Fabrication and Optimization of a Nanoporous Platinum Electrode and a Non-enzymatic Glucose Micro-sensor on Silicon
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Chemicals
2.2. Design and Fabrication
3. Experimental Results and Discussions
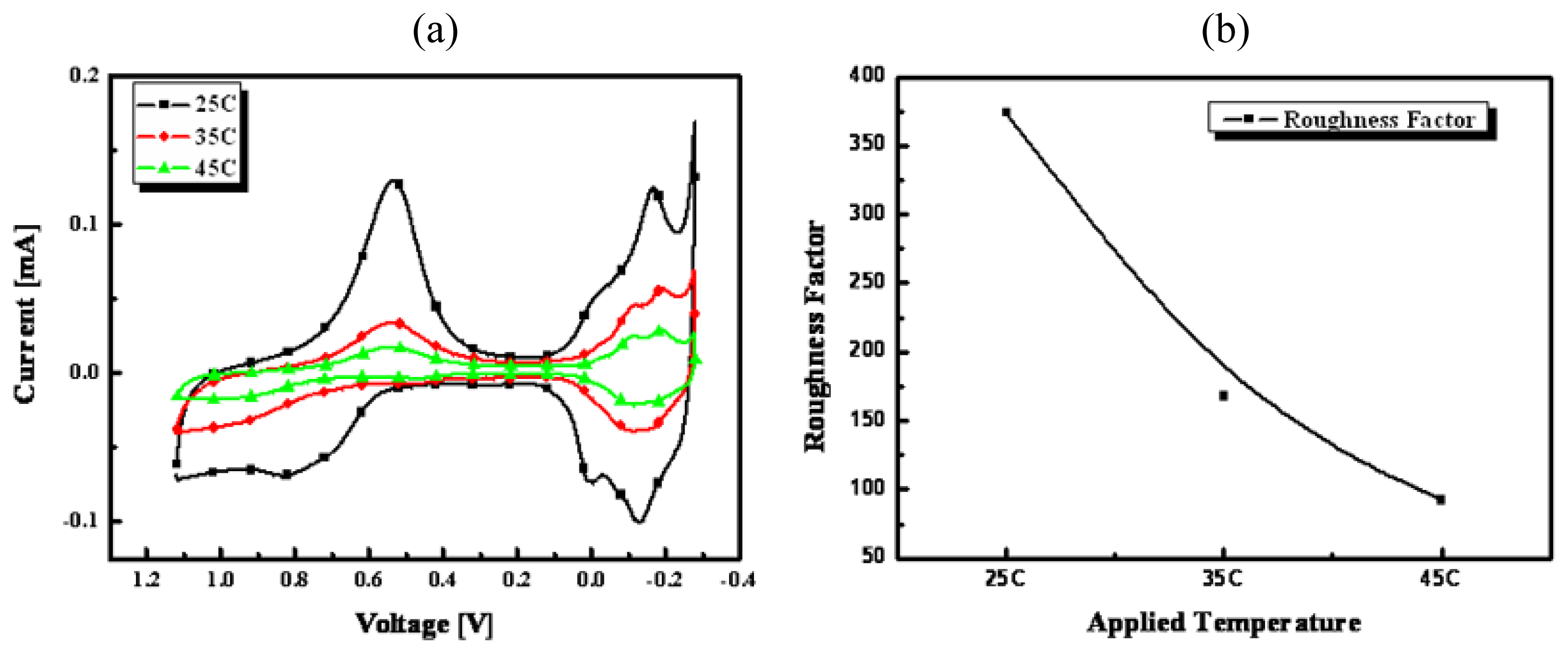
3.1. AFM analysis
3.2. Electrochemical analysis
3.2.1 Optimization of nanoporous Pt electrode
3.2.2 Non-enzymatic glucose micro-sensor
4. Conclusions
Acknowledgments
References and Notes
- Woderer, S.; Henninger, N.; Garthe, C.D.; Kloetzer, H.M.; Hajnsek, M.; Kamecke, U.; Gretz, N. Continuous glucose monitoring in interstitial fluid using glucose oxidase-based sensor compared to established blood glucose measurement in rats. Anal. Chim. Acta 2007, 581, 7–12. [Google Scholar]
- Kang, X.H.; Mai, Z.B.; Zou, X.Y.; Cai, P.X.; Mo, J.Y. A novel sensitive non-enzymatic glucose sensor. Chin. Chemi. Lett. 2007, 18, 189–191. [Google Scholar]
- Guan, W.J.; Li, Y.; Chen, Y.Q.; Zhang, X.B.; Hu, G.Q. Glucose biosensor based on multi-wall carbon nanotubes and screen printed carbon electrodes. Biosens. Bioelectron. 2005, 21, 508–512. [Google Scholar]
- Deng, C.; Li, M.; Xie, Z.; Liu, M.; Tan, Y.; Xu, X.; Yao, S. New glucose biosensor based on a poly(o-phenylendiamine)/glucose oxidase-glutaraldehyde/Prussian blue/Au electrode with QCM monitoring of various electrode-surface modifications. Anal. Chem. 2006, 557, 85–94. [Google Scholar]
- Beden, B.; Largeaud, F.; Kokoh, K.B.; Lamy, C. Fourier transform infrared reflectance spectroscopic investigation of the electrocatalytic oxidation of D-glucose: identification of reactive intermediates and reaction products. Electrochim. Acta 1996, 41, 701–709. [Google Scholar]
- Wei, X.L.; Shen, P.K. Anodic oxidation of glucose on platinum in alkaline media. Chin. J. Chem. Phys. 2003, 16, 395–400. [Google Scholar]
- Matsumoto, F.; Harada, M.; Koura, N.; Uesugi, S. Electrochemical oxidation of glucose at Hg adatom-modified Au electrode in alkaline aqueous solution. Electrochem. Commun. 2003, 5, 42–46. [Google Scholar]
- Lee, S.H.; Fang, H.Y.; Chen, W.C. Amperometric glucose biosensor based on screen-printed carbon electrodes mediated with hexacyanoferrate-chitosan oligomers mixture. Sens. Actu. B 2006, 117, 236–243. [Google Scholar]
