A Nonoxidative Electrochemical Sensor Based on a Self-Doped Polyaniline/Carbon Nanotube Composite for Sensitive and Selective Detection of the Neurotransmitter Dopamine: A Review
Abstract
:1. Introduction
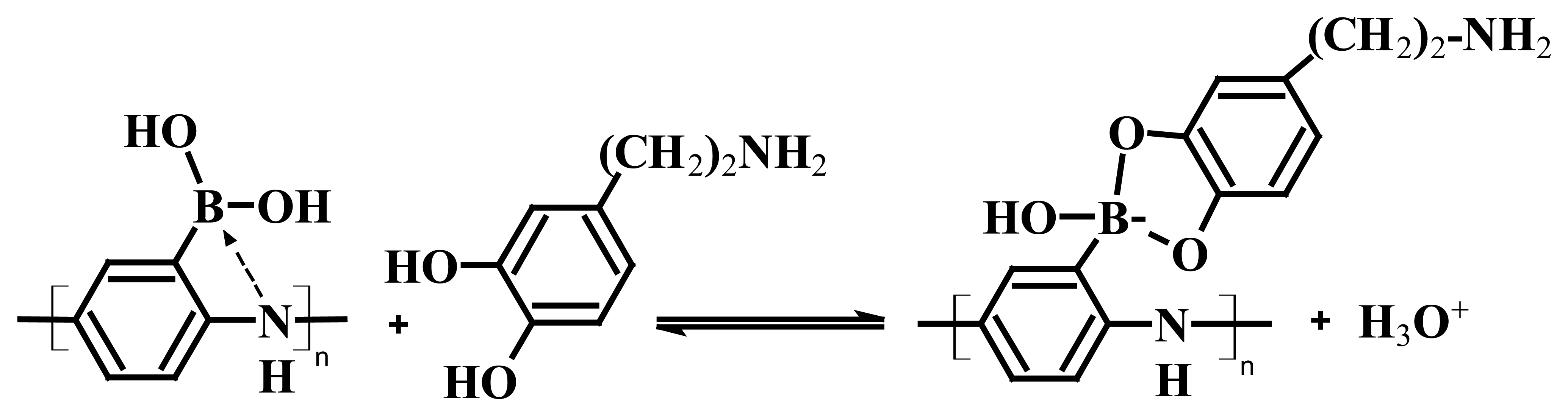
2. Development of a nonoxidative dopamine sensor based on a