Lactoferrin Isolation Using Monolithic Column Coupled with Spectrometric or Micro-Amperometric Detector
Abstract
:1. Introduction
1.1 Biological functions of lactoferrin
1.2 Lactoferrin and intestine inflammation
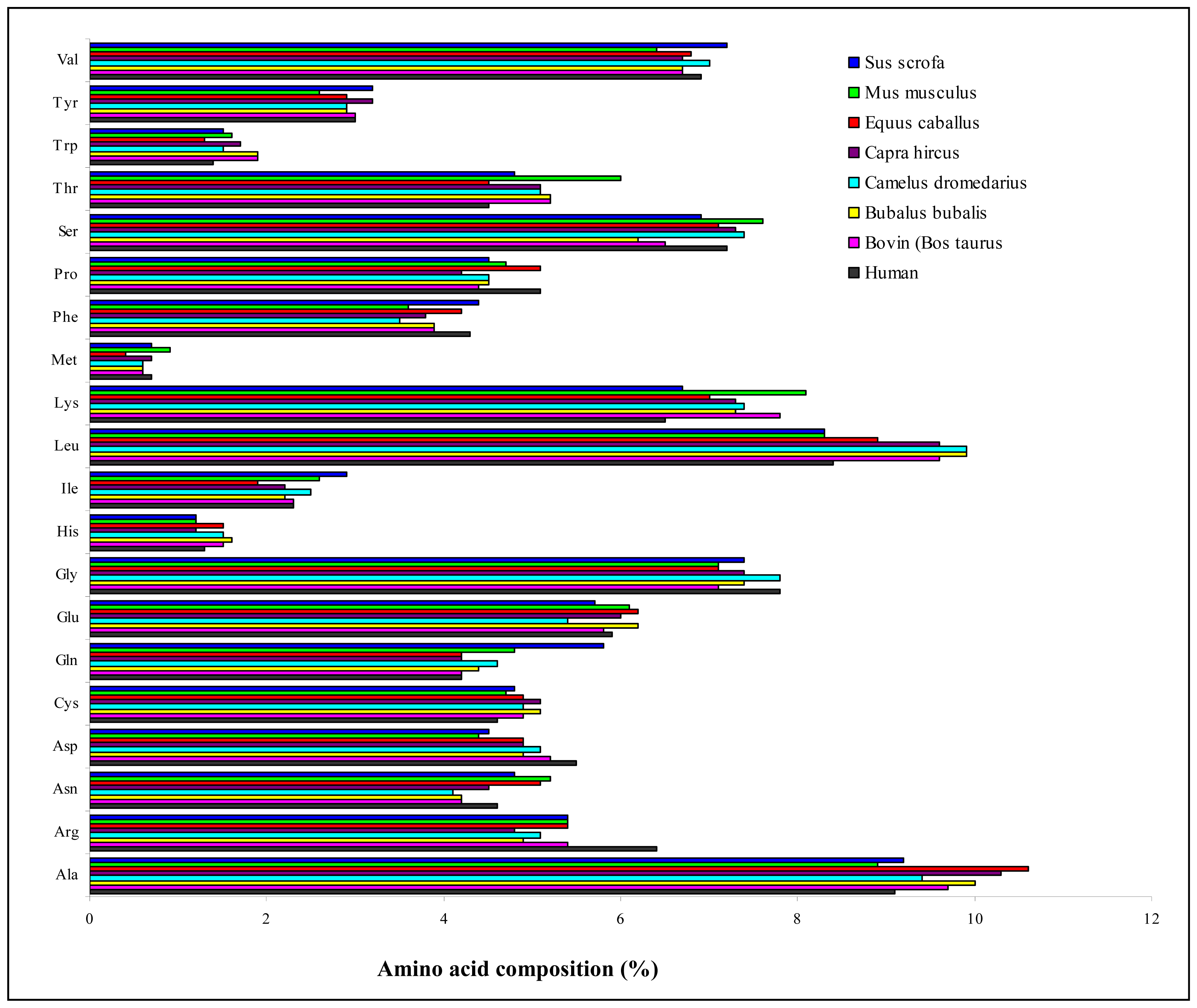
1.3 Lactoferrin detection
2. Experimental Section
2.1 Chemicals
2.2 Biological samples
2.2.1 Faeces
2.2.2 Cow colostrums
2.3 Flow injection analysis with electrochemical detection
2.4 Linear sweep voltammetry at carbon paste electrodes
2.5 Miniaturized amperometric device using screen printed electrodes
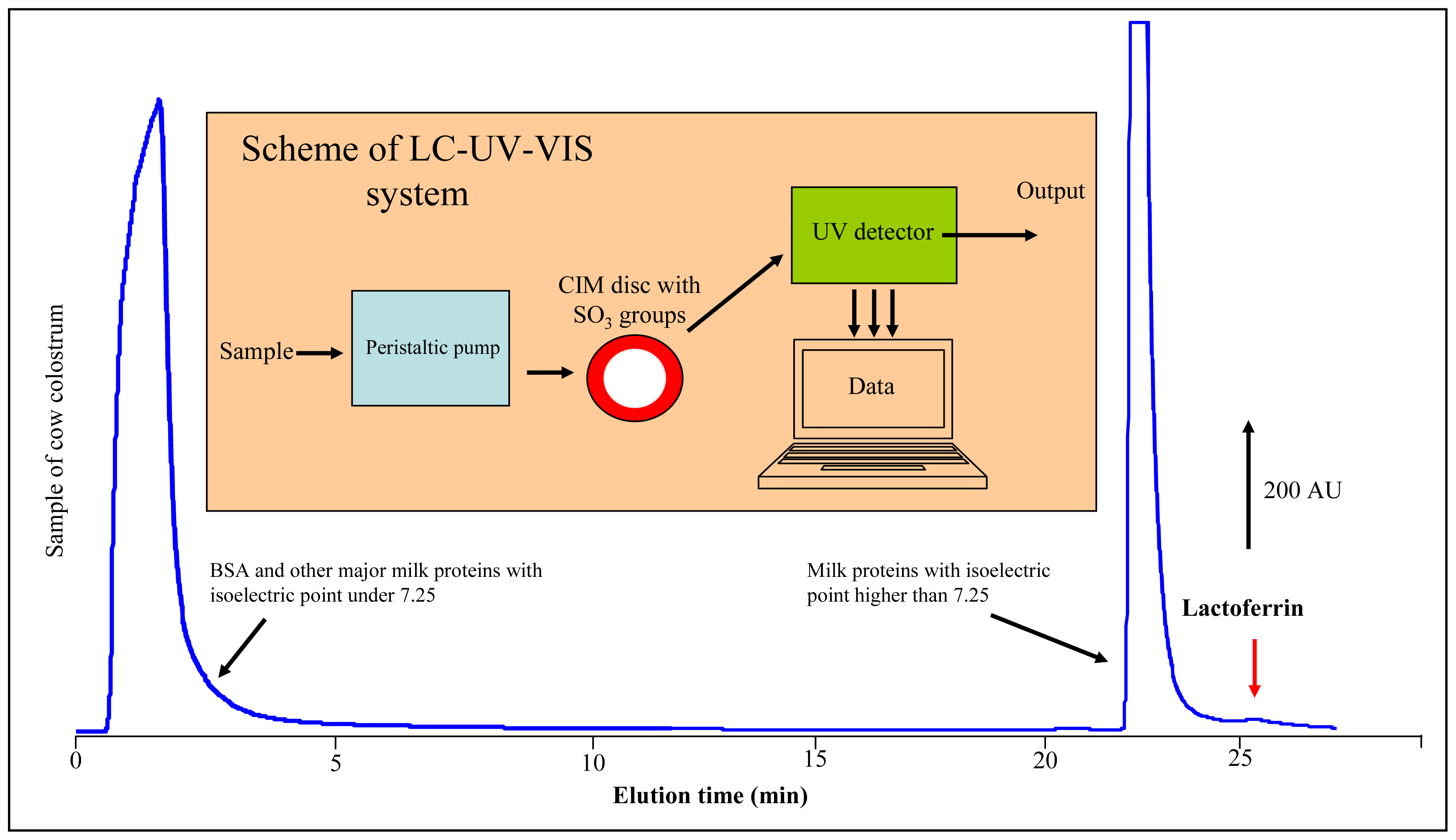
2.6 Liquid chromatography coupled with UV-VIS detector
2.7 Chip capillary electrophoresis – Experion
2.8 Sodium dodecyl sulphate - polyacrylamide gel electrophoresis
2.9 Statistical analysis
3. Results and Discussion
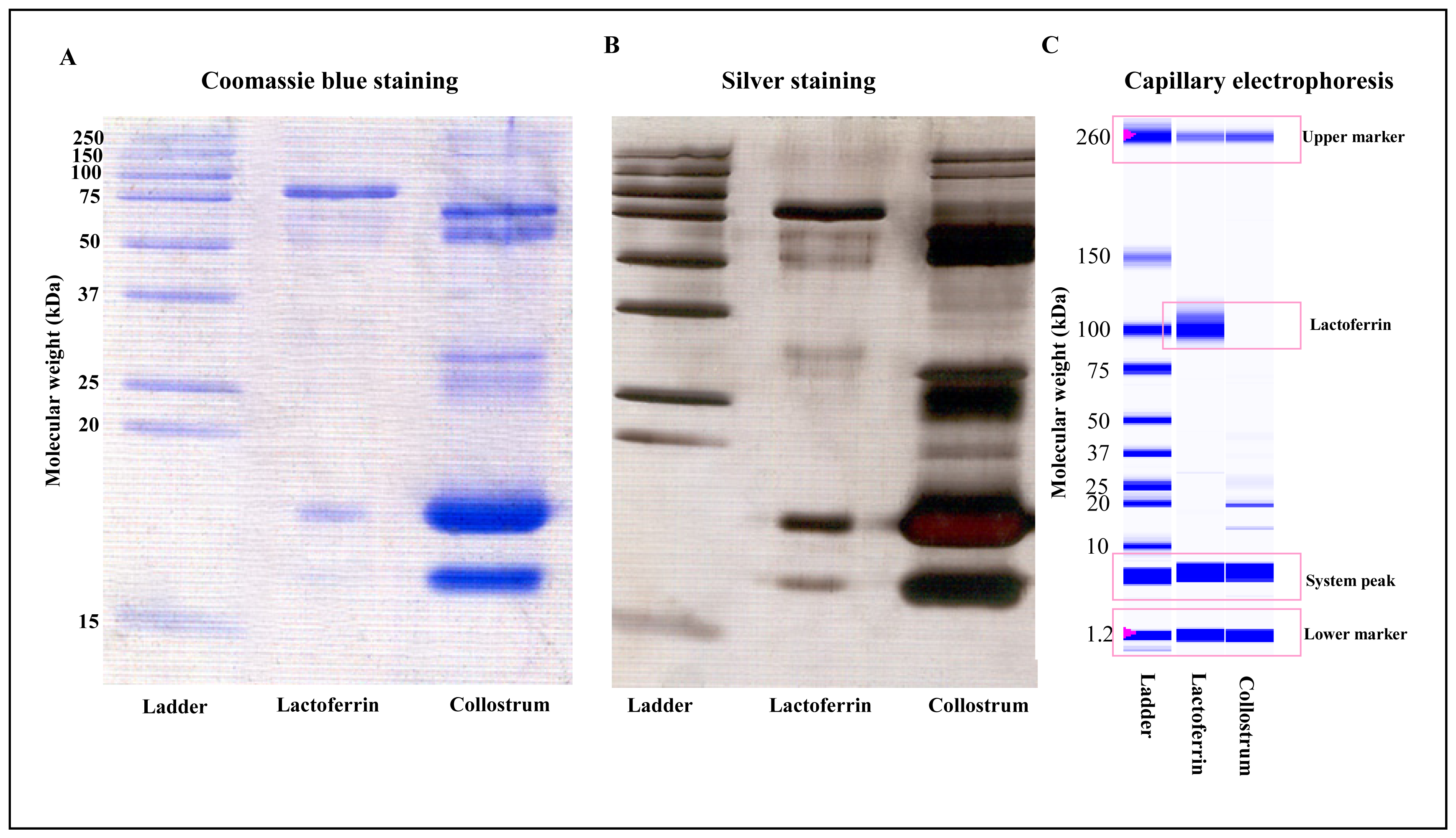
3.1 SDS-PAGE and Experion analysis of lactoferrin
3.1 Lactoferrin separation using monolithic column
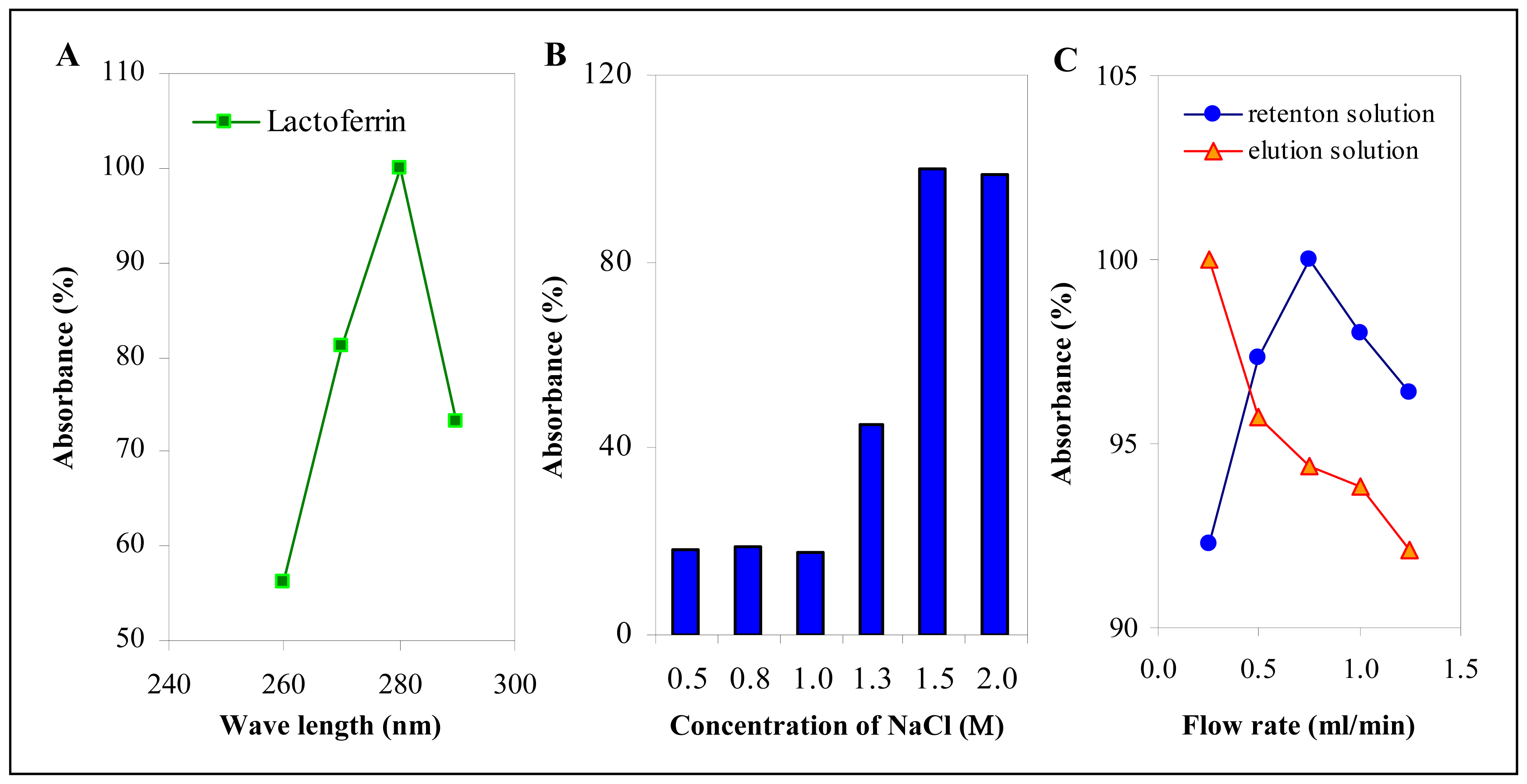
3.2 Optimization of lactoferrin isolation at monolithic column
3.3 Detection limits
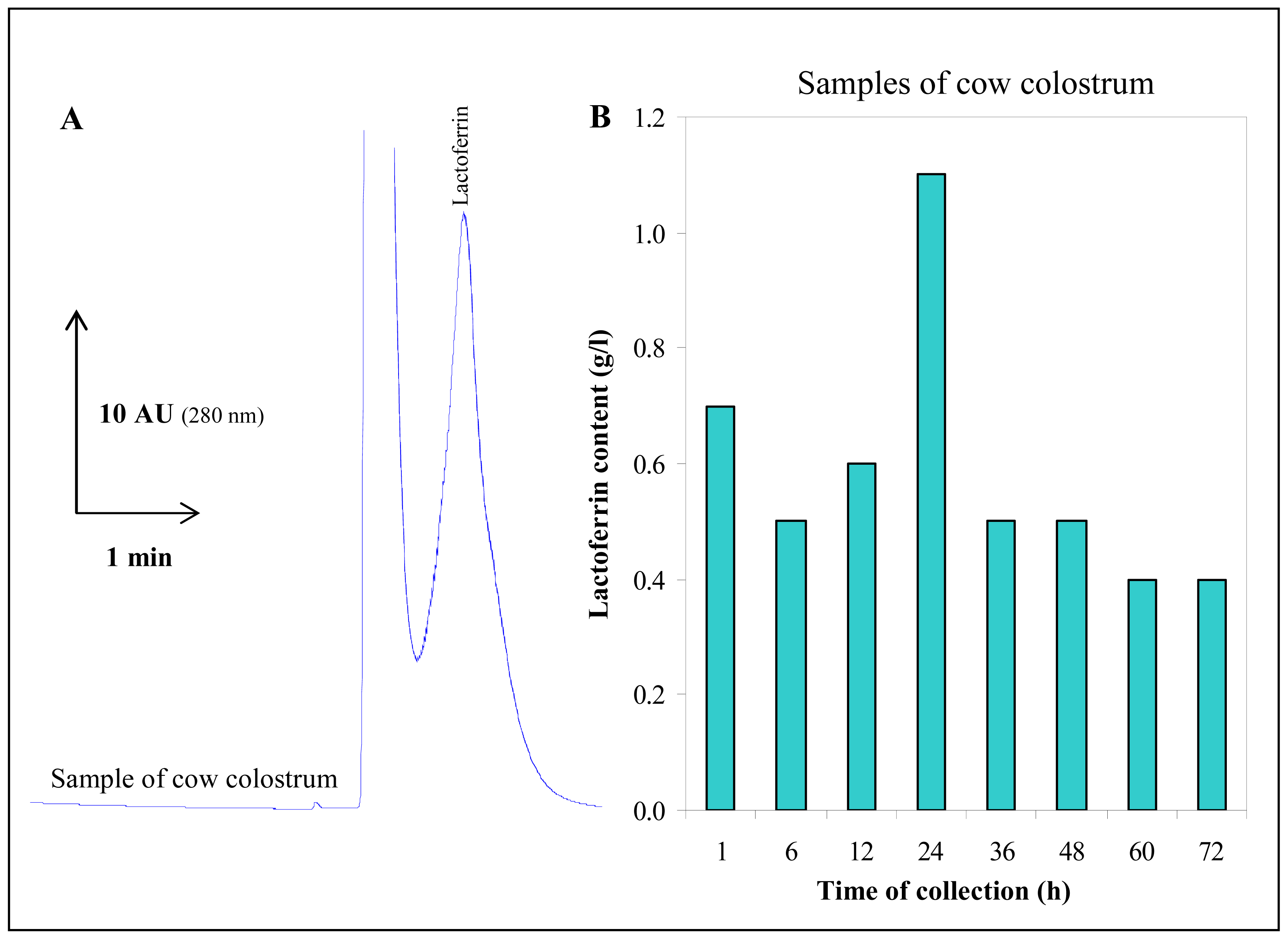
3.4 Lactoferrin isolation from cow colostrum
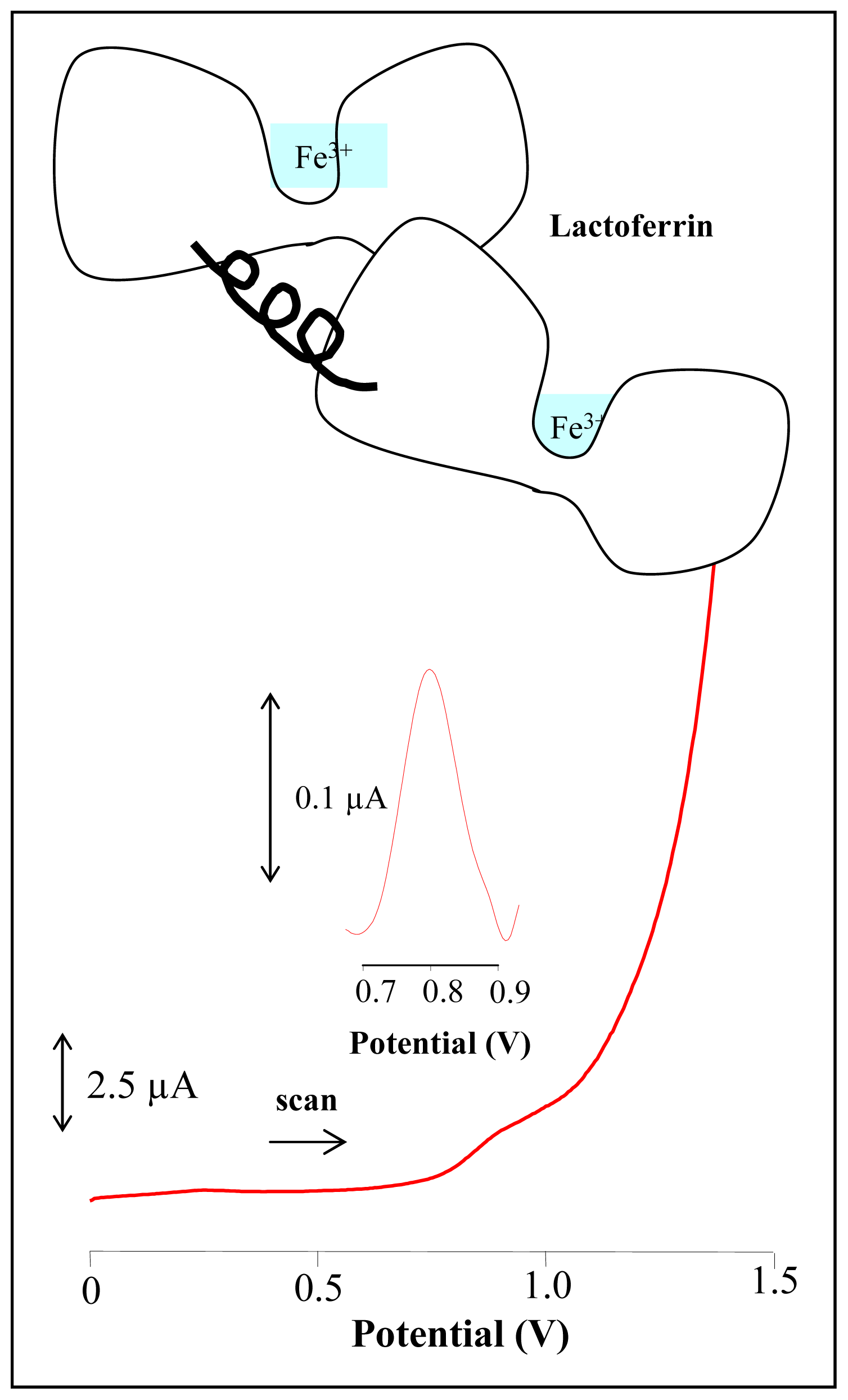
3.5 Electrochemical detection of lactoferrin
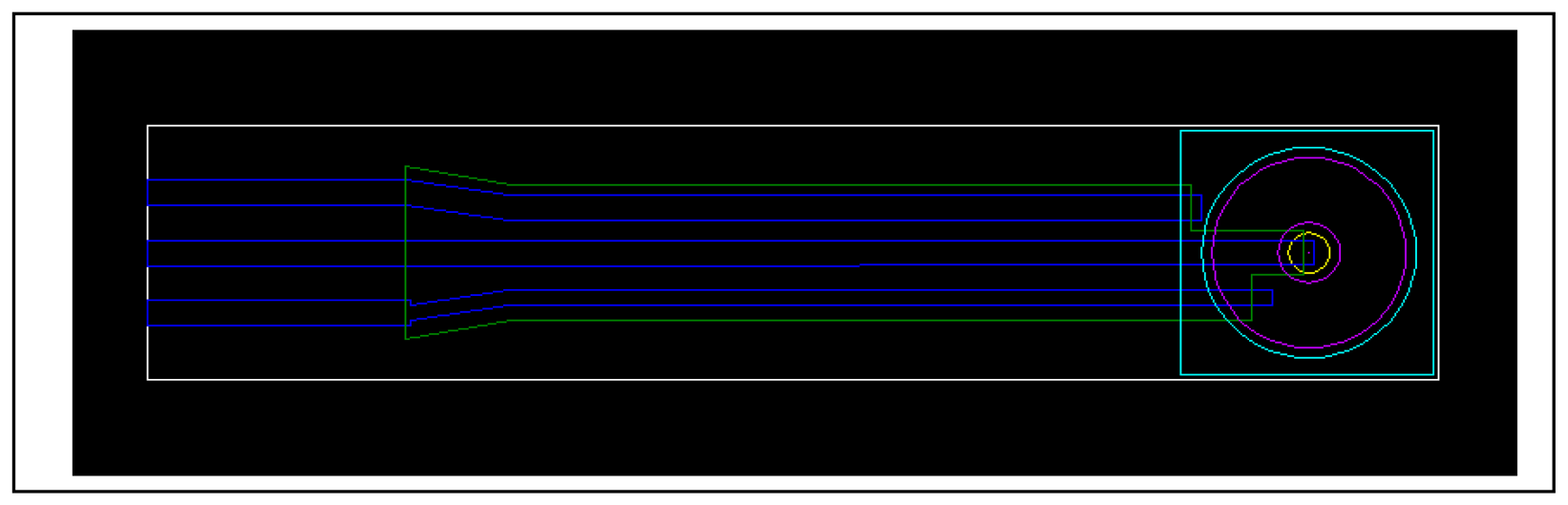
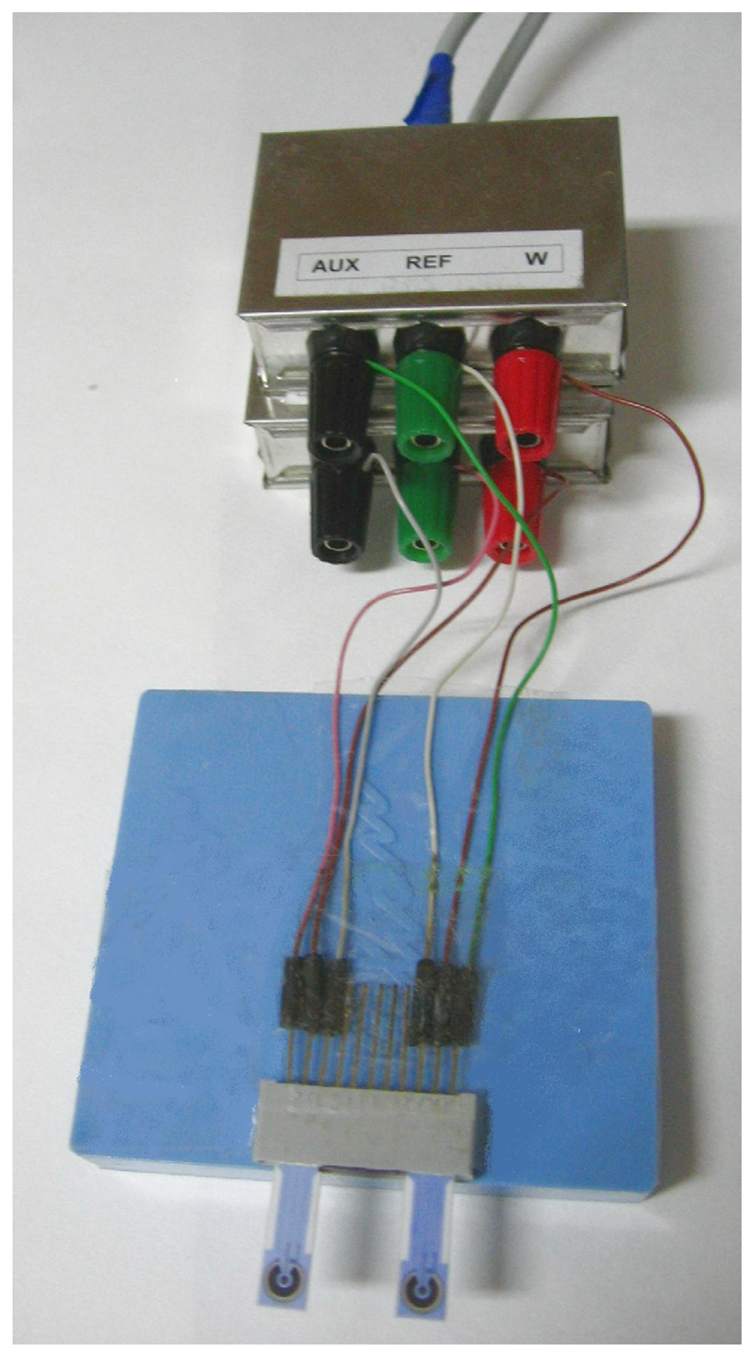
3.6 Fabrication of miniaturised screen-printed electrodes
3.7 A detection of lactoferrin using screen-printed electrodes
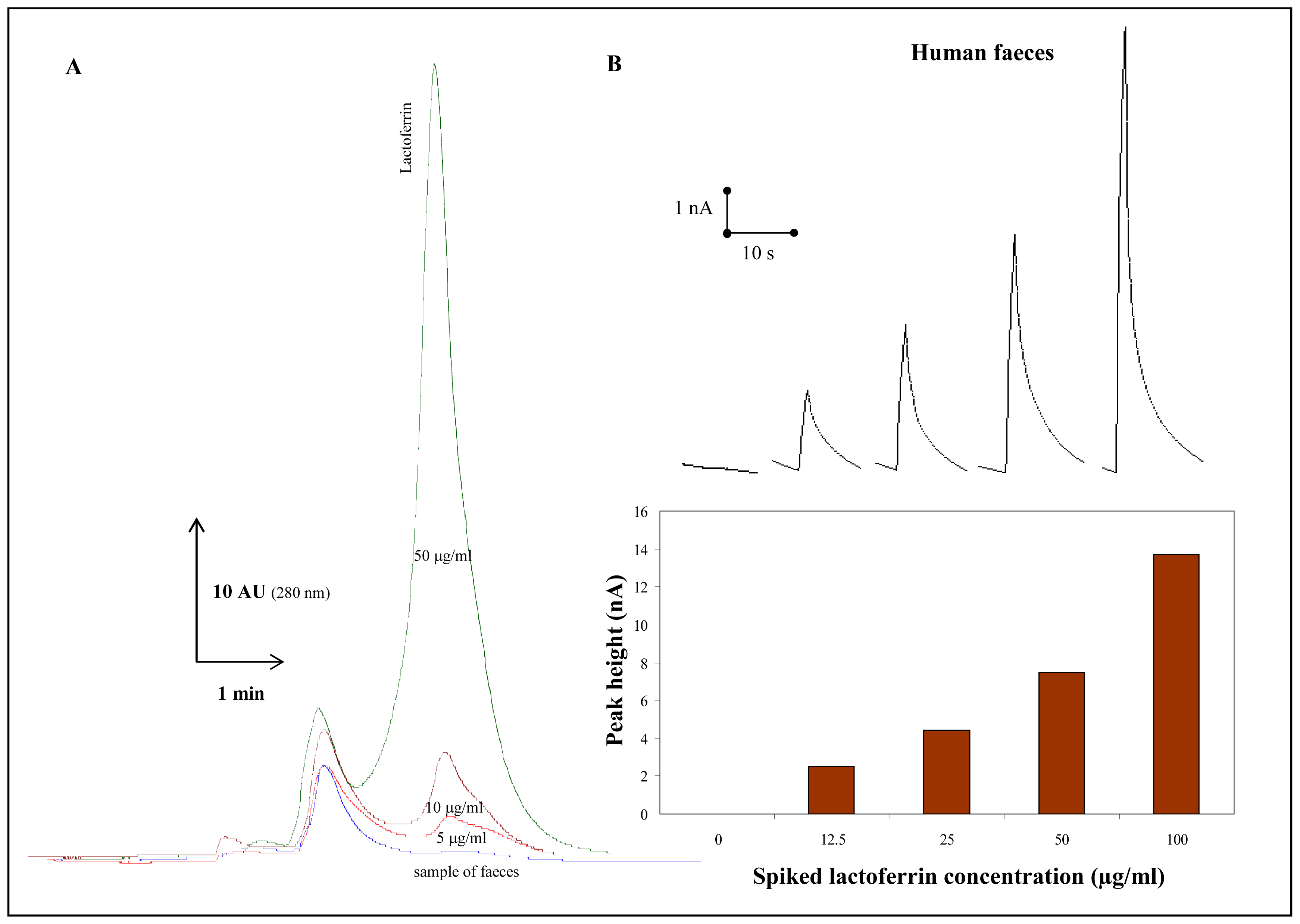
3.8 Lactoferrin detection in faeces samples
4. Conclusion
Acknowledgments
References
- Bezkorovainy, A. Human milk and colostrum proteins - review. J. Dairy Sci 1977, 60, 1023–1037. [Google Scholar]
- Sanchez, L.; Aranda, P.; Perez, M.D.; Calvo, M. Concentration of Lactoferrin and Transferrin Throughout Lactation in Cows Colostrum and Milk. Biol. Chem. Hoppe-Seyler 1988, 369, 1005–1008. [Google Scholar]
- Sanchez, L.; Calvo, M.; Brock, J.H. Biological Role of Lactoferrin. Arch. Dis. Child 1992, 67, 657–661. [Google Scholar]
- Sanchez, L.; Peiro, J.M.; Castillo, H.; Perez, M.D.; Ena, J.M.; Calvo, M. Kinetic-Parameters for Denaturation of Bovine-Milk Lactoferrin. J. Food Sci 1992, 57, 873–879. [Google Scholar]
- Sharp, P.; Srai, S.K. Molecular mechanisms involved in intestinal iron absorption. World J. Gastroenterol 2007, 13, 4716–4724. [Google Scholar]
- Levay, P.F.; Viljoen, M. Lactoferrin - a General-Review. Haematologica 1995, 80, 252–267. [Google Scholar]
- Brisson, G.; Britten, M.; Pouliot, Y. Heat-induced aggregation of bovine lactoferrin at neutral pH: Effect of iron saturation. Int. Dairy J 2007, 17, 617–624. [Google Scholar]
- Bernal, V.; Jelen, P. Thermal-Stability of Whey Proteins - a Calorimetric Study. J. Dairy Sci 1985, 68, 2847–2852. [Google Scholar]
- Baker, E.N.; Baker, H.M. Molecular structure, binding properties and dynamics of lactoferrin. Cell. Mol. Life Sci 2005, 62, 2531–2539. [Google Scholar]
- Baker, H.M.; Baker, E.N. Lactoferrin and iron: structural and dynamic aspects of binding and release. Biometals 2004, 17, 209–216. [Google Scholar]
- Weinberg, E.D. Antibiotic properties and applications of lactoferrin. Curr. Pharm. Design 2007, 13, 801–811. [Google Scholar]
- Struff, W.G.; Sprotte, G. Bovine colostrum as a biologic in clinical medicine: a review - Part I: Biotechnological standards, pharmacodynamic and pharmacolkinetic characteristics and principles of treatment. Int. J. Clin. Pharmacol. Ther 2007, 45, 193–202. [Google Scholar]
- Pan, Y.; Rowney, M.; Guo, P.; Hobman, P. Biological properties of lactoferrin: an overview. Aust. J. Dairy Technol 2007, 62, 31–42. [Google Scholar]
- Berlov, M.N.; Korableva, E.S.; Andreeva, Y.V.; Ovchinnikova, T.V.; Kokryakov, V.N. Lactoferrin from canine neutrophils: Isolation and physicochemical and antimicrobial properties. Biochem. -Moscow 2007, 72, 445–451. [Google Scholar]
- Piccinini, R.; Gabai, G.; Bailoni, L.; Simonetto, A.; Dapra, V.; Zecconi, A. Influence of the suppression of dry period on several blood and milk markers in dairy cows. Milchwiss.-Milk Sci. Int 2007, 62, 367–370. [Google Scholar]
- Hwang, S.A.; Wilk, K.M.; Budnicka, M.; Olsen, M.; Bangale, Y.A.; Hunter, R.L.; Kruzel, M.L.; Actor, J.K. Lactoferrin enhanced efficacy of the BCG vaccine to generate host protective responses against challenge with virulent Mycobacterium tuberculosis. Vaccine 2007, 25, 6730–6743. [Google Scholar]
- Hwang, S.A.; Wilk, K.M.; Bangale, Y.A.; Kruzel, M.L.; Actor, J.K. Lactoferrin modulation of IL-12 and IL-10 response from activated murine leukocytes. Med. Microbiol. Immunol 2007, 196, 171–180. [Google Scholar]
- Yoshise, R.E.; Matsumoto, M.; Chiji, H.; Kuwata, H.; Shin, K.; Yamauchi, K.; Tamura, Y.; Tanaka, T.; Kumura, H.; Shimazaki, K. Profiles of bovine lactoferrin in the gastrointestinal tracts of rats as observed by ELISA, Western blotting and SELDI-affinity MS. Milchwiss.-Milk Sci. Int 2007, 62, 446–450. [Google Scholar]
- Caraher, E.M.; Gumulapurapu, K.; Taggart, C.C.; Murphy, P.; McClean, S.; Callaghan, M. The effect of recombinant human lactoferrin on growth and the antibiotic susceptibility of the cystic fibrosis pathogen Burkholderia cepacia complex when cultured planktonically or as biofilms. J. Antimicrob. Chemother 2007, 60, 546–554. [Google Scholar]
- Beeckman, D.S.A.; Van Droogenbroeck, C.; De Cock, B.J.A.; Van Oostveltdt, P.; Vanrompay, D.C.G. Effect of ovotransferrin and lactoferrins on Chlamydophila psittaci adhesion and invasion in HD11 chicken macrophages. Vet. Res 2007, 38, 729–739. [Google Scholar]
- Hanson, L.A. Breast-feeding and immune function. Proc. Nutr. Soc 2007, 66, 384–396. [Google Scholar]
- Senkovich, O.; Cook, W.J.; Mirza, S.; Hollingshead, S.K.; Protasevich, II; Briles, D.E.; Chattopadhyay, D. Structure of a complex of human lactoferrin N-lobe with pneumococcal surface protein A provides insight into microbial defense mechanism. J. Mol. Biol 2007, 370, 701–713. [Google Scholar]
- Flach, C.F.; Qadri, F.; Bhuiyan, T.R.; Alam, N.H.; Jennische, E.; Lonnroth, I.; Holmgren, J. Broad up-regulation of innate defense factors during acute cholera. Infect. Immun 2007, 75, 2343–2350. [Google Scholar]
