Changes in Spectral Properties, Chlorophyll Content and Internal Mesophyll Structure of Senescing Populus balsamifera and Populus tremuloides Leaves
Abstract
:1. Introduction
2. Methods
2.1 Leaf spectral reflectance and chlorophyll content
2.2 Instruments used in this study
2.3 SPAD measurements and chlorophyll content analysis (samples collected during 2002)
2.4 Leaf spectral reflectance measurements (samples collected during 2002)
2.5 Histology (samples collected during 2003)
2.5.1 Sample collection
2.5.2 Leaf fixation and thin section analysis
2.6 Data analysis
2.6.1 Indices
2.6.2 Histological analysis
2.6.3 Correlation analysis
3. Results
3.1 Results
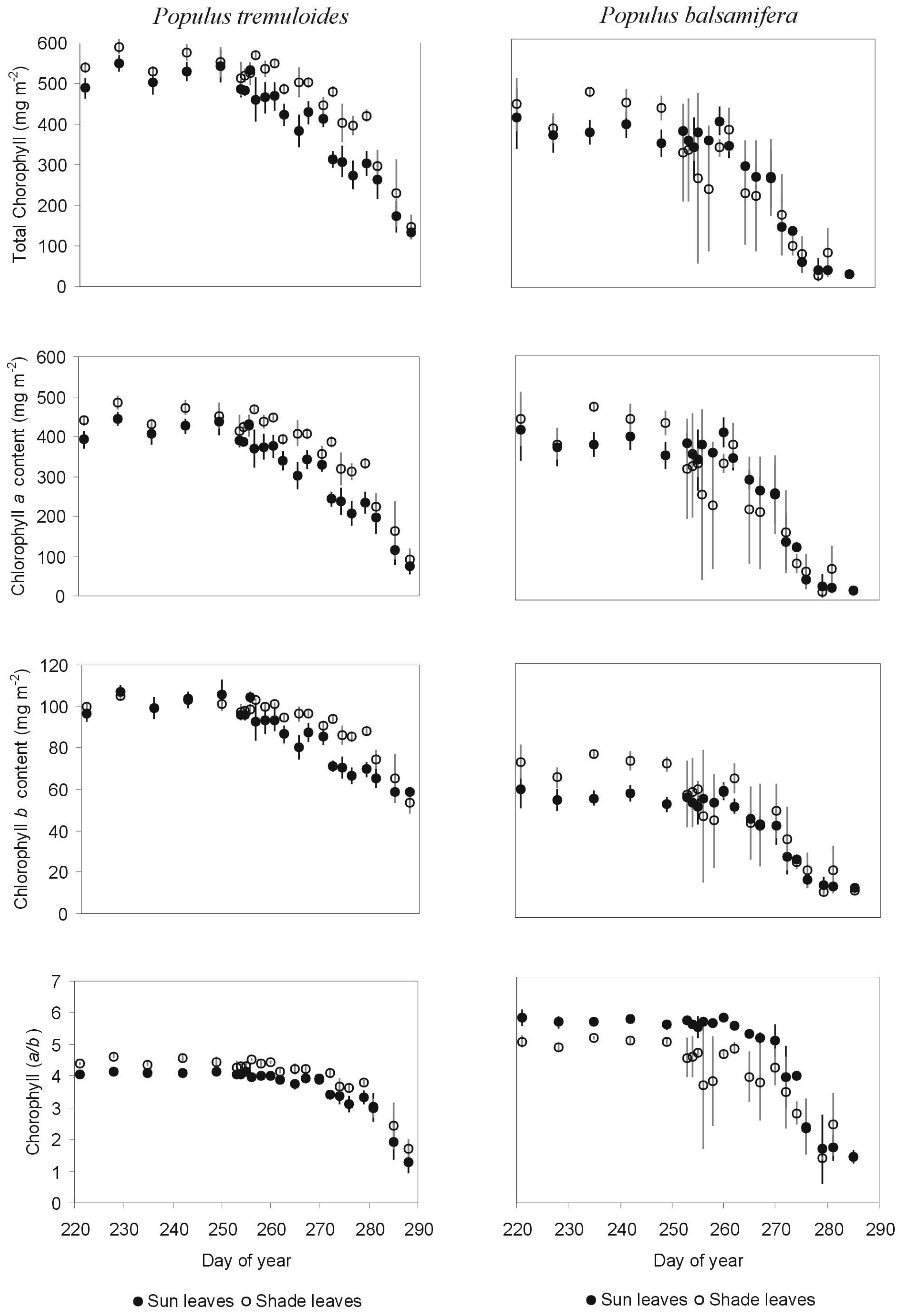
3.1.1 Indices and chlorophyll content
3.1.2 Histological analysis
- P. tremuloides leaves are thinner than P. balsamifera leaves
- P. tremuloides leaves have lower percent intercellular space in the spongy mesophyll than P. balsamifera leaves
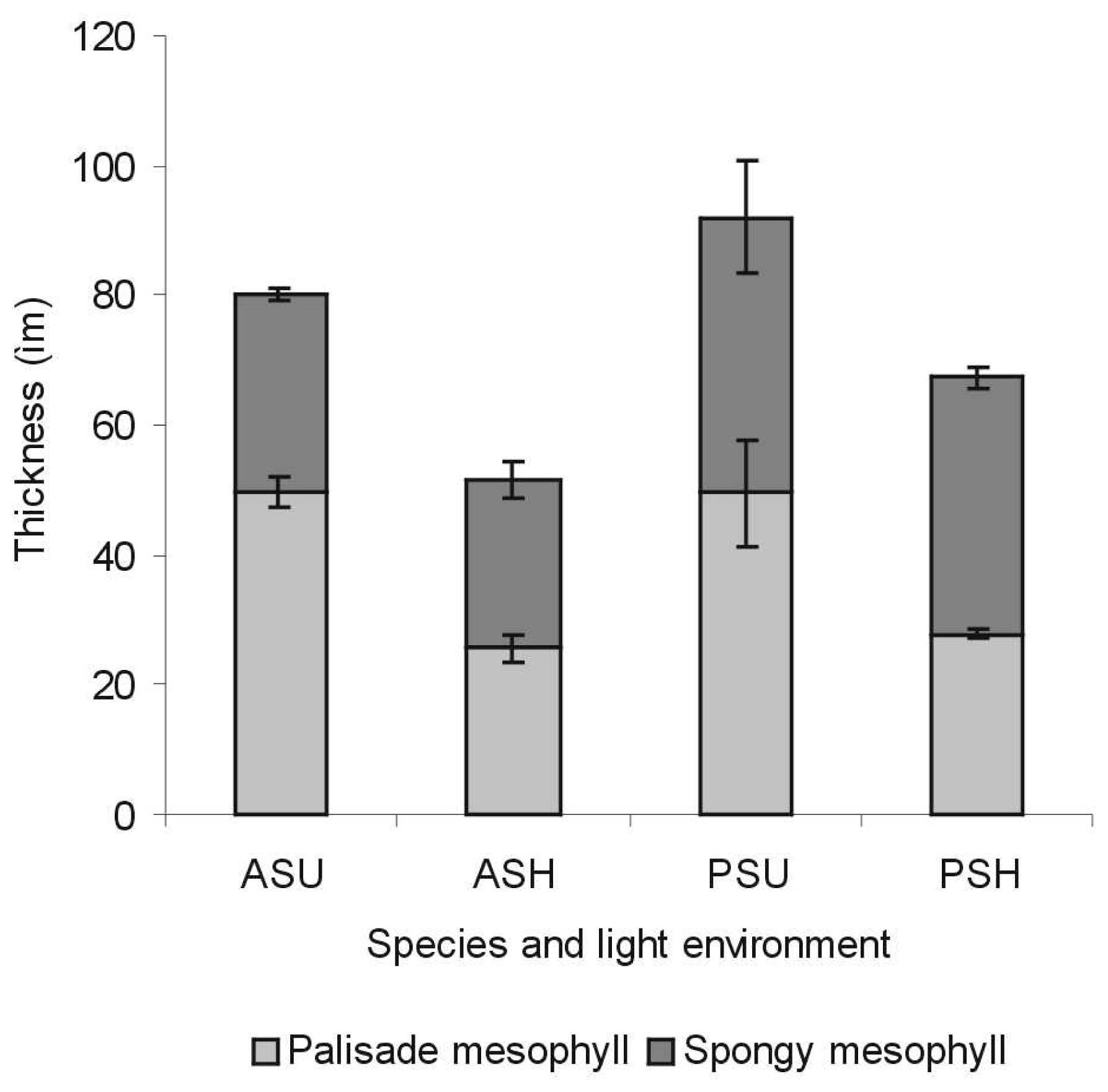
- Sun leaves are thicker than shade leaves (Figure 3)
- For sun leaves, the palisade mesophyll is thicker than the spongy mesophyll (Figure 3)
- For P. tremuloides shade leaves, the palisade mesophyll is thicker than the spongy mesophyll but for P. balsamifera shade leaves, the spongy mesophyll is thicker than the palisade mesophyll (Figure 3)
- Both sun and shade leaves of P. tremuloides and P. balsamifera had two palisade cell layers
- For mature leaves, percent intercellular space was greater in shade leaves than for sun leaves, notably for P. tremuloides
- Comparisons between mature and senescing leaves
- For sun leaves, percent intercellular space is higher in senescing leaves
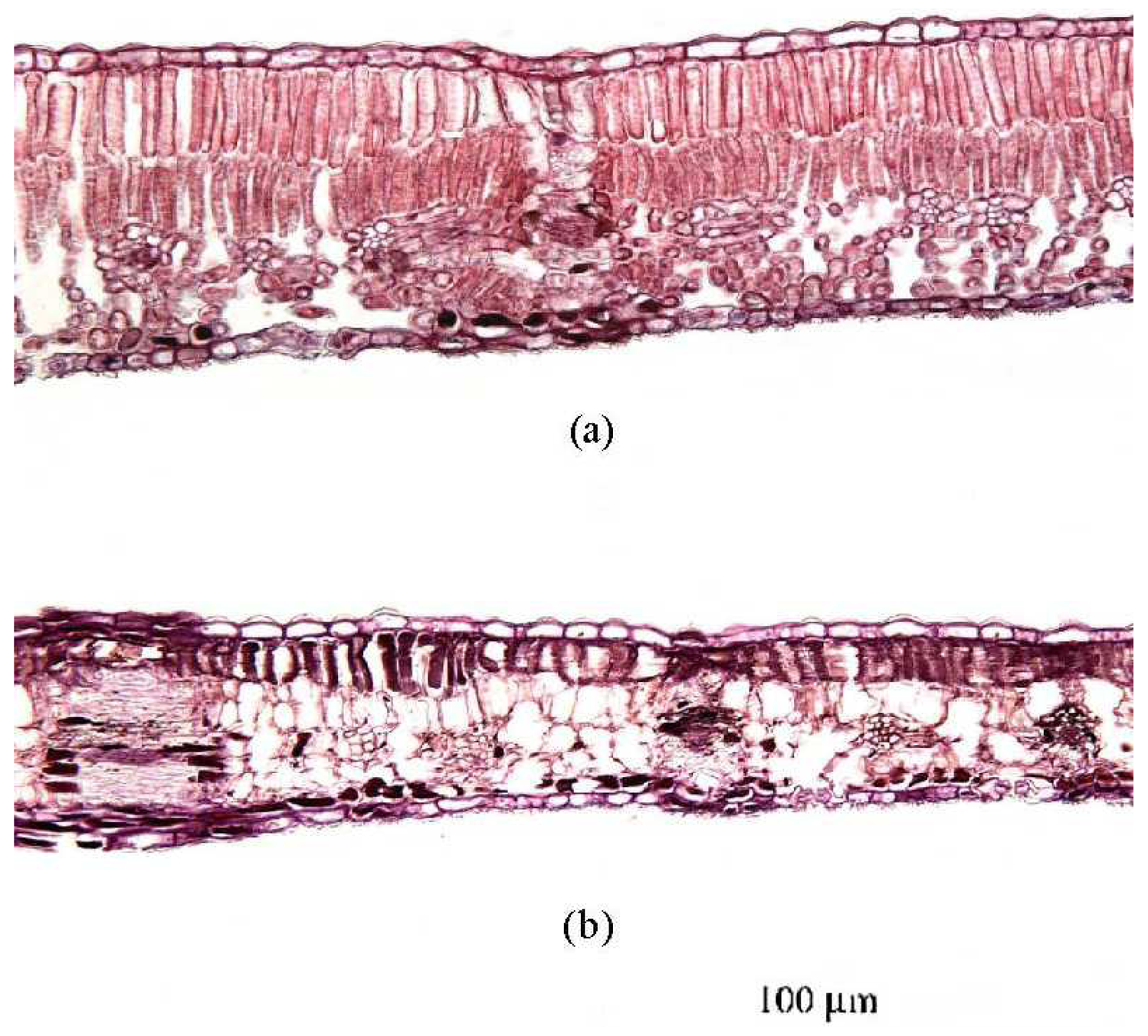
- Cell disintegration in the spongy mesophyll was evident in late stages of senescence (Figure 4).
3.1.3 Correlation analyses
4. Discussion
4.1 Comparison of species