nanocomposite of carbon nanotube/poly(anilineboronic acid)
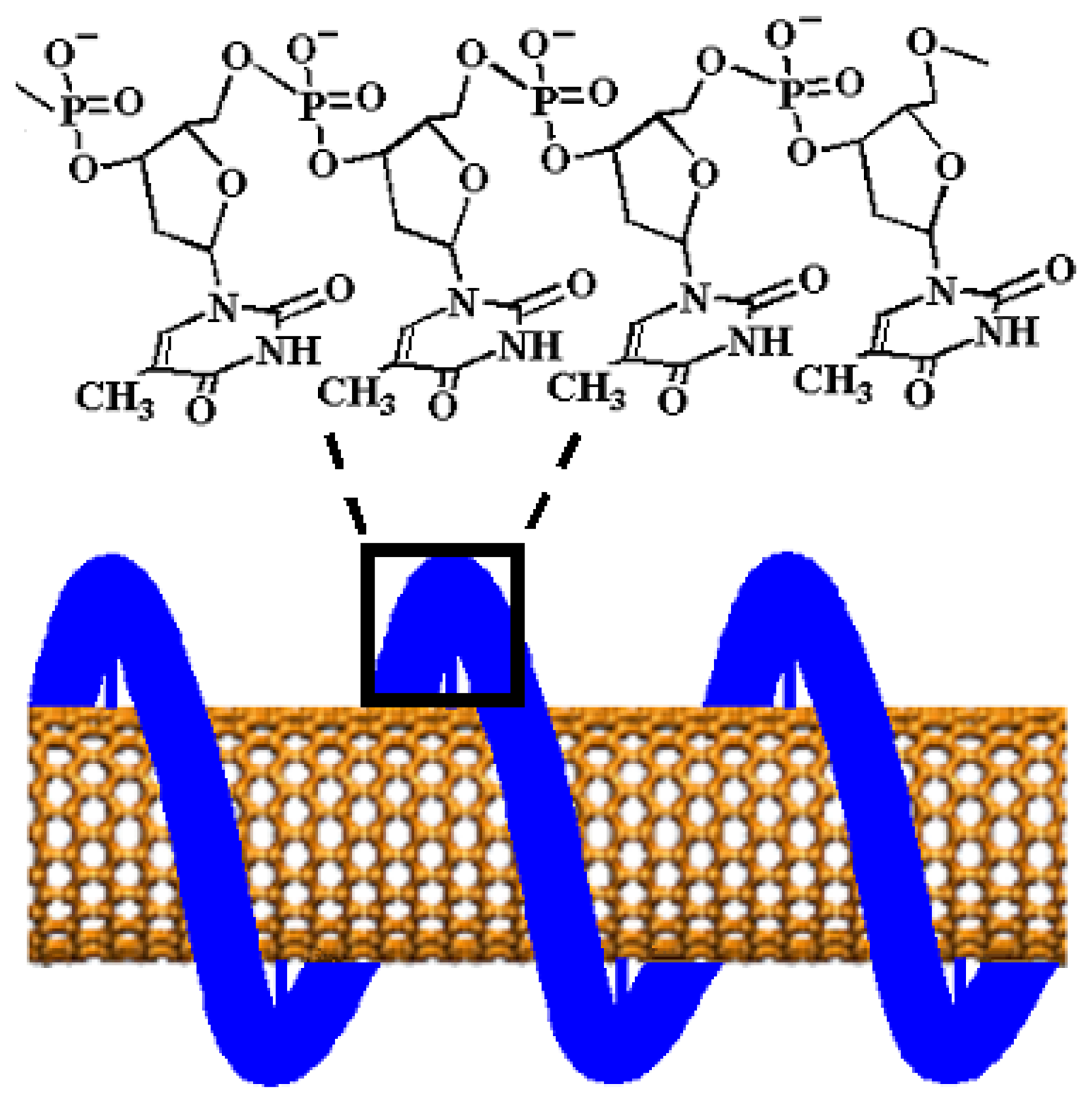
2.1. Carbon nanotube/poly(anilineboronic acid) composites, multiple roles of single walled carbon nanotubes dispersed and functionalized by single stranded DNA
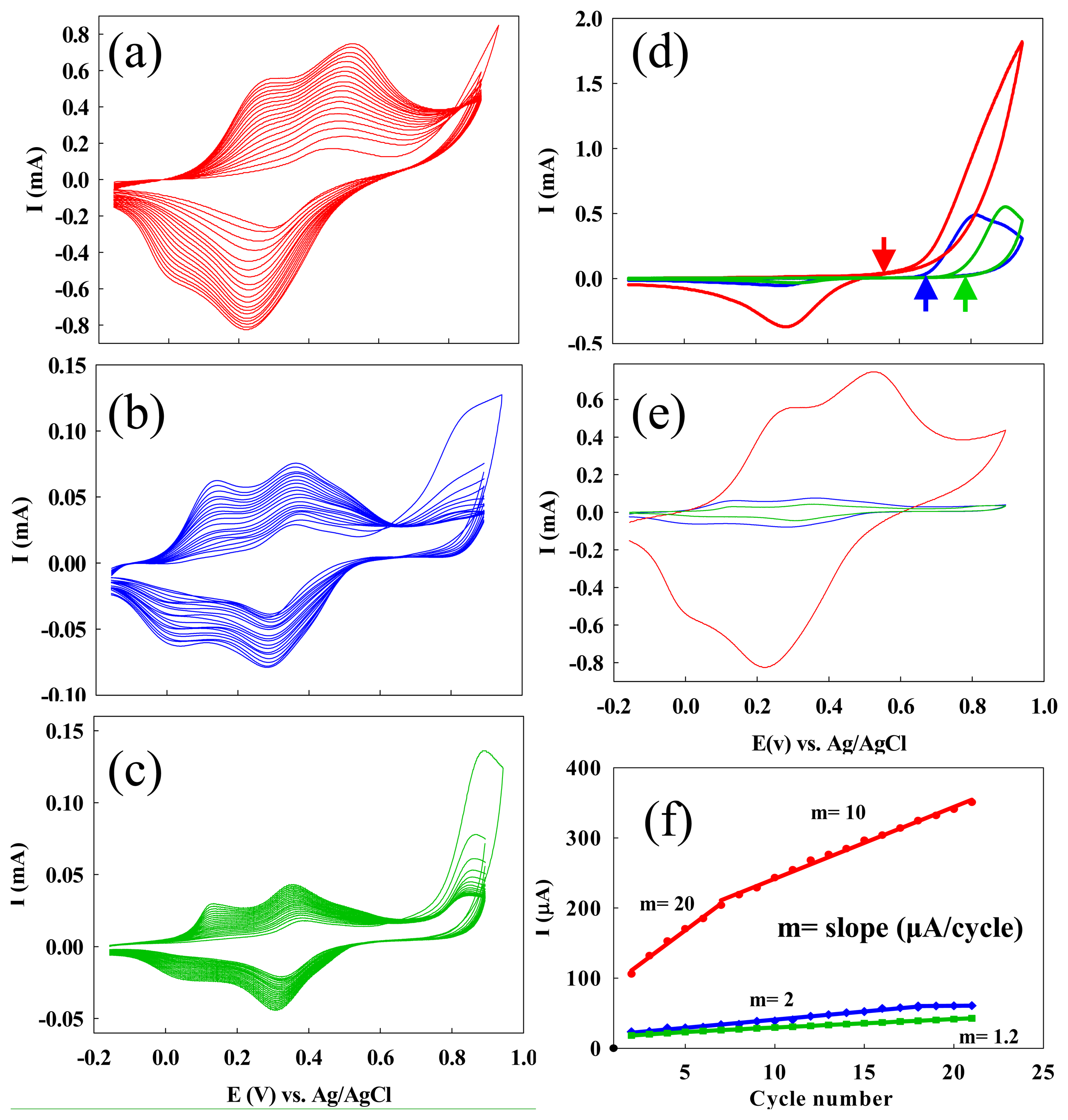