- Zullo, A.; De Francesco, V.; Scaccianoce, G.; Manes, G.; Efrati, C.; Hassan, C.; Maconi, G.; Pighonica, D.; Cannaviello, C.; Panella, C.; Morini, S.; Lerardi, E. Helicobacter pylori eradication with either quadruple regimen with lactoferrin or levofloxacin-based triple therapy: A multicentre study. Dig. Liver Dis 2007, 39, 806–810. [Google Scholar]
- Tursi, A.; Elisei, W.; Brandimarte, G.; Giorgetti, G.M.; Modeo, M.E.; Aiello, F. Effect of lactoferrin supplementation on the effectiveness and tolerability of a 7-day quadruple therapy after failure of a first attempt to cure Helicobacter pylori infection. Med. Sci. Monitor 2007, 13, CR187–CR190. [Google Scholar]
- Mistry, N.; Drobni, P.; Nasland, J.; Sunkari, V.G.; Jenssen, H.; Evander, M. The anti-papillomavirus activity of human and bovine lactoferricin. Antiviral Res 2007, 75, 258–265. [Google Scholar]
- Patel, S.; Hazrati, E.; Cheshenko, N.; Galen, B.; Yang, H.Y.; Guzman, E.; Wang, R.; Herold, B.C.; Keller, M.J. Seminal plasma reduces the effectiveness of topical polyanionic microbicides. J. Infect. Dis 2007, 196, 1394–1402. [Google Scholar]
- Kaito, M.; Iwasa, M.; Fujita, N.; Kobayashi, Y.; Kojima, Y.; Ikoma, J.; Imoto, I.; Adachi, Y.; Hamano, H.; Yamauchi, K. Effect of lactoferrin in patients with chronic hepatitis C: Combination therapy with interferon and ribavirin. J. Gastroenterol. Hepatol 2007, 22, 1894–1897. [Google Scholar]
- Redwan, E.M.; Tabll, A. Camel lactoferrin markedly inhibits hepatitis C virus genotype 4 infection of human peripheral blood leukocytes. J. Immunoass. Immunoch 2007, 28, 267–277. [Google Scholar]
- Novak, R.M.; Donoval, B.A.; Graham, P.J.; Boksa, L.A.; Spear, G.; Hershow, R.C.; Chen, H.Y.; Landay, A. Cervicovaginal levels of lactoferrin, secretory leukocyte protease inhibitor, and RANTES and the effects of coexisting vaginoses in human immunodeficiency virus (HIV)-seronegative women with a high risk of heterosexual acquisition of HIV infection. Clin. Vaccine Immunol 2007, 14, 1102–1107. [Google Scholar]
- Shaw, J.L.V.; Smith, C.R.; Diamandis, E.P. Proteomic analysis of human cervico-vaginal fluid. J. Proteome Res 2007, 6, 2859–2865. [Google Scholar]
- Tang, L.J.; De Seta, F.; Odreman, F.; Venge, P.; Piva, C.; Guaschino, S.; Garcia, R.C. Proteomic analysis of human cervical-vaginal fluids. J. Proteome Res 2007, 6, 2874–2883. [Google Scholar]
- Lupetti, A.; Brouwer, C.; Bogaards, S.J.P.; Welling, M.M.; de Heer, E.; Campa, M.; van Dissel, J.T.; Friesen, R.H.E.; Nibbering, P.H. Human lactoferrin-derived peptide's antifungal activities against disseminated Candida albicans infection. J. Infect. Dis 2007, 196, 1416–1424. [Google Scholar]
- Enrique, M.; Marcos, J.F.; Yuste, M.; Martinez, M.; Valles, S.; Manzanares, P. Antimicrobial action of synthetic peptides towards wine spoilage yeasts. Int. J. Food Microbiol 2007, 118, 318–325. [Google Scholar]
- Parodi, P.W. A role for milk proteins and their peptides in cancer prevention. Curr. Pharm. Design 2007, 13, 813–828. [Google Scholar]
- Buccigrossi, V.; De Marco, G.; Bruzzese, E.; Ombrato, L.; Bracale, I.; Polito, G.; Guarino, A. Lactoferrin induces concentration-dependent functional modulation of intestinal proliferation and differentiation. Pediatr. Res 2007, 61, 410–414. [Google Scholar]
- Mohan, K.; Gunasekaran, P.; Varalakshmi, E.; Hara, Y.; Nagini, S. In vitro evaluation of the anticancer effect of lactoferrin and tea polyphenol combination on oral carcinoma cells. Cell Biol. Int 2007, 31, 599–608. [Google Scholar]
- Kehoe, S.I.; Jayarao, B.M.; Heinrichs, A.J. A survey of bovine colostrum composition and colostrum management practices on Pennsylvania dairy farms. J. Dairy Sci 2007, 90, 4108–4116. [Google Scholar]
- Piccinini, R.; Binda, E.; Belotti, M.; Dapra, V.; Zecconi, A. Evaluation of milk components during whole lactation in healthy quarters. J. Dairy Res 2007, 74, 226–232. [Google Scholar]
- Shan, T.; Wang, Y.; Wang, Y.; Liu, J.; Xu, Z. Effect of dietary lactoferrin on the immune functions and serum iron level of weanling piglets. J. Anim. Sci 2007, 85, 2140–2146. [Google Scholar]
- Wang, Y.Z.; Shan, T.Z.; Xu, Z.R.; Feng, J.; Wang, Z.Q. Effects of the lactoferrin (LF) on the growth performance, intestinal microflora and morphology of weanling pigs. Anim. Feed Sci. Technol 2007, 135, 263–272. [Google Scholar]
- El-Hatmi, H.; Girardet, J.M.; Gaillard, J.L.; Yahyaoui, M.H.; Attia, H. Characterisation of whey proteins of camel (Camelus dromedarius) milk and colostrum. Small Ruminant Res 2007, 70, 267–271. [Google Scholar]
- Park, Y.W.; Juarez, M.; Ramos, M.; Haenlein, G.F.W. Physico-chemical characteristics of goat and sheep milk. Small Ruminant Res 2007, 68, 88–113. [Google Scholar]
- Han, Z.S.; Li, Q.W.; Zhang, Z.Y.; Xiao, B.; Gao, D.W.; Wu, S.Y.; Li, J.; Zhao, H.W.; Jiang, Z.L.; Hu, J.H. High-level expression of human lactoferrin in the milk of goats by using replication-defective adenoviral vectors. Protein Expr. Purif 2007, 53, 225–231. [Google Scholar]
- Schoepfer, A.M.; Trummler, M.; Seeholzer, P.; Criblez, D.H.; Seibold, F. Accuracy of four fecal assays in the diagnosis of colitis. Dis. Colon Rectum 2007, 50, 1697–1706. [Google Scholar]
- Angriman, I.; Scarpa, M.; D'Inca, R.; Basso, D.; Ruffolo, C.; Polese, L.; Sturniolo, G.C.; D'Amico, D.F.; Plebani, M. Enzymes in feces: Useful markers of chronic inflammatory bowel disease. Clin. Chim. Acta 2007, 381, 63–68. [Google Scholar]
- Zou, H.; Harrington, J.J.; Sugumar, A.; Klatt, K.K.; Smyrk, T.C.; Ahlquist, D.A. Detection of colorectal disease by stool defensin assay: An exploratory study. Clin. Gastroenterol. Hepatol 2007, 5, 865–868. [Google Scholar]
- Scarpa, M.; D'Inca, R.; Basso, D.; Ruffolo, C.; Polese, L.; Bertin, E.; Luise, A.; Frego, M.; Plebani, M.; Sturniolo, G.C.; D'Amico, D.F.; Angriman, I. Fecal lactoferrin and calprotectin after ileocolonic resection for Crohn's disease. Dis. Colon Rectum 2007, 50, 861–869. [Google Scholar]
- Hirata, I.; Hoshimoto, M.; Saito, O.; Kayazawa, M.; Nishikawa, T.; Murano, M.; Toshina, K.; Wang, F.Y.; Matsuse, R. Usefulness of fecal lactoferrin and hemoglobin in diagnosis of colorectal diseases. World J. Gastroenterol 2007, 13, 1569–1574. [Google Scholar]
- Walker, T.R.; Land, M.L.; Kartashov, A.; Saslowsky, T.M.; Lyerly, D.M.; Boone, J.H.; Rufo, P.A. Fecal lactoferrin is a sensitive and specific marker of disease activity in children and young adults with inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr 2007, 44, 414–422. [Google Scholar]
- D'Inca, R.; Dal Pont, E.; Di Leo, V.; Ferronato, A.; Fries, W.; Vettorato, M.G.; Martines, D.; Sturniolo, G.C. Calprotectin and lactoferrin in the assessment of intestinal inflammation and organic disease. Int. J. Colorectal Dis 2007, 22, 429–437. [Google Scholar]
- Giuffre, G.; Barresi, V.; Skliros, C.; Barresi, G.; Tuccari, G. Immunoexpression of lactoferrin in human sporadic renal cell carcinomas. Oncol. Rep 2007, 17, 1021–1026. [Google Scholar]
- Sabatucci, A.; Vachette, P.; Vasilyev, V.B.; Beltramini, M.; Sokolov, A.; Pulina, M.; Salvato, B.; Angelucci, C.B.; Maccarronel, M.; Cozzani, I.; Dainese, E. Structural characterization of the ceruloplasmin: Lactoferrin complex in solution. J. Mol. Biol 2007, 371, 1038–1046. [Google Scholar]
- Sokolov, A.V.; Pulina, M.O.; Ageeva, K.V.; Ayrapetov, M.I.; Berlov, M.N.; Volgin, G.N.; Markov, A.G.; Yablonsky, P.K.; Kolodkin, N.I.; Zakharova, E.T.; Vasilyev, V.B. Interaction of ceruloplasmin, lactoferrin, and myeloperoxidase. Biochem. -Moscow 2007, 72, 409–415. [Google Scholar]
- Chen, L.; Guo, C.; Guan, Y.P.; Liu, H.Z. Isolation of lactoferrin from acid whey by magnetic affinity separation. Sep. Purif. Technol 2007, 56, 168–174. [Google Scholar]
- Lu, R.R.; Xu, S.Y.; Wang, Z.; Yang, R.J. Isolation of lactoferrin from bovine colostrum by ultrafiltration coupled with strong cation exchange chromatography on a production scale. J. Membr. Sci 2007, 297, 152–161. [Google Scholar]
- Andersson, J.; Mattiasson, B. Simulated moving bed technology with a simplified approach for protein purification - Separation of lactoperoxidase and lactoferrin from whey protein concentrate. J. Chromatogr. A 2006, 1107, 88–95. [Google Scholar]
- Uchida, T.; Dosako, S.; Sato, K.; Kawakami, H. Sequential separation of lactoferrin, lactoperoxidase, and secretory component by sulfate-linked ion-exchange chromatography. Milchwiss.-Milk Sci. Int 2003, 58, 482–486. [Google Scholar]
- Yoshida, A.; Wei, Z.; Shinmura, Y.; Fukunaga, N. Separation of lactoferrin-a and -b from bovine colostrum. J. Dairy Sci 2000, 83, 2211–2215. [Google Scholar]
- Grasselli, M.; Cascone, O. Separation of lactoferrin from bovine whey by dye affinity chromatography. Neth. Milk Dairy J 1996, 50, 551–561. [Google Scholar]
- Almashikhi, S.A.; Lichan, E.; Nakai, S. Separation of Immunoglobulins and Lactoferrin from Cheese Whey by Chelating Chromatography. J. Dairy Sci 1988, 71, 1747–1755. [Google Scholar]
- Noh, K.H.; Rhee, M.S.; Imm, J.Y. Separation of lactoferrin from model whey protein mixture by reverse micelles formed by cationic surfactant. Food Sci. Biotechnol 2005, 14, 131–136. [Google Scholar]
- Zitka, O.; Horna, A.; Stejskal, K.; Zehnalek, J.; Adam, V.; Havel, L.; Zeman, L.; Kizek, R. Study of structural changes of lactoferrin using flow injection analysis with electrochemical detection on glassy carbon electrode. Acta Chim. Slov 2007, 54, 68–73. [Google Scholar]
- Billova, S.; Kizek, R.; Jelen, F.; Novotna, P. Square-wave voltammetric determination of cefoperazone in a bacterial culture, pharmaceutical drug, milk, and urine. Anal. Bioanal. Chem 2003, 377, 362–369. [Google Scholar]
- Billova, S.; Kizek, R.; Palecek, E. Differential pulse adsorptive stripping voltammetry of osmium-modified peptides. Bioelectrochemistry 2002, 56, 63–66. [Google Scholar]
- Kizek, R.; Masarik, M.; Kramer, K.J.; Potesil, D.; Bailey, M.; Howard, J.A.; Klejdus, B.; Mikelova, R.; Adam, V.; Trnkova, L.; Jelen, F. An analysis of avidin, biotin and their interaction at attomole levels by voltammetric and chromatographic techniques. Anal. Bioanal. Chem 2005, 381, 1167–1178. [Google Scholar]
- Kizek, R.; Trnkova, L.; Palecek, E. Determination of metallothionein at the femtomole level by constant current stripping chronopotentiometry. Anal. Chem 2001, 73, 4801–4807. [Google Scholar]
- Kizek, R.; Trnkova, L.; Sevcikova, S.; Smarda, J.; Jelen, F. Silver electrode as a sensor for determination of zinc in cell cultivation medium. Anal. Biochem 2002, 301, 8–13. [Google Scholar]
- Palecek, E.; Masarik, M.; Kizek, R.; Kuhlmeier, D.; Hassmann, J.; Schulein, J. Sensitive electrochemical determination of unlabeled MutS protein and detection of point mutations in DNA. Anal. Chem 2004, 76, 5930–5936. [Google Scholar]
- Potesil, D.; Mikelova, R.; Adam, V.; Kizek, R.; Prusa, R. Change of the protein p53 electrochemical signal according to its structural form - Quick and sensitive distinguishing of native, denatured, and aggregated form of the “guardian of the genome”. Protein J 2006, 25, 23–32. [Google Scholar]
- Adam, V.; Beklova, M.; Pikula, J.; Hubalek, J.; Trnkova, L.; Kizek, R. Shapes of differential pulse voltammograms and level of metallothionein at different animal species. Sensors 2007, 7, 2419–2429. [Google Scholar]
- Adam, V.; Mikelova, R.; Hubalek, J.; Hanustiak, P.; Beklova, M.; Hodek, P.; Horna, A.; Trnkova, L.; Stiborova, M.; Zeman, L.; Kizek, R. Utilizing of square wave voltammetry to detect flavonoids in the presence of human urine. Sensors 2007, 7, 2402–2418. [Google Scholar]
- Adam, V.; Zehnalek, J.; Petrlova, J.; Potesil, D.; Sures, B.; Trnkova, L.; Jelen, F.; Vitecek, J.; Kizek, R. Phytochelatin modified electrode surface as a sensitive heavy metal ions biosensor. Sensors 2005, 5, 70–84. [Google Scholar]
- Babula, P.; Huska, D.; Hanustiak, P.; Baloun, J.; Krizkova, S.; Adam, V.; Hubalek, J.; Havel, L.; Zemlicka, M.; Horna, A.; Beklova, M.; Kizek, R. Flow injection analysis coupled with carbon electrodes as the tool for analysis of naphthoquinones with respect to their content and functions in biological samples. Sensors 2006, 11, 1466–1482. [Google Scholar]
- Hubalek, J.; Hradecky, J.; Adam, V.; Krystofova, O.; Huska, D.; Masarik, M.; Trnkova, L.; Horna, A.; Klosova, K.; Adamek, M.; Zehnalek, J.; Kizek, R. Spectrometric and voltammetric analysis of urease – Nickel nanoelectrode as an electrochemical sensor. Sensors 2007, 7, 1238–1255. [Google Scholar]
- Krizkova, S.; Beklova, M.; Pikula, J.; Adam, V.; Horna, A.; Kizek, R. Hazards of secondary bromadiolone intoxications evaluated using high-performance liquid chromatography with electrochemical detection. Sensors 2007, 7, 1271–1286. [Google Scholar]
- Prasek, J.; Adamek, M.; Hubalek, J.; Adam, V.; Trnkova, L.; Kizek, R. New hydrodynamic electrochemical arrangement for cadmium ions detection using thick-film chemical sensor electrodes. Sensors 2006, 11, 1498–1512. [Google Scholar]
- Supalkova, V.; Huska, D.; Diopan, V.; Hanustiak, P.; Zitka, O.; Stejskal, K.; Baloun, J.; Pikula, J.; Havel, L.; Zehnalek, J.; Adam, V.; Trnkova, L.; Beklova, M.; Kizek, R. Electroanalysis of plant thiols. Sensors 2007, 7, 932–959. [Google Scholar]
- Supalkova, V.; Petrek, J.; Baloun, J.; Adam, V.; Bartusek, K.; Trnkova, L.; Beklova, M.; Diopan, V.; Havel, L.; Kizek, R. Multi-instrumental investigation of affecting of early somatic embryos of Spruce by cadmium(II) and lead(II) ions. Sensors 2007, 7, 743–759. [Google Scholar]
- Supalkova, V.; Petrek, J.; Havel, L.; Krizkova, S.; Petrlova, J.; Adam, V.; Potesil, D.; Babula, P.; Beklova, M.; Horna, A.; Kizek, R. Electrochemical sensors for detection of acetylsalicylic acid. Sensors 2006, 11, 1483–1497. [Google Scholar]
- Zitka, O.; Huska, D.; Krizkova, S.; Adam, V.; Chavis, G.J.; Trnkova, L.; Horna, A.; Hubalek, J.; Kizek, R. An investigation of glutathione-platinum(II) interactions by means of the flow injection analysis using glassy carbon electrode. Sensors 2007, 7, 1256–1270. [Google Scholar]
- Adam, V.; Hanustiak, P.; Krizkova, S.; Beklova, M.; Zehnalek, J.; Trnkova, L.; Horna, A.; Sures, B.; Kizek, R. Palladium biosensor. Electroanalysis 2007, 19, 1909–1914. [Google Scholar]
- Adam, V.; Krizkova, S.; Zitka, O.; Trnkova, L.; Petrlova, J.; Beklova, M.; Kizek, R. A determination of apo-metallothionein using adsorptive transfer stripping technique in connection with differential pulse voltammetry. Electroanalysis 2007, 19, 339–347. [Google Scholar]
- Adam, V.; Petrlova, J.; Potesil, D.; Zehnalek, J.; Sures, B.; Trnkova, L.; Jelen, F.; Kizek, R. Study of metallothionein modified electrode surface behaviour in the presence of heavy metal ions -biosensor. Electroanalysis 2005, 17, 1649–1657. [Google Scholar]
- Petrlova, J.; Krizkova, S.; Zitka, O.; Hubalek, J.; Prusa, R.; Adam, V.; Wang, J.; Beklova, M.; Sures, B.; Kizek, R. Utilizing a chronopotentiometric sensor technique for metallothionein determination in fish tissues and their host parasites. Sens. Actuator B-Chem 2007, 127, 112–119. [Google Scholar]
- Petrlova, J.; Masarik, M.; Potesil, D.; Adam, V.; Trnkova, L.; Kizek, R. Zeptomole detection of streptavidin using carbon paste electrode and square wave voltammetry. Electroanalysis 2007, 19, 1177–1182. [Google Scholar]
- Petrlova, J.; Potesil, D.; Mikelova, R.; Blastik, O.; Adam, V.; Trnkova, L.; Jelen, F.; Prusa, R.; Kukacka, J.; Kizek, R. Attomole voltammetric determination of metallothionein. Electrochim. Acta 2006, 51, 5112–5119. [Google Scholar]
- Petrlova, J.; Potesil, D.; Zehnalek, J.; Sures, B.; Adam, V.; Trnkova, L.; Kizek, R. Cisplatin electrochemical biosensor. Electrochim. Acta 2006, 51, 5169–5173. [Google Scholar]
- Potesil, D.; Petrlova, J.; Adam, V.; Vacek, J.; Klejdus, B.; Zehnalek, J.; Trnkova, L.; Havel, L.; Kizek, R. Simultaneous femtomole determination of cysteine, reduced and oxidized glutathione, and phytochelatin in maize (Zea mays L.) kernels using high-performance liquid chromatography with electrochemical detection. J. Chromatogr. A 2005, 1084, 134–144. [Google Scholar]
- Stejskal, K.; Krizkova, S.; Adam, V.; Sures, B.; Trnkova, L.; Zehnalek, J.; Hubalek, J.; Beklova, M.; Hanustiak, P.; Svobodova, Z.; Horna, A.; Kizek, R. Bio-assessing of environmental pollution via monitoring of metallothionein level using electrochemical detection. IEEE Sens. J. 2008, in press. [Google Scholar]
- Supalkova, V.; Beklova, M.; Baloun, J.; Singer, C.; Sures, B.; Adam, V.; Huska, D.; Pikula, J.; Rauscherova, L.; Havel, L.; Zehnalek, J.; Kizek, R. Affecting of aquatic vascular plant Lemna minor by cisplatin revealed by voltammetry. Bioelectrochemistry 2008. [Google Scholar] [CrossRef]
- Zitka, O.; Stejskal, K.; Kleckerova, A.; Adam, V.; Beklova, M.; Horna, A.; Havel, L.; Kizek, R. Utilizing of electrochemical techniques for detection of biological samples. Chem. Listy 2007, 101, 225–231. [Google Scholar]
- Kizek, R.; Vacek, J.; Trnkova, L.; Klejdus, B.; Havel, L. Application of catalytic reactions on a mercury electrode for electrochemical detection of metallothioneins. Chem. Listy 2004, 98, 166–173. [Google Scholar]
- Mikelova, R.; Baloun, J.; Petrlova, J.; Adam, V.; Havel, L.; Petrek, J.; Horna, A.; Kizek, R. Electrochemical determination of Ag-ions in environment waters and their action on plant embryos. Bioelectrochemistry 2007, 70, 508–518. [Google Scholar]
- Prusa, R.; Potesil, D.; Masarik, M.; Adam, V.; Kizek, R.; Jelen, F. Fast and sensitive electrochemical detection of native, denatured, and aggregated forms of tumor suppressor protein p53. Mol. Biol. Cell 2004, 15, 249A–249A. [Google Scholar]
- Laemmli, U.K. Cleavage of Structural Proteins During Assembly of Head of Bacteriophage-T4. Nature 1970, 227, 680–685. [Google Scholar]
- Diezel, W.; Hofmann, E.; Kopperschlager, G. Improved Procedure for Protein Staining in Polyacrylamide Gels with a New Type of Coomassie Brilliant Blue. Anal. Biochem 1972, 48, 617–620. [Google Scholar]
- Long, G.L.; Winefordner, J.D. Limit of Detection. Anal. Chem 1983, 55, A712–A724. [Google Scholar]
- Lavagnini, I.; Antiochia, R.; Magno, F. A calibration-base method for the evaluation of the detection limit of an electrochemical biosensor. Electroanalysis 2007, 19, 1227–1230. [Google Scholar]


© 2008 by MDPI Reproduction is permitted for noncommercial purposes.
Share and Cite
Adam, V.; Zitka, O.; Dolezal, P.; Zeman, L.; Horna, A.; Hubalek, J.; Sileny, J.; Krizkova, S.; Trnkova, L.; Kizek, R. Lactoferrin Isolation Using Monolithic Column Coupled with Spectrometric or Micro-Amperometric Detector. Sensors 2008, 8, 464-487. https://doi.org/10.3390/s8010464
Adam V, Zitka O, Dolezal P, Zeman L, Horna A, Hubalek J, Sileny J, Krizkova S, Trnkova L, Kizek R. Lactoferrin Isolation Using Monolithic Column Coupled with Spectrometric or Micro-Amperometric Detector. Sensors. 2008; 8(1):464-487. https://doi.org/10.3390/s8010464
Chicago/Turabian StyleAdam, Vojtech, Ondrej Zitka, Petr Dolezal, Ladislav Zeman, Ales Horna, Jaromir Hubalek, Jan Sileny, Sona Krizkova, Libuse Trnkova, and Rene Kizek. 2008. "Lactoferrin Isolation Using Monolithic Column Coupled with Spectrometric or Micro-Amperometric Detector" Sensors 8, no. 1: 464-487. https://doi.org/10.3390/s8010464