- Chen, S.H.; Yuan, R.; Chai, Y.Q.; Zhang, L.G.; Wang, N.; Li, X.L. Amperometric third-generation hydrogen peroxide biosensor based on the immobilization of hemoglobin on multiwall carbon nanotubes and gold colloidal nanoparticles. Biosen. Bioelectron. 2007, 22, 1268–1274. [Google Scholar]
- Wu, S.U.; Zhao, H.T.; Ju, H.X.; Shi, C.G.; Zhao, J.W. Electrodeposition of silver-DNA hybrid nanoparticles for electrochemical sensing of hydrogen peroxide and glucose. Electrochem. Commun. 2006, 8, 1197–1203. [Google Scholar]
- Choi, S.J.; Choi, B.G.; Park, S.M. Electrochemical sensor for electrochemically lnactive β-D(+)-glucose using α-cyclodextrin template molecules. Anal. Chem. 2002, 74, 1998–2002. [Google Scholar]
- Hsiao, M.W.; Adzic, R.R.; Yeager, E.B. Electrochemical oxidation of glucose on single crystal and polycrystalline gold surfaces in phosphate buffer. J. Electrochem. Soc. 1996, 143, 759–767. [Google Scholar]
- Rong, L.Q.; Yang, C.; Qian, Q.Y.; Xia, X.H. Study of the nonenzymatic glucose sensor based on highly dispersed Pt nanoparticles supported on carbon nanotubes. Talanta 2007, 72, 819–824. [Google Scholar]
- Yuan, J.; Wang, K.; Xia, X. Highly ordered platinum-nanotubule arrays for amperometric glucose sensing. Adv. Func. Mater. 2005, 15, 803–809. [Google Scholar]
- Wang, K.; Zhang, D.; Zhou, T.; Xia, X.H. A dual-electrode approach for highly selective detection of glucose based on diffusion layer theory: experiments and simulation. Chem. Eur. J. 2005, 11, 1341–1347. [Google Scholar]
- Attard, G.S.; Bartlett, P.N.; Coleman, N.R.B.; Elliott, J.M.; Owen, J.R.; Wang, J.H. Mesoporous platinum films from lyotropic liquid crystalline phases. Science 1997, 278, 838–840. [Google Scholar]
- Seo, H.K.; Park, D.J.; Park, J.Y. Fabrication and characterization of nano-hole-arrayed Pt electrode on silicon CMOS integrated non-disposable biosensor applications. J. Kor. Phy. Soc. 2006, 49, S812–S815. [Google Scholar]
- Seo, H.K.; Park, D.J.; Park, J.Y. Nanofabrication of enzymeless micro-biosensors with mesoporous platinum and platinum black working electrodes. J. Kor. Phy. Soc. 2007, 51, S284–S288. [Google Scholar]
- Seo, H.K.; Park, D.J.; Park, J.Y. Comparion of micro- and nano-pore platinum working electrodes for CMOS Integrated nondisposable biosensor applications. IEEE SENSORS J. 2007, 7, 945–946. [Google Scholar]
- Biegler, T.; Rand, D.A.J.; Woods, R. Limiting oxygen coverage on platinized platinum; relevance to determination of real platinum area by hydrogen adsorption. J. Electroanal. Chem. 1971, 29, 269–277. [Google Scholar]
- Park, S.J.; Boo, H.K.; Chung, T.D. Electrochemical non-enzymatic glucose sensors. Anal. Chim. Acta 2006, 556, 46–57. [Google Scholar]
- Cui, H.F.; Ye, J.S.; Zhang, W.D.; Li, C.M.; Luong, H.T.; Sheu, F.S. Selective and sensitive electrochemical detection of glucose in neutral solution using platinum-lead alloy nanoparticle/carbon nanotube nanocomposites. Anal. Chim. Acta 2007, 594, 175–183. [Google Scholar]
- Li, Y.; Song, Y.Y.; Yang, C.; Xia, X.H. Hydrogen bubble dynamic template synthesis of porous gold for nonenzymatic electrochemical detection of glucose. Electrochem. Commun. 2007, 9, 981–988. [Google Scholar]
- Wang, G.; Thai, N.M.; Yau, S.T. Preserved enzymatic activity of glucose oxidase immobilized on unmodified electrodes for glucose detection. Biosen. Bioelectron. 2007, 22, 2158–2164. [Google Scholar]
- Zhao, H.; Ju, H. Multilayer membranes for glucose biosensing via layer-by-layer assembly of multiwall carbon nanotubes and glucose oxidase. Anal. Biochem. 2006, 350, 138–144. [Google Scholar]









© 2008 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lee, Y.-J.; Park, D.-J.; Park, J.-Y.; Kim, Y. Fabrication and Optimization of a Nanoporous Platinum Electrode and a Non-enzymatic Glucose Micro-sensor on Silicon. Sensors 2008, 8, 6154-6164. https://doi.org/10.3390/s8106154
Lee Y-J, Park D-J, Park J-Y, Kim Y. Fabrication and Optimization of a Nanoporous Platinum Electrode and a Non-enzymatic Glucose Micro-sensor on Silicon. Sensors. 2008; 8(10):6154-6164. https://doi.org/10.3390/s8106154
Chicago/Turabian StyleLee, Yi-Jae, Dae-Joon Park, Jae-Yeong Park, and Younghun Kim. 2008. "Fabrication and Optimization of a Nanoporous Platinum Electrode and a Non-enzymatic Glucose Micro-sensor on Silicon" Sensors 8, no. 10: 6154-6164. https://doi.org/10.3390/s8106154