4.2 Comparison of sun and shade leaves
4.3 Comparison of mature and senescing leaves
5. Conclusions
Acknowledgments
References
- Anderson, A.; Keskutalo, J.; Sjodin, A.; Bhalerao, R.; Sterkym, F.; Wissel, K.; Tandre, K.; Aspeborg, H.; Moyle, R.; Ohmiya, Y.; Brunner, A.; Gustafsson, P.; Karlsson, J.; Lundeberg, J.; Nilsson, O.; Sandberg, G.; Strauss, S.; Sundberg, B.; Uhlen, M.; Jansson, D.; Nilsson, P. A transcriptional timetable of autumn senescence. Genome Biol. 2005, 5(R24), 1–13. [Google Scholar]
- Matile, P.; Duggelin, T.; Rentsch, M.; Bortlik, K.; Peiker, C.; Thomas, H. How and why is chlorophyll broken down in senescent leaves. Plant Physiol. Biochem. 1989, 27, 595–604. [Google Scholar]
- Masoni, A.; Ercoli, L.; Mariotti, M.; Barberi, P. Changes in spectral properties of aging and senescing maize and sunflower leaves. Physiol. Planta. 1994, 91, 334–338. [Google Scholar]
- Collier, D. E.; Thibodeau, B. A. Changes in respiration and chemical content during autumnal senescence of Populus tremuloides and Quercus rubra leaves. Tree Physiol. 1995, 15, 759–764. [Google Scholar]
- Merzlyak, M. N.; Gitelson, A. Why and what for the leaves are yellow in autumn? On the interpretation of optical spectra of senescing leaves (Acer platanoides L.). J. Plant Physiol. 1995, 145, 315–320. [Google Scholar]
- Wolf, F. T. Changes in chlorophyll-a and chlorophyll-b in autumn leaves. American Journal of Botany 1956, 43, 714–718. [Google Scholar]
- Sanger, J. E. Quantitative investigations of leaf pigments from their inception in buds through autumn coloration to decomposition in falling leaves. Ecology 1971, 52, 1075–1089. [Google Scholar]
- Watts, D. F.; Eeley, J. H. Changes in the chlorophyll a-b ratio during autumn coloration of Populus sargentii. Bull. Torrey Botan. Club 1981, 108, 379–382. [Google Scholar]