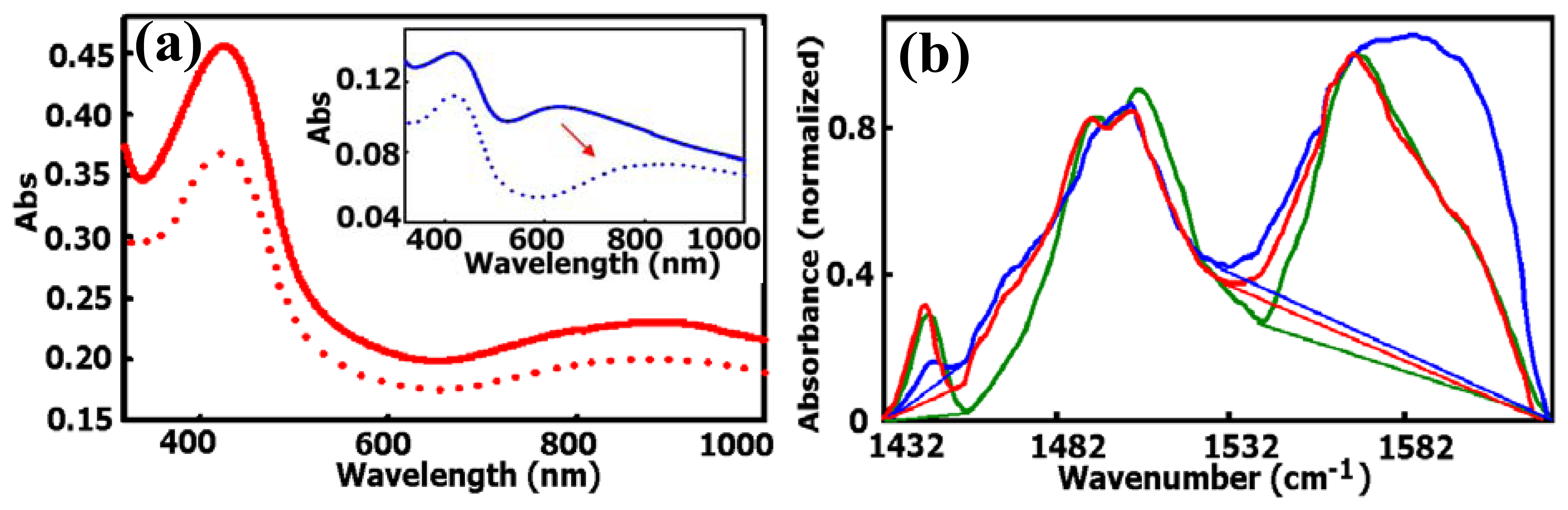
2.1.1. Increased electrochemical polymerization speed: ss-DNA-SWNTs acted as catalytic molecular templates
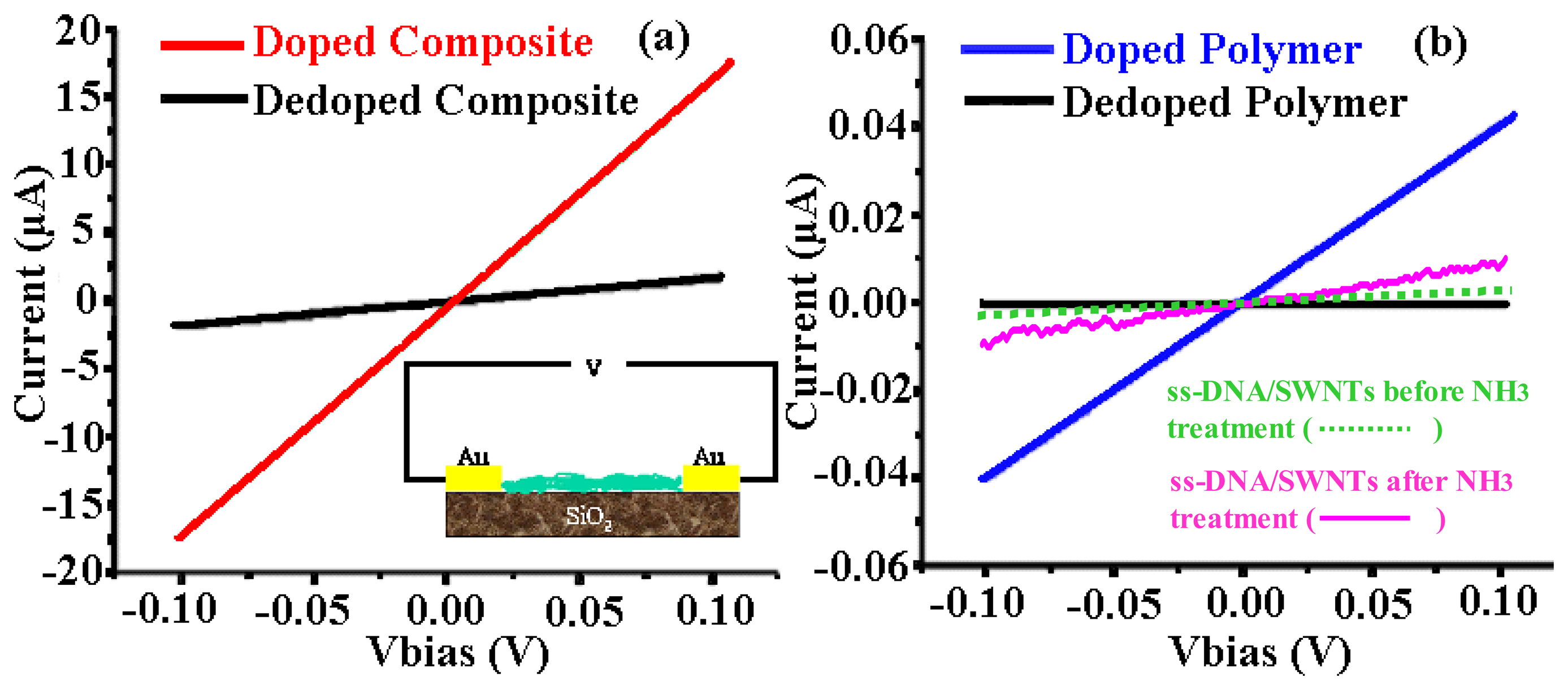
2.1.2. Improved conductivity: ss-DNA-SWNTs acted as a unique conductive doping agent
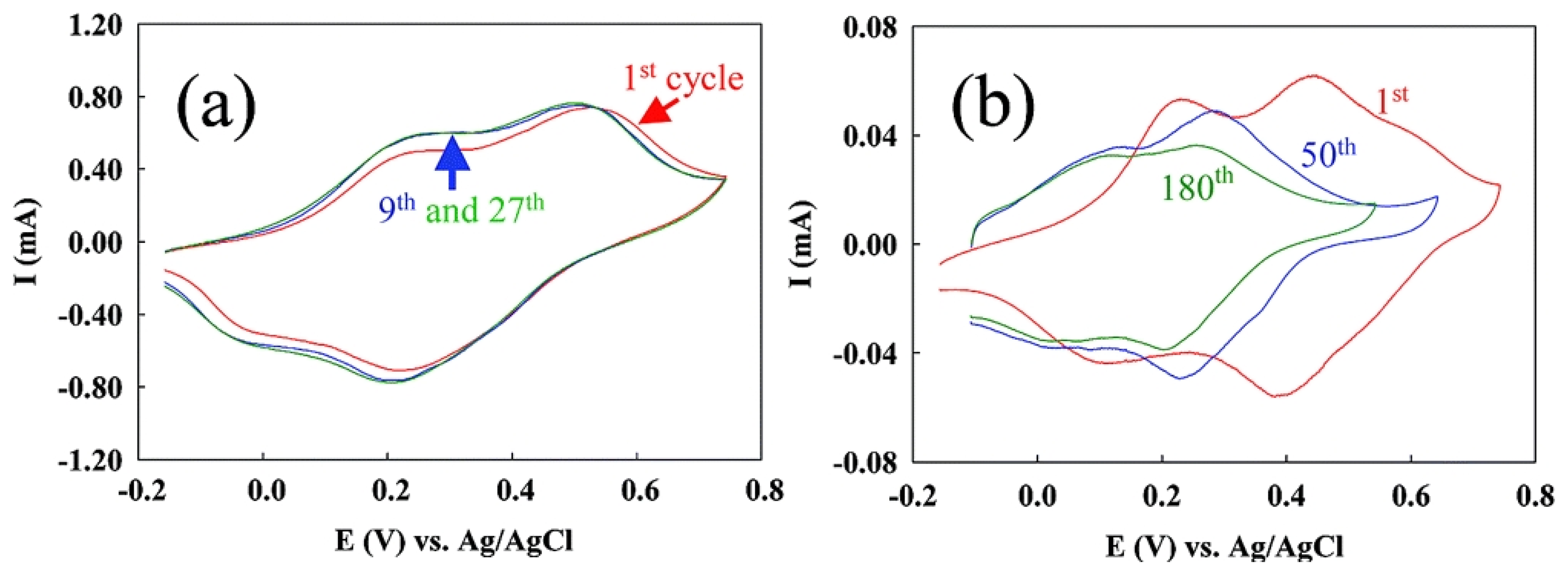
