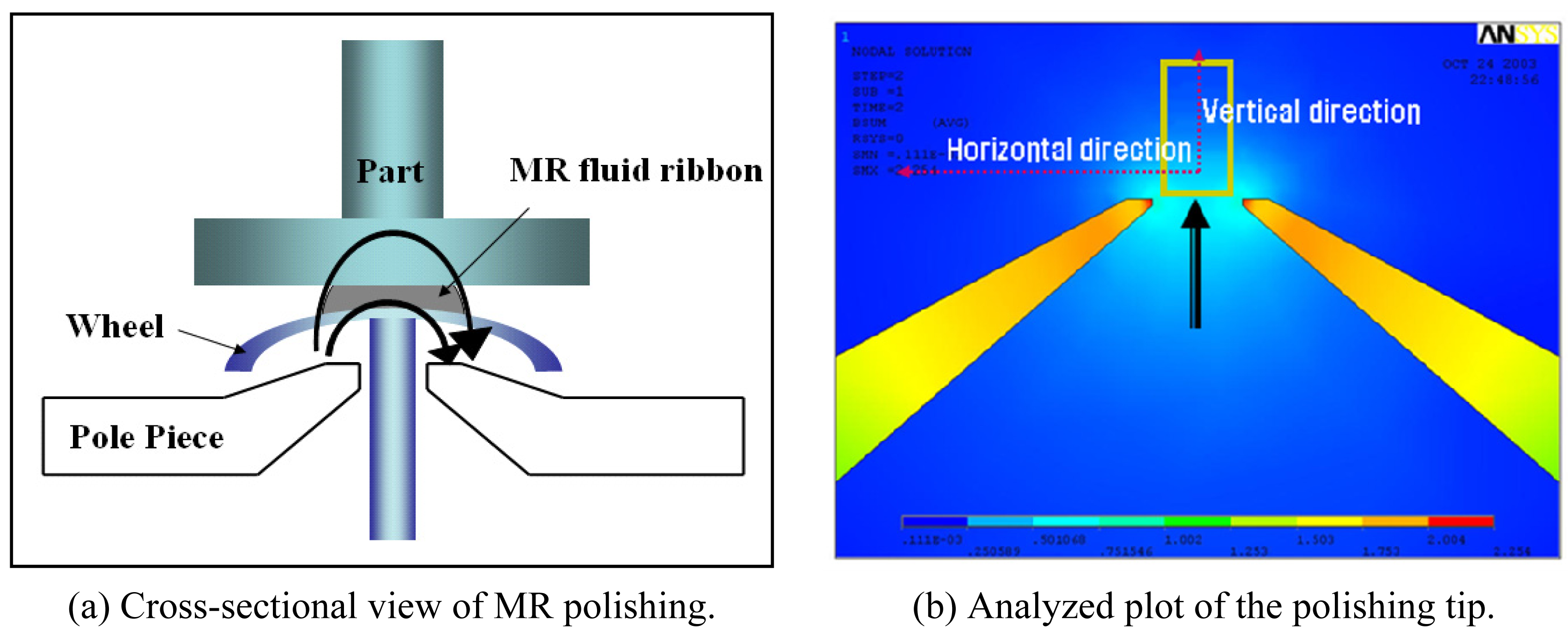
2.1. Polishing Principle Using MR Fluids
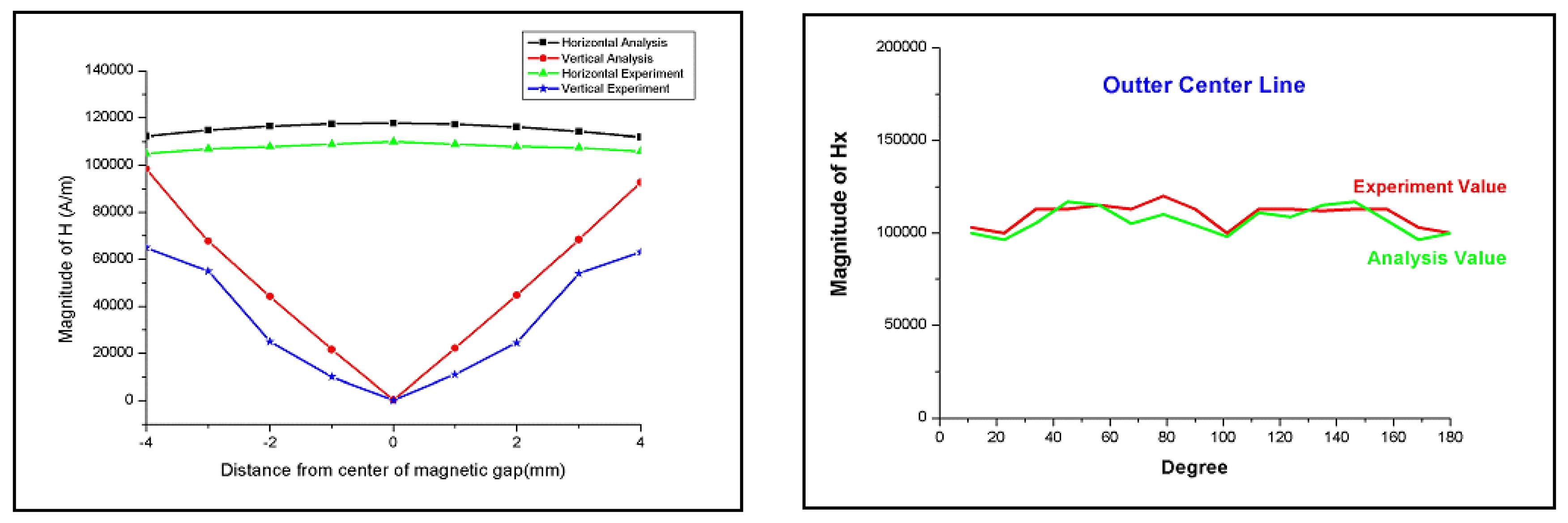
2.2. Flow Characteristics and Material Removal Rate in MR Polishing
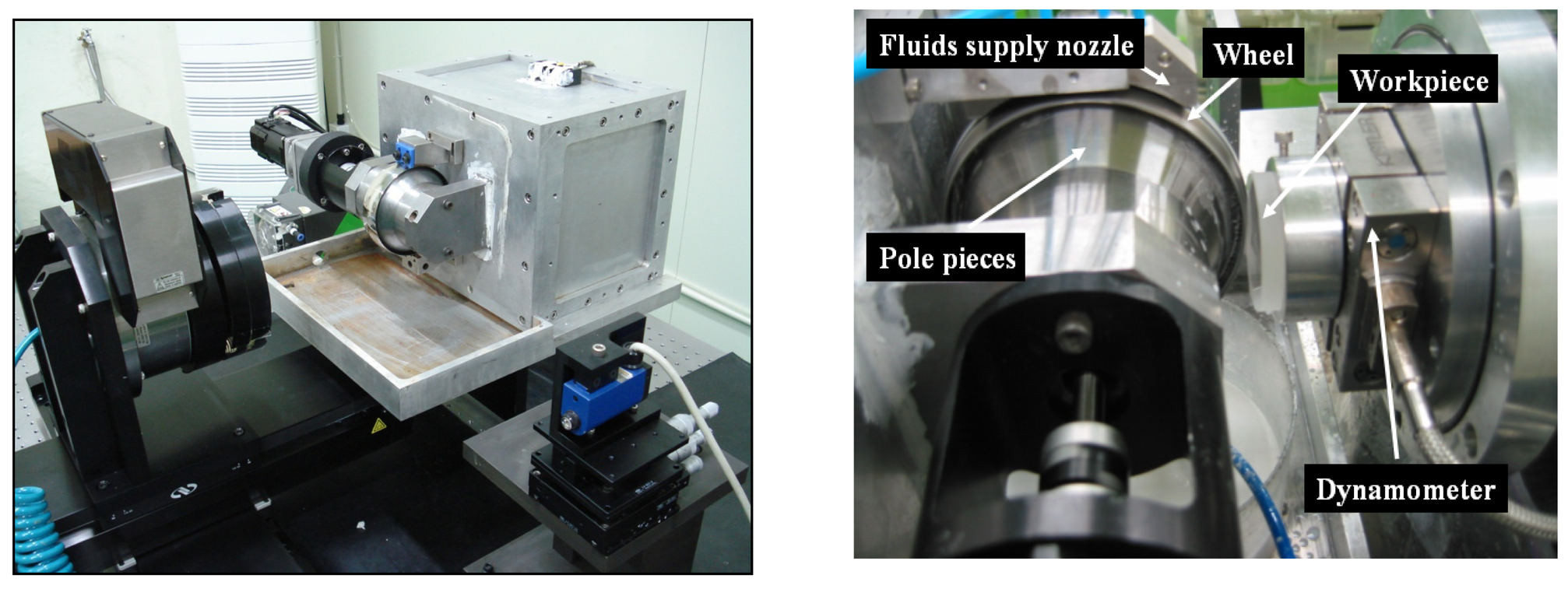
3. Experimental Setup and Polishing Conditions
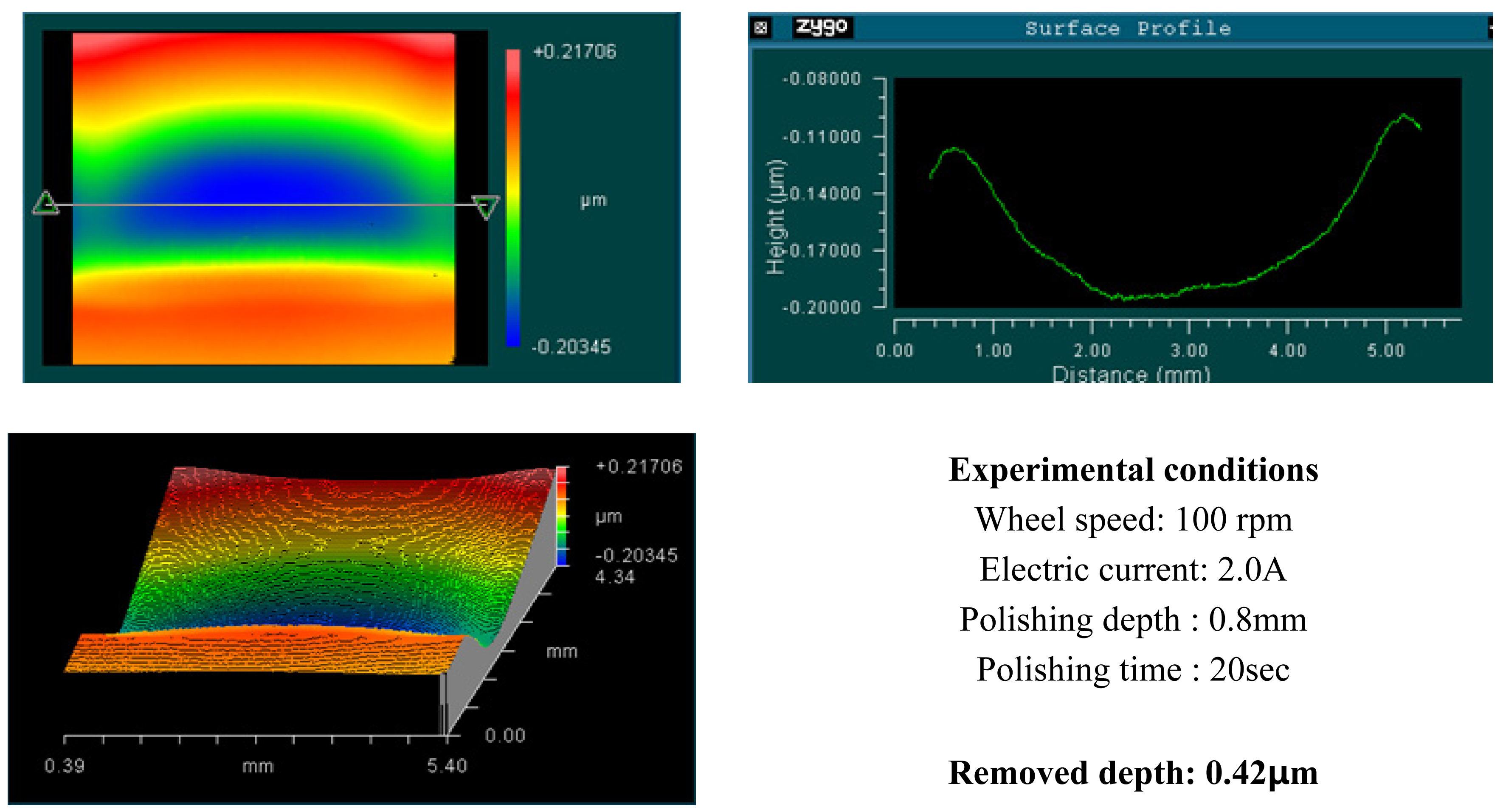
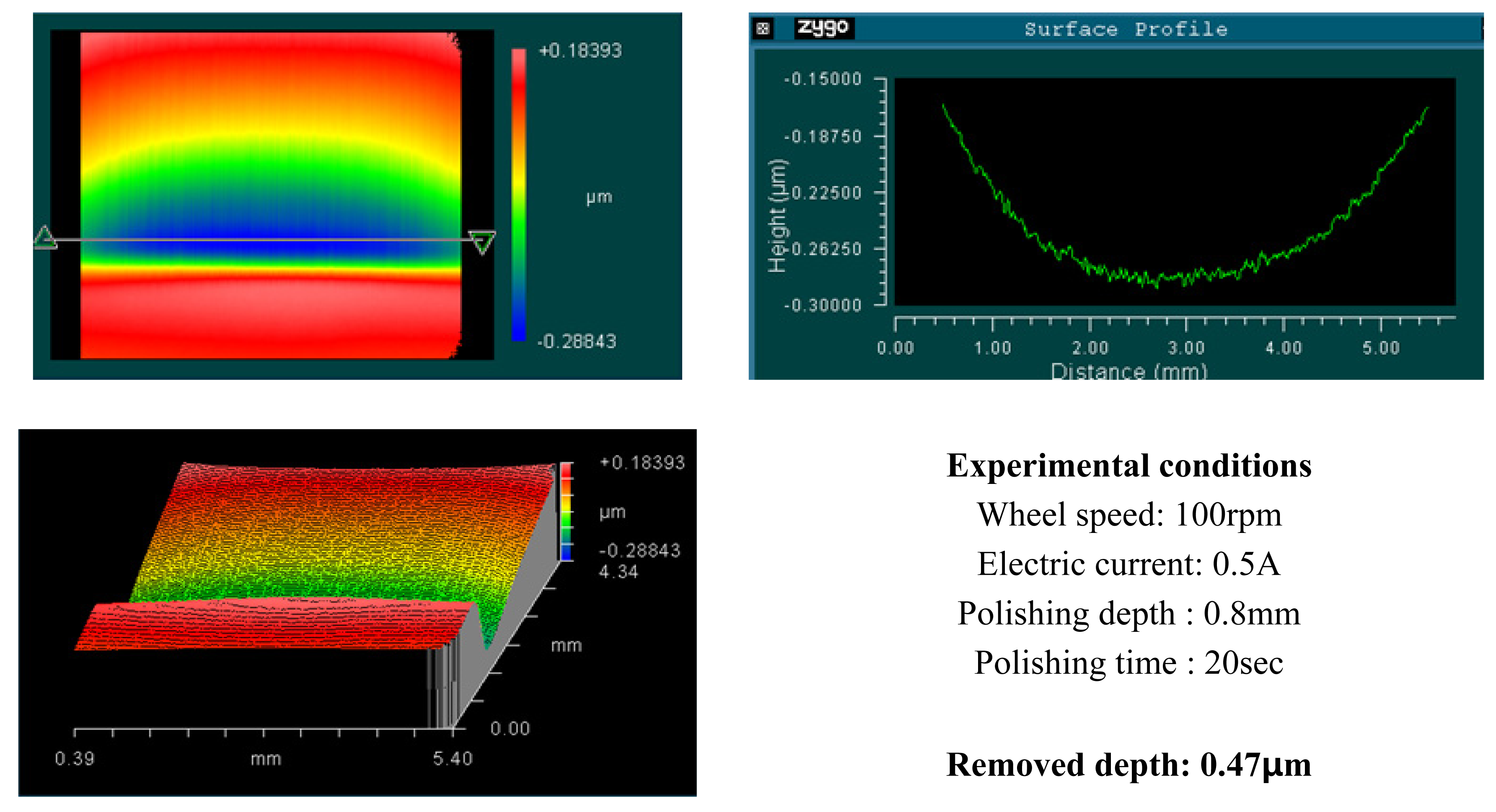
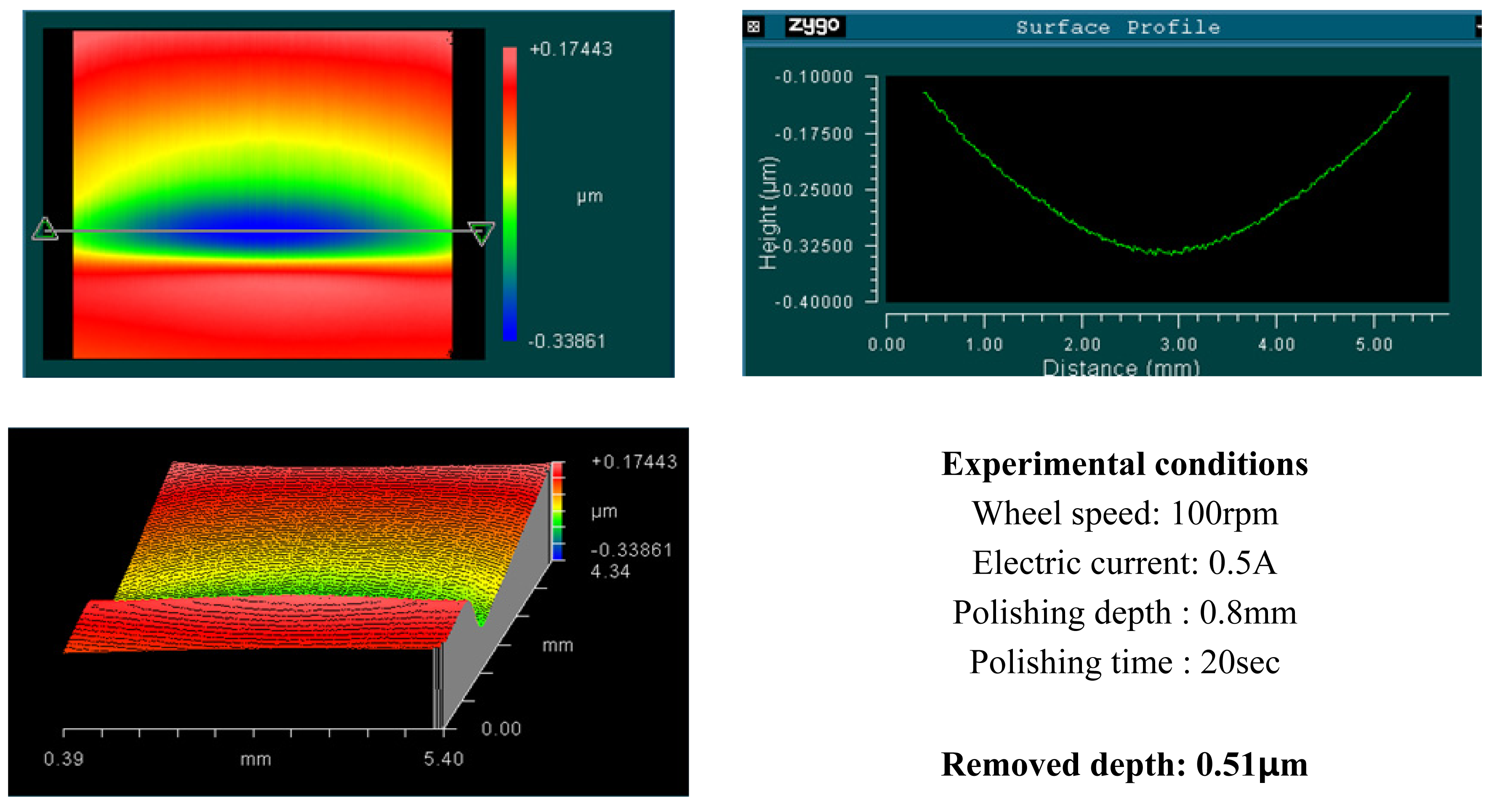
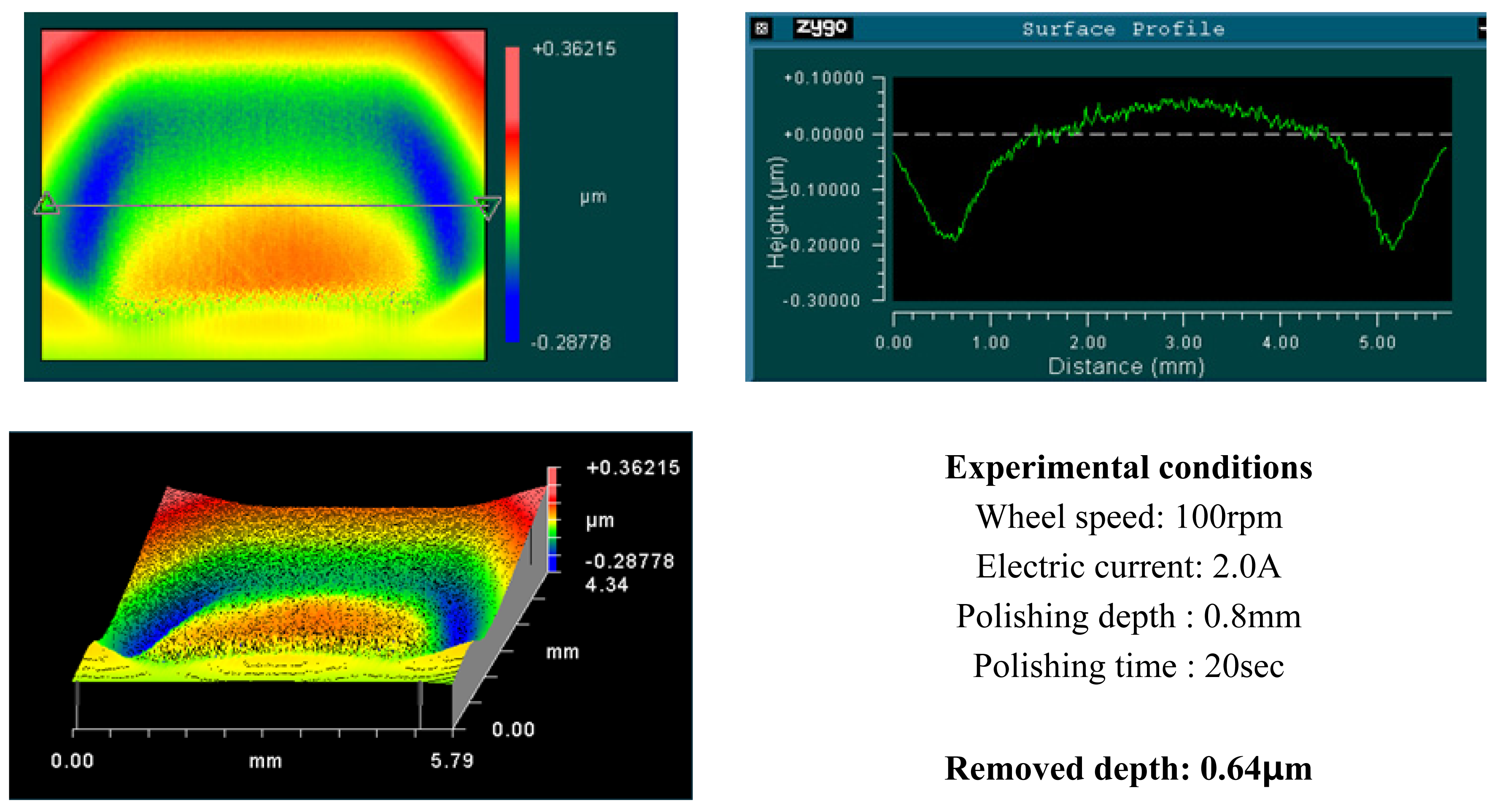
4. Experimental Results and Analysis
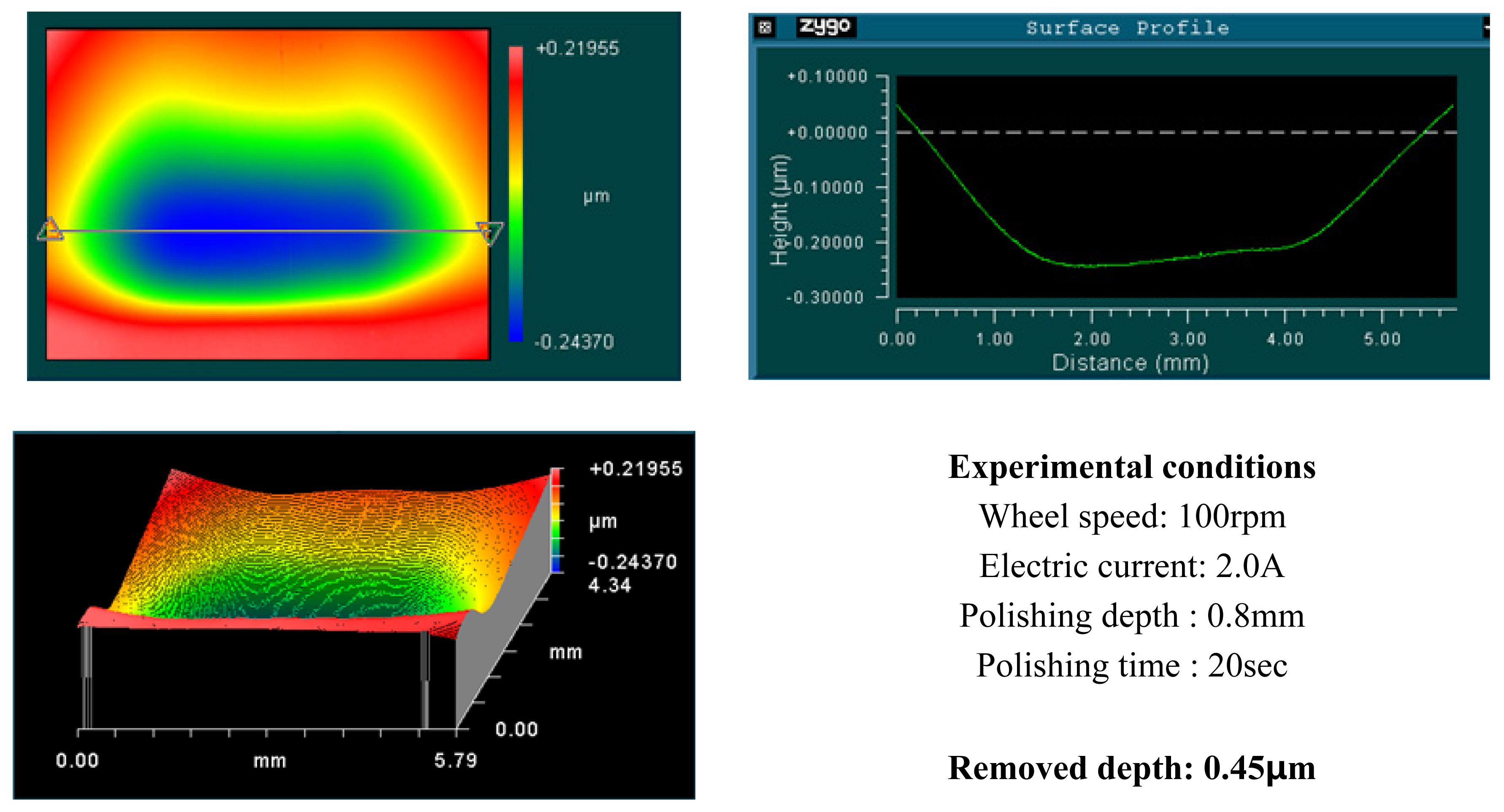
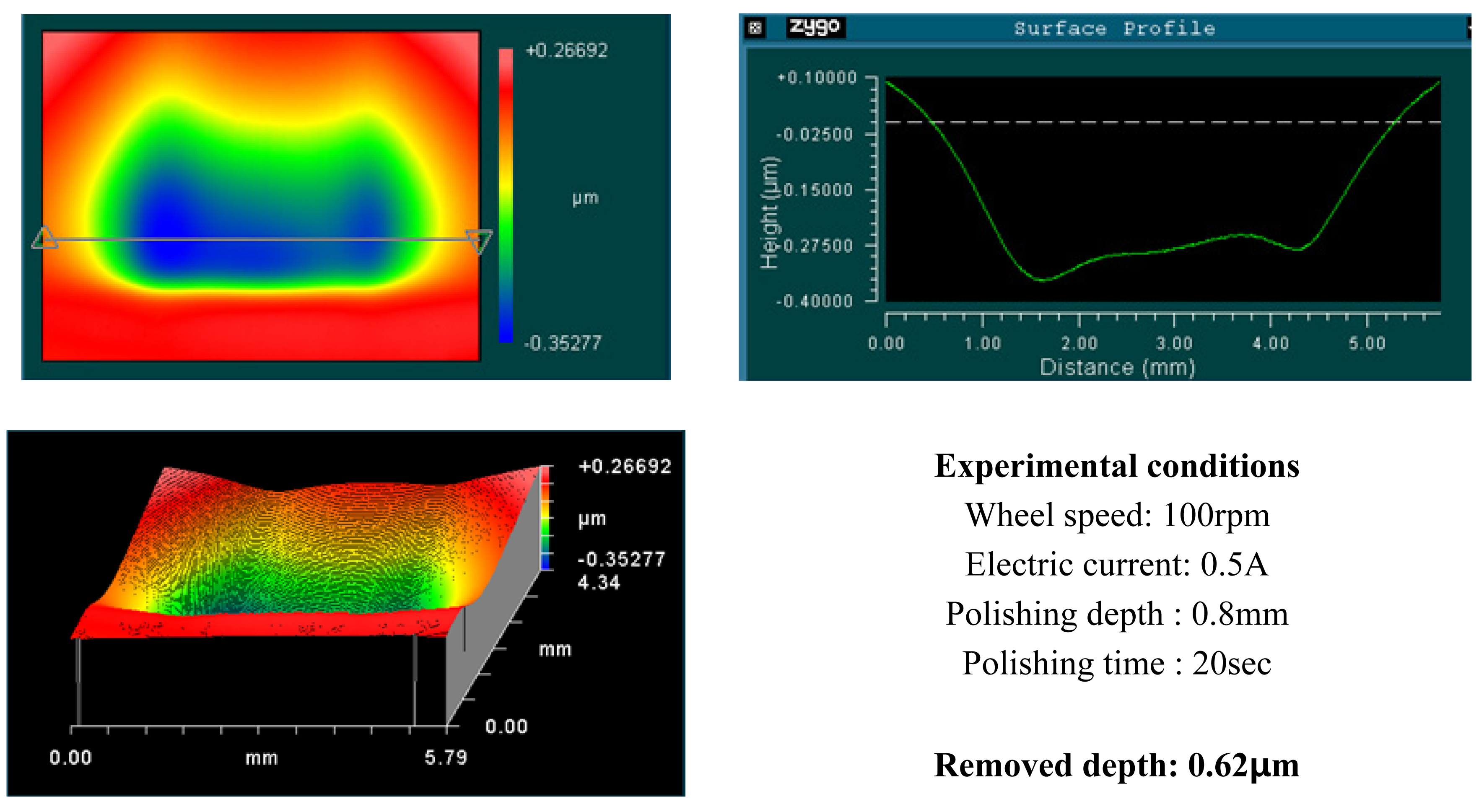
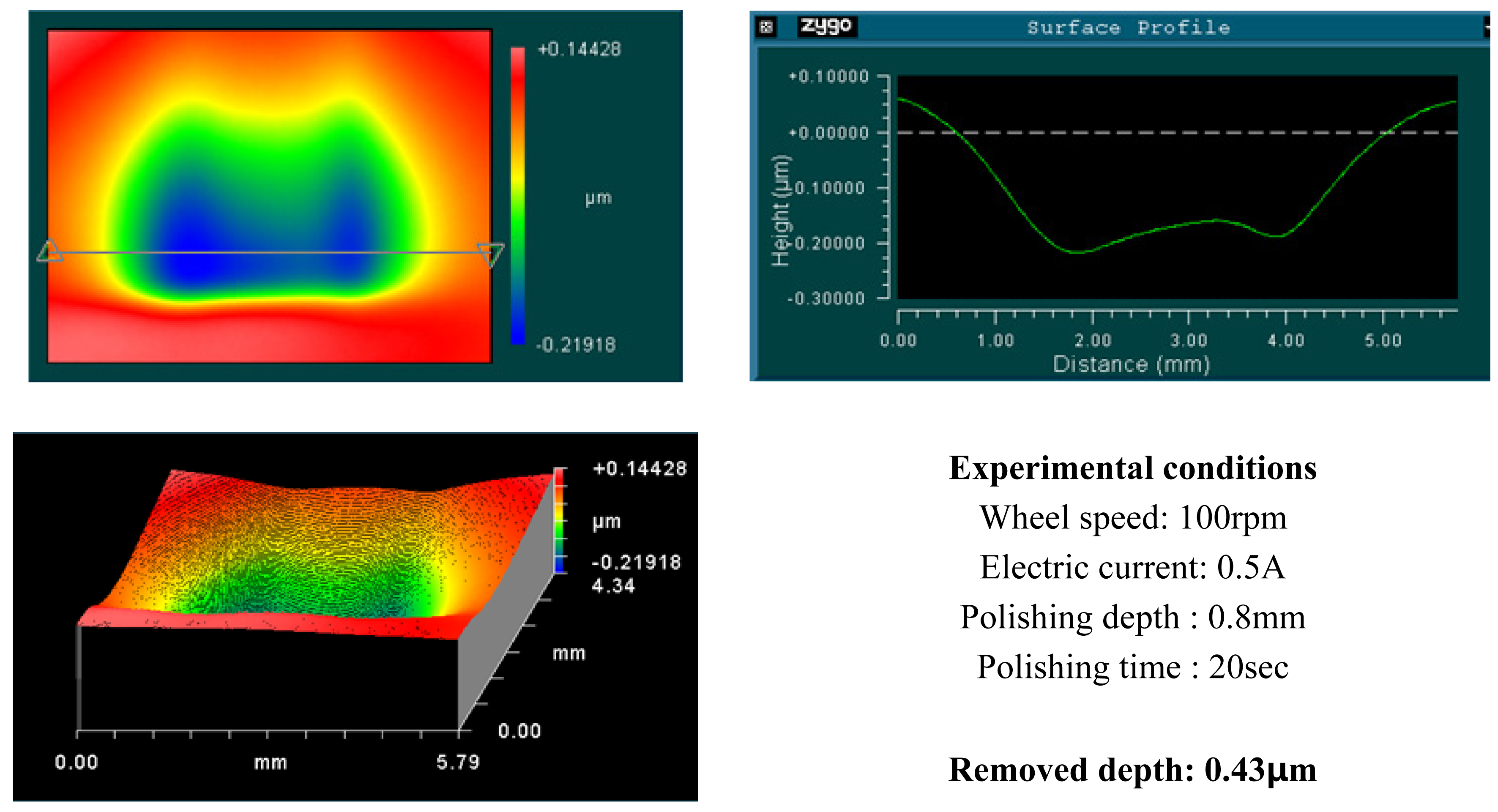
4.1. Basic Experimental Results
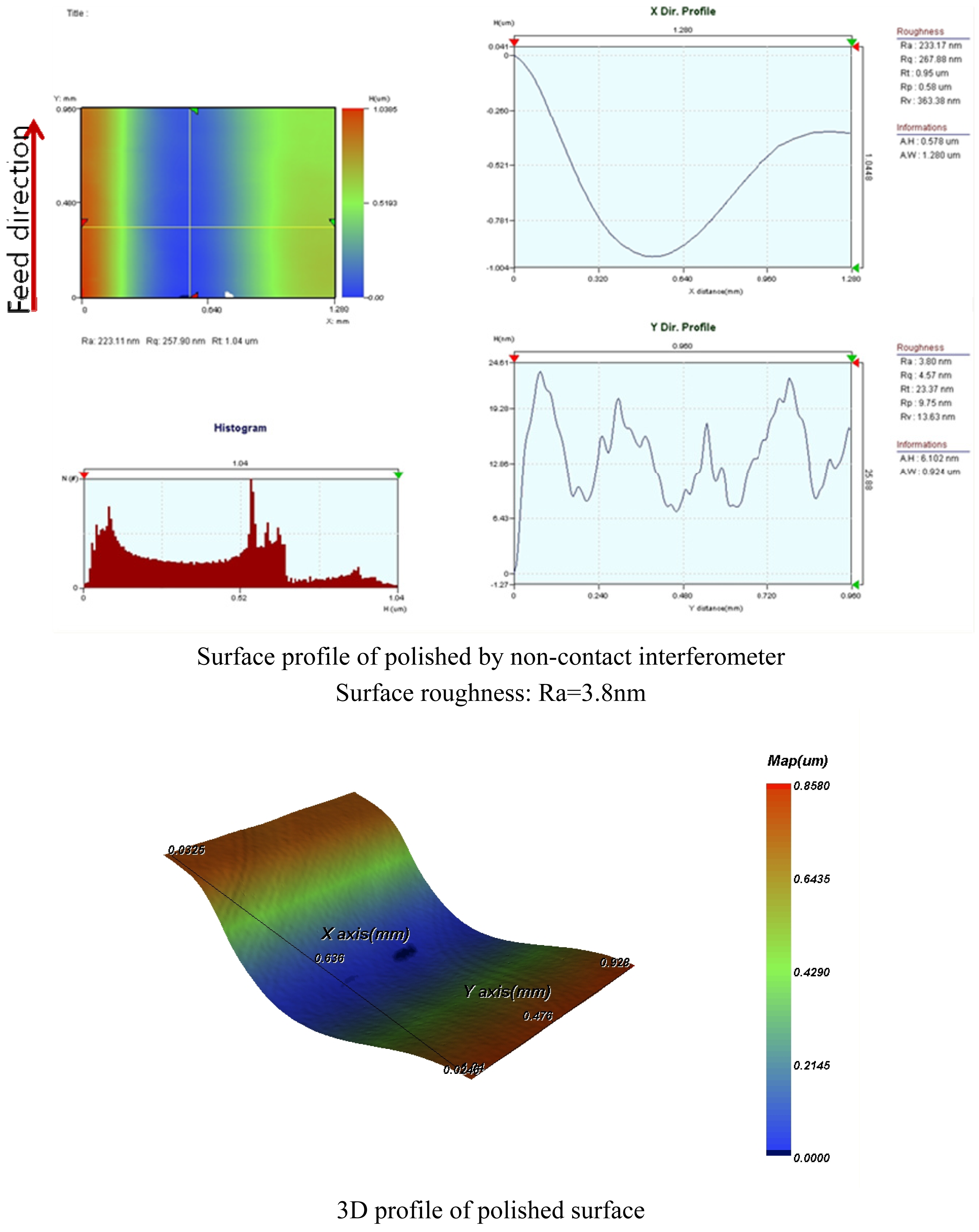
4.2. Groove Polishing Using Slurry No.3
5. Conclusions
- (1)
- Effects of alumina abrasive particle behavior for MR polishing on BK7 glass were experimentally studied.
- (2)
- Viscosity of the MR fluids increased with increased imposed magnetic fields and electric currents.
- (3)
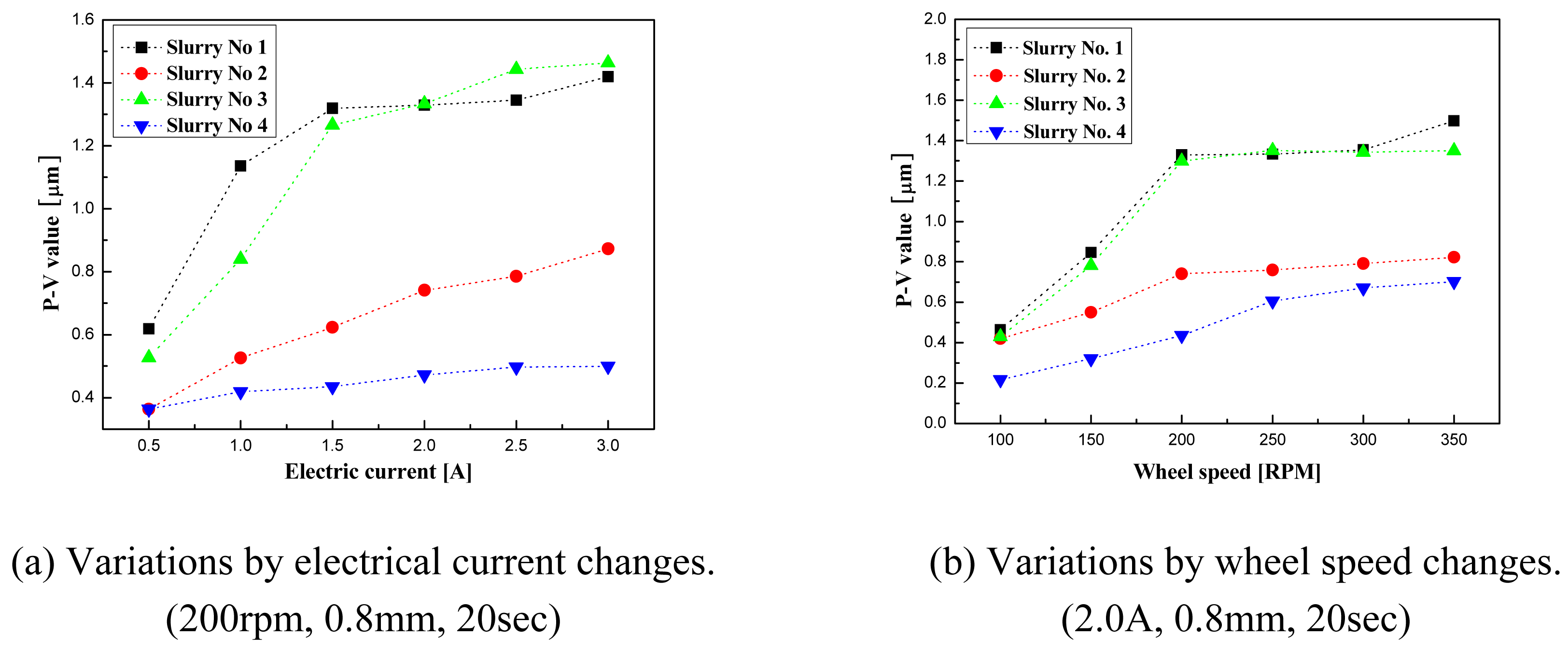
- Material removal rate increases with increased wheel speed and electric current; however, the rate of increase decelerated when the values exceeded certain levels. This phenomenon is due to chain structure breakage between the MR particles, and slipping between the wheel and the MR fluids by excessive shear stress.
- (4)
- Alumina slurry No.3 showed the best results. Using the conditions prescribed for this slurry, groove polishing on BK7 glass was performed. Outstanding surface roughness of Ra=3.8nm was obtained.
- (5)
- From the experimental results, it can be seen that the MR polishing method can be applied to fabricate micro parts, especially in MEMS.
- (6)
- It will be possible to obtain more advanced results through appropriate combinations of MR particles, micro abrasives, and process parameters.
References
- Akagami, Y.; Ogasawara, Y.; Nisimura, S. Characterization of particle motion for polishing and texturing under AC field by using particle dispersion type ER fluid. Smart structures and materials 1998, 593–598. [Google Scholar]
- Kim, K.D. A Controllable Micro Damper Using Magneto-Rheological Fluids. Journal of the KSPE 2000, 17, 41–45. [Google Scholar]
- Kormann, C.; Laun, H.M.; Laun, H.J. MR Fluids with nano-magnetic particles. International Journal of Modern Physics B 1996, 10, 3167–3172. [Google Scholar]
- Laun, S.D.; Arrasmith, S.R.; Kozhinova, I.A.; Gregg, L.; Shorey, A.B.; Romanofsky, H.J.; Golini, D. Studies of Material Removal in MRF from Polishing Spots. Ceramic Transactions 2000, 102, 201–212. [Google Scholar]
- Kordonski, W.I.; Jacobs, S.D. Magnetorheological Finishing. International Journal of Modern Physics B 1996, 10, 2837–2848. [Google Scholar]
- Golini, D.; Kordonski, W.I.; Dumas, P. Magnetorheological finishing(MRF) in commercial precision Optics Manufacturing. Optical Manufacturing and Testing 1999, 3782, 80–91. [Google Scholar]
- Kordonski, W.I.; Golini, D. Magnetorheological suspension–based finishing technology. Proceedings of SPIE-the international society for optical engineering 1998, 3326, 527–535. [Google Scholar]
- Kordonski, W.I.; Golini, D. Progress update in magnetorheological finishing. International Journal of Modern Physics B 1999, 13, 2205–2212. [Google Scholar]

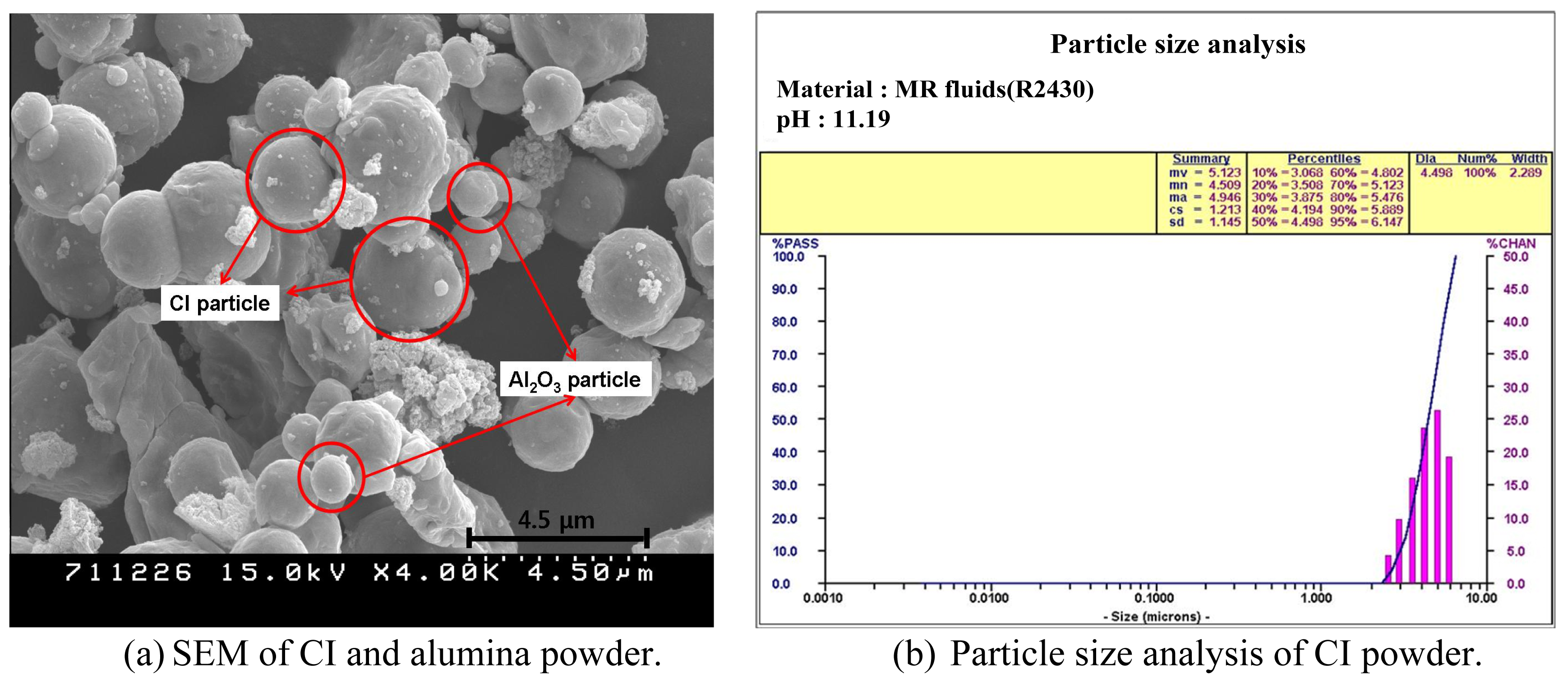

| Structure | Single-crystal cubic c-cut |
| Water solubility | 0.0017 |
| Surface hardness (GPa) | 2.47 |
| Young's module (GPa) | 110 |
| Fracture toughness(KIC) | 0.33 |
| Micro powder iron grade R-2430 | |
|---|---|
| Color | Gray |
| Apparent density | 2.0 - 3.0 |
| True density | 7.7 Maximum |
| Average particle diameter | 4-6 microns |
| % iron | 99.5 max |
| % carbon | 0.05 max |
| % Oxygen | 0.5 max |
| % Nitrogen | 0.1 max |
| Al2O3 | DI water | Glycerol | Na2CO3 |
| 30% | 67.7% | 2% | 0.3% |
| Slurry No. | Slurry condition (wt %) |
|---|---|
| Alumina slurry No.1 | Al2O3 particle(0.05μm) 10% + DI water 88.5% + Glycerin 0.5% + Na2CO3 1% |
| Alumina slurry No.2 | Al2O3 particle 3% + commercial stabilizer(Virgo 222YT-P) 5%+ DI water 92% |
| Alumina slurry No.3 | Al2O3 particle 5% + commercial stabilizer(Virgo 222YT-P) 5%+ DI water 90% |
| Alumina slurry No.4 | Al2O3 particle 10% + commercial stabilizer(Virgo 222YT-P) 5%+ DI water 85% |
| No. | Wheel speed (rpm) | Electric current (A) | Polishing Time | Polishing depth |
|---|---|---|---|---|
| 1 | 100 | 0.5,1,∼ 3 | 20sec | 0.8mm |
| 2 | 150 | 0.5,1,∼ 3 | ||
| 3 | 200 | 0.5,1,∼ 3 | ||
| 4 | 250 | 0.5,1,∼ 3 | ||
| 5 | 300 | 0.5,1,∼ 3 | ||
| 6 | 350 | 0.5,1,∼ 3 |
© 2008 by MDPI Reproduction is permitted for noncommercial purposes.
Share and Cite
Kim, D.-W.; Cho, M.-W.; Seo, T.-I.; Shin, Y.-J. Experimental Study on the Effects of Alumina Abrasive Particle Behavior in MR Polishing for MEMS Applications. Sensors 2008, 8, 222-235. https://doi.org/10.3390/s8010222
Kim D-W, Cho M-W, Seo T-I, Shin Y-J. Experimental Study on the Effects of Alumina Abrasive Particle Behavior in MR Polishing for MEMS Applications. Sensors. 2008; 8(1):222-235. https://doi.org/10.3390/s8010222
Chicago/Turabian StyleKim, Dong-Woo, Myeong-Woo Cho, Tae-Il Seo, and Young-Jae Shin. 2008. "Experimental Study on the Effects of Alumina Abrasive Particle Behavior in MR Polishing for MEMS Applications" Sensors 8, no. 1: 222-235. https://doi.org/10.3390/s8010222