- Peterson, E. B.; Peterson, N. M. Ecology, management, and use of aspen and balsam poplar in the prairie provinces. Special Report 1; Forestry Canada Northwest Region Northern Forestry Centre: Edmonton, AB, Canada, 1992. [Google Scholar]
- Okada, K.; Inoue, Y.; Satoh, K.; Katoh, S. Effects of light on degradation of chlorophyll and proteins during senescence of detached rice leaves. Plant Cell Physiol. 1992, 33, 1183–1191. [Google Scholar]
- Masclaux, C.; Valadier, M. H.; Brugiere, N.; Morot-Gaudry, J. F.; Hirel, B. Characterization of the sink/source transition in tobacco (Nicotiana tabacum L.) shoots in relation to nitrogen management and leaf senescence. Planta 2000, 211, 510–518. [Google Scholar]
- Hortensteiner, S.; Feller, U. Nitrogen metabolism and remobilization during senescence. J. Exp. Bot. 2002, 53, 927–937. [Google Scholar]
- Boyer, M.; Miller, J.; Belanger, M.; Hare, E.; Wu, J. Y. Senescence and spectral reflectance in leaves of Northern Pin Oak (Quercus palustris Muenchh). Remote Sens. Environ. 1998, 25, 71–87. [Google Scholar]
- Knipling, E. B. Physical and physiological basis for the reflectance of visible and near-infrared radiation from vegetation. Remote Sens. Environ. 1970, 1, 155–159. [Google Scholar]
- Kmipling, E. B. Physical and physiological basis for differences in reflectance of healthy and diseased plants. In Proc. Workshop on Infrared Color Photography and diseased plants; Florida Dept. of Agriculture: Winter Haven, Florida, 1967. [Google Scholar]
- Gausman, H. W. Plant leaf optical properties in visible and near-infrared light; Graduate Studies No. 29; Texas Tech Press: Lubbock, Texas, 1985. [Google Scholar]
- Gitelson, A.; Merzlyak, M. N. Spectral reflectance changes associated with autumn senescence of Aesculus hippocastanum L and Acer platanoides L leaves - spectral features and relation to chlorophyll estimation. J. Plant Physiol. 1994, 143, 286–292. [Google Scholar]
- Merzlyak, M. N.; Gitelson, A. A.; Chivkunova, O. B.; Rakitin, V. Y. Non-destructive optical detection of pigment changes during leaf senescence and fruit ripening. Physiol. Planta. 1999, 106, 135–141. [Google Scholar]
- StJacques, C.; Labrecque, M.; Bellefleur, P. Plasticity of leaf absorbency in some broadleaf tree seedlings. Bot. Gaz. 1991, 152, 195–202. [Google Scholar]
- Ashton, P.M. S.; Berlyn, G. P. Leaf adaptations of some Shorea species to sun and shade. New Phytol. 1992, 121, 587–596. [Google Scholar]
- Feng, Y. L.; Cao, K. F.; Zhang, J. L. Photosynthetic characteristics, dark respiration, and leaf mass per unit area in seedlings of four tropical tree species grown under three irradiances. Photosynthetica 2004, 42, 431–437. [Google Scholar]
- Gamage, H. K.; Ashton, M. S.; Singhakumara, B. M. R. Leaf structure of Syzygium spp. (Myrtaceae) in relation to site affinity within a tropical rain forest. Bot. J. Linn. Soc. 2003, 141, 365–377. [Google Scholar]
- Ashton, P. M. S.; Olander, L. P.; Berlyn, G. P.; Thadani, R.; Cameron, I. R. Changes in leaf structure in relation to crown position and tree size of Betula papyrifera within fire-origin stands of interior cedar-hemlock. Can. J. Bot.-Rev. Can. Bot. 1998, 76, 1180–1187. [Google Scholar]
- Roth, I. Stratification of tropical forests as seen in leaf structure; Dr. W. Junk Publishers: The Hague, 1984. [Google Scholar]
- Gausman, H. W. Evaluation of factors causing reflectance differences between sun and shade leaves. Remote Sens. Environ. 1984, 15, 177–181. [Google Scholar]
- Gamon, J. A; Surfus, J. S. Assessing leaf pigments content and activity with a reflectometer. New Phytol. 1999, 143, 105–117. [Google Scholar]
- Oberbauer, S. F; Strain, B. R. Effects of canopy position and irradiance on the leaf physiology and morphology of Pentaclethra macroloba (Mimosaceae). Am. J. Bot. 1986, 73, 409–416. [Google Scholar]
- Givnish, T. J. Adapatations to sun and shade: A whole-plant perspective. In Ecology of Photosynthesis in Sun and Shade; Evans, J. R., vonCaemmerer, S., Adams, W. W., III, Eds.; CSIRO: Australia, 1988; pp. 63–92. [Google Scholar]
- Lei, T. T.; Tabuchi, R.; Kitao, M.; Koike, T. Functional relationship between chlorophyll content and leaf reflectance, and light-capturing efficiency of Japanese forest species. Physiol. Planta. 1996, 96, 411–418. [Google Scholar]
- Lee, D. W.; Graham, R. Leaf optical-properties of rain-forest sun and extreme shade plants. Am. J. Bot. 1986, 73, 1100–1108. [Google Scholar]
- Knapp, A. K.; Carter, G. A. Variability in leaf optical properties among 26 species from a broad range of habitats. Am. J. Bot. 1998, 85, 940–946. [Google Scholar]
- Demarez, V.; Gastellu-Etchegorry, J. P.; Mougin, E.; Marty, G.; Proisy, C.; Dufrene, E.; Le Dantec, V. Seasonal variation of leaf chlorophyll content of a temperate forest. Inversion of the PROSPECT model. Int. J. Remote Sens. 1999, 20, 879–894. [Google Scholar]
- Dean, M. A.; Letner, C. A.; Eley, J. H. Effect of autumn foliar senescence on chlorophyll a-b ratio and respiratory enzymes of Populus tremuloides. Bull. Torrey Bot. Club 1993, 120, 269–274. [Google Scholar]
- Rauscher, H. M.; Perala, D. A.; Worth, C. V. The ecology and management of aspen. AI Appl. 1995, 9, 59–59. [Google Scholar]
- Farrar, J. L. Trees in Canada; Fitzhenry & Whiteside Ltd. and the Canadian Forest Service Natural Resources Canada: Ottawa, Canada, 1995. [Google Scholar]
- Amlin, N. M.; Rod, S. B. Drought stress and recovery of riparian cottonwoods due to water table alteration along Willow Creek, Alberta. Trees-Struct. Funct. 2003, 17, 351–358. [Google Scholar]
- Mitton, J. B.; Grant, M. C. Observations on the ecology and evolution of quaking aspen, Populus tremuloides, in the Colorado Front Range. Am. J. Bot. 1980, 67, 202–209. [Google Scholar]
- Peterson, E. B.; Peterson, N. M. Ecology, management, and use of aspen and balsam poplar in the prairie provinces. Special Report 1. Forestry Canada Northwest Region Northern Forestry Centre 1992, 252. [Google Scholar]
- Garcia-Plazaola, J. I.; Hernandez, A.; Becerril, J. M. Antioxidant and pigment composition during autumnal leaf senescence in woody deciduous species differing in their ecological traits. Plant Biol. 2003, 5, 557–566. [Google Scholar]
- Slaton, M. R.; Hunt, E. R.; Smith, W. K. Estimating near-infrared leaf reflectance from leaf structural characteristics. Am. J. Bot. 2001, 88, 278–284. [Google Scholar]
- Castro-Esau, K. L.; Sanchez-Azofeifa, G. A.; Rivard, B.; Wright, S. J.; Quesada, M. Variability in leaf optical properties of Mesoamerican trees and the potential for species classification. Am. J. Bot. 2006, 93, 517–530. [Google Scholar]
- Sims, D. A.; Gamon, J. A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar]
- Madeira, A. C.; Mendonca, A.; Ferreira, M. E.; Torborda, M. D. Relationship between spectroradiometric and chlorophyll measurements in green beans. Commun. Soil Sci. Plant Anal. 2000, 31, 631–643. [Google Scholar]
- Hiscox, J. D.; Israelstam, G. F. Method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot.-Rev. Can. Bot. 1979, 57, 1332–1334. [Google Scholar]
- Pandey, S.; Ranade, S. A.; Nagar, P. K.; Kumar, N. Role of polynamines and ethylene as modulators of plant senescene. J. Biosci. 2000, 25, 291–299. [Google Scholar]

| Variable | Mature (Total Chl>400mgm-2) | Senescing (Total Chl<250mgm-2) | ||
|---|---|---|---|---|
| ASU (n=4) | ASH (n=8) | ASU (n=3) | ASH | |
| Total thickness (μm) | 88.43(1.42) | 68.09(7.09) | 84.73(16.52) | NA |
| Thickness palisade (μm) | 49.35(2.61) | 31.02(4.76) | 48.13(12.24) | NA |
| Thickness spongy (μm) | 29.90(0.58) | 27.19(3.47) | 26.88(4.88) | NA |
| Intercellular space (%) | 28.35(4.00) | 37.06(5.94) | 41.28(10.97) | NA |
| R800.7 | 56.07(6.87) | 54.46(4.06) | 52.63(3.27) | NA |
| PSU (n=2) | PSH (n=4) | PSU (n=5) | PSH (n=3) | |
| Total thickness (μm) | 100.70(21.07) | 80.24(8.05) | 109.24(18.62) | 77.83(13.61) |
| Thickness palisade (μm) | 49.50(9.90) | 28.47(0.66) | 44.12(8.59) | 35.27(13.74) |
| Thickness spongy (μm) | 42.60(10.39) | 42.25(7.89) | 55.12(10.43) | 39.50(11.36) |
| Intercellular space (%) | 46.62(6.55) | 50.66(5.46) | 51.08(9.85) | 48.22(12.78) |
| R800.7 | 58.71(0.10) | 55.59(3.65) | 56.41(2.43) | 55.03(1.55) |
| Leaf type | Index | Chl a | Chl b | Chl a/b | Total Chl |
|---|---|---|---|---|---|
| ASU | mSR705 | 0.919** | 0.961** | 0.711* | 0.930** |
| mND705 | 0.925** | 0.910** | 0.790** | 0.929** | |
| ASH | mSR705 | 0.985** | 0.982** | 0.971** | 0.985** |
| mND705 | 0.980** | 0.981** | 0.981** | 0.980** | |
| PSU | mSR705 | 0.992** | 0.990** | 0.911** | 0.992** |
| mND705 | 0.982** | 0.985** | 0.962** | 0.982** | |
| PSH | mSR705 | 0.974** | 0.960** | 0.859** | 0.972** |
| mND705 | 0.982** | 0.977** | 0.908** | 0.982** |
© 2008 by MDPI Reproduction is permitted for noncommercial purposes.
Share and Cite
Castro, K.L.; Sanchez-Azofeifa, G.A. Changes in Spectral Properties, Chlorophyll Content and Internal Mesophyll Structure of Senescing Populus balsamifera and Populus tremuloides Leaves. Sensors 2008, 8, 51-69. https://doi.org/10.3390/s8010051
Castro KL, Sanchez-Azofeifa GA. Changes in Spectral Properties, Chlorophyll Content and Internal Mesophyll Structure of Senescing Populus balsamifera and Populus tremuloides Leaves. Sensors. 2008; 8(1):51-69. https://doi.org/10.3390/s8010051
Chicago/Turabian StyleCastro, Karen L., and G. Arturo Sanchez-Azofeifa. 2008. "Changes in Spectral Properties, Chlorophyll Content and Internal Mesophyll Structure of Senescing Populus balsamifera and Populus tremuloides Leaves" Sensors 8, no. 1: 51-69. https://doi.org/10.3390/s8010051