2.1.3. Enhanced Stability: ss-DNA/SWNTs acted as active stabilizer
2.2. Non oxidative electrochemical detection of dopamine with enhanced sensitivity: ss-DNA-SWNTs increased effective electrode surface area
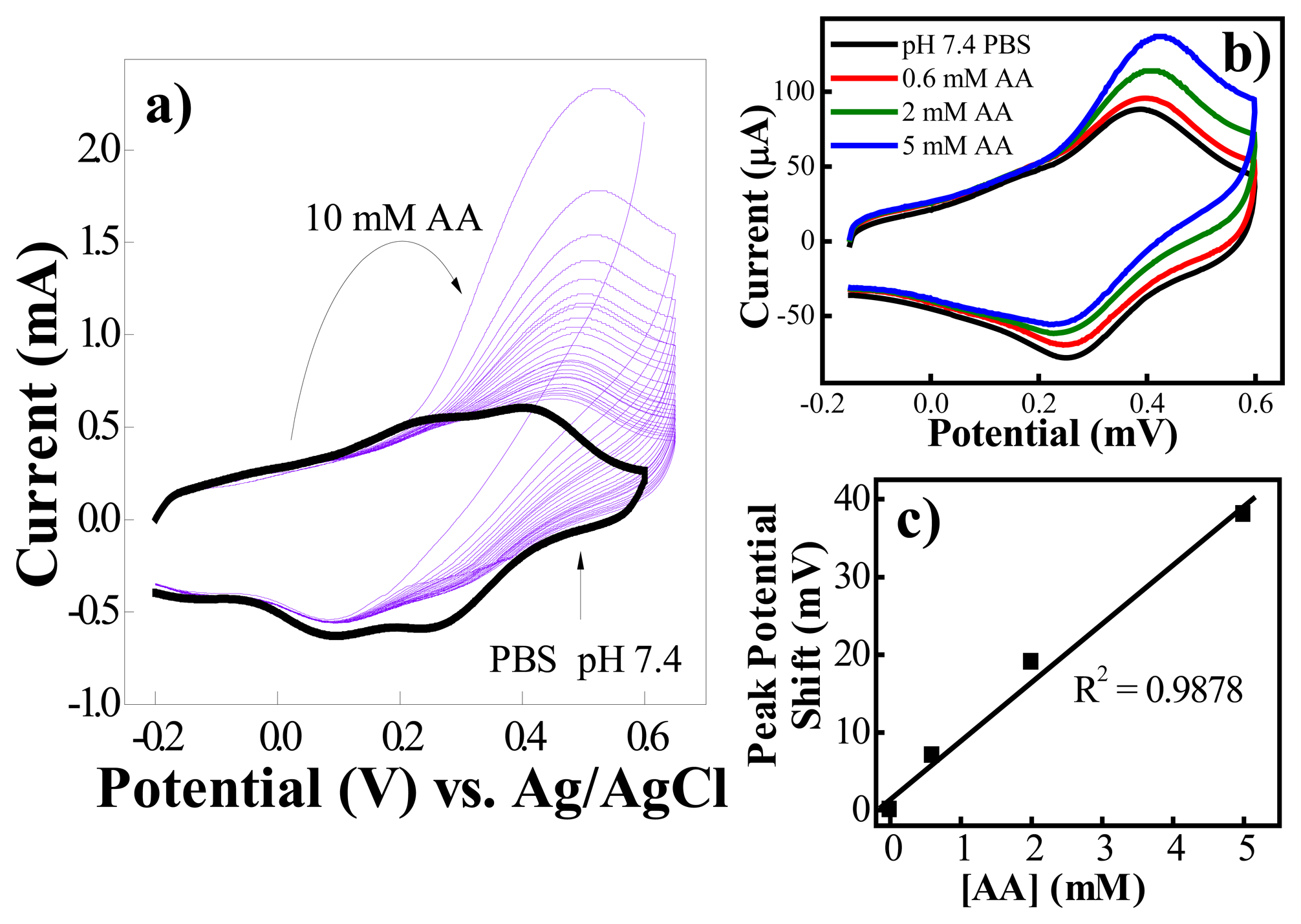
2.3. Interference by Ascorbic Acid
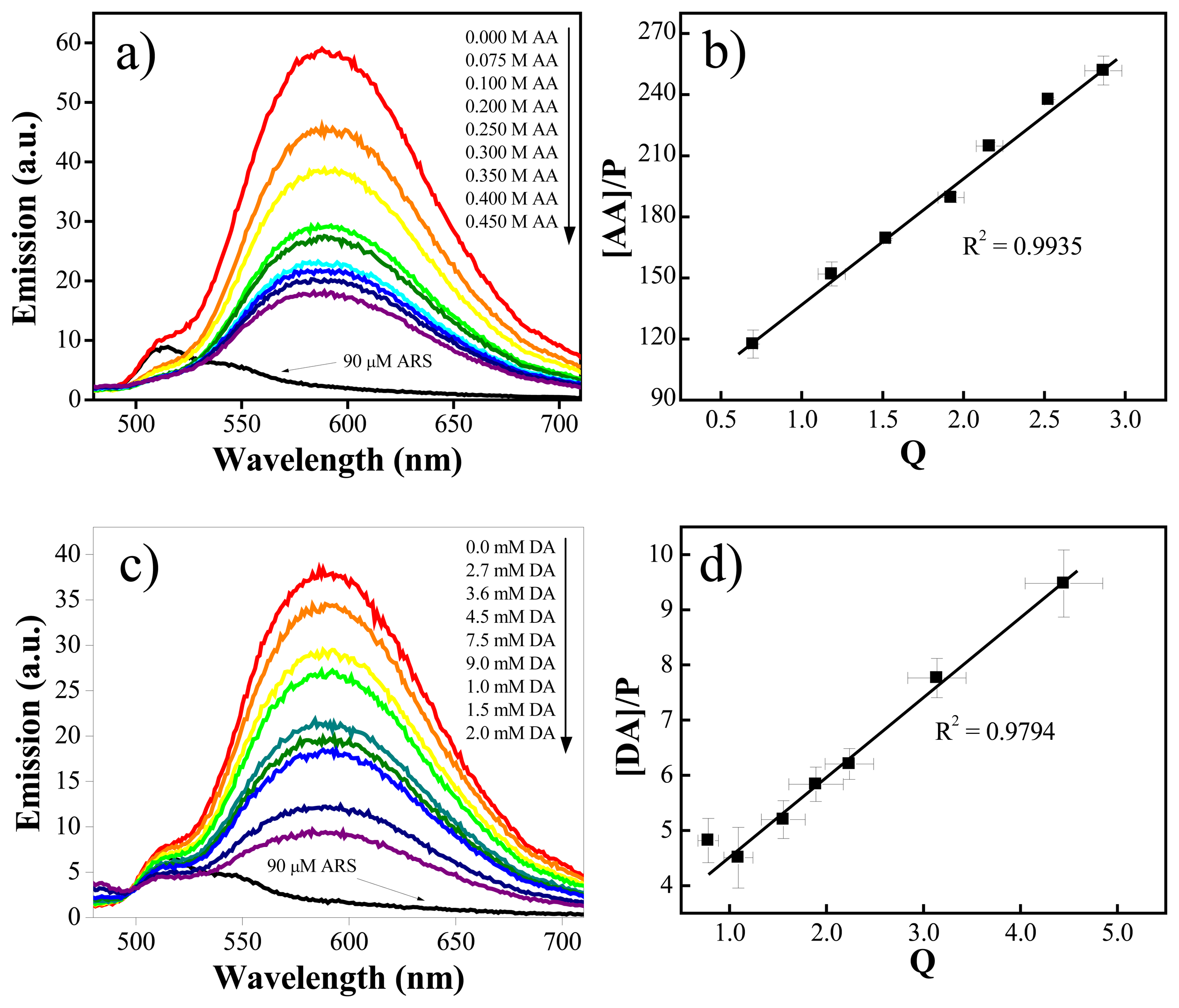
2.4. Further study of the interference of ascorbic acid: a fluorescence binding assay
2.5. Elimination of the Ascorbic Acid Interference with Nafion
3. Conclusions
Acknowledgments
References and Notes
- Robinson, D.L.; Hermans, A.; Seipel, A.T.; Wightman, R.M. Monitoring Rapid Chemical Communication in the Brain. Chem. Rev. 2008, 108, 2554–2584. [Google Scholar]
- Venton, B.J.; Wightman, R.M. Psychoanalytical Electrochemistry: Dopamine and Behavior. Anal. Chem. 2003, 75, 414A–421A. [Google Scholar]
- Wightman, R.M.; May, L.J.; Michael, A.C. Detection of Dopamine Dynamics in the Brain. Anal. Chem. 1988, 60, 769A–779A. [Google Scholar]
- Troyer, K.P.; Heien, M.L.A.V.; Venton, B.J.; Wightman, R.M. Neurochemistry and Electroanalytical Probes. Curr. Opin. Chem. Biol. 2002, 6, 696–703. [Google Scholar]
- Heien, M.L.A.V.; Khan, A.S.; Ariansen, J.L.; Cheer, J.F.; Phillips, P.E.M.; Wassum, K.M.; Wightman, R.M. Real-time Measurement of Dopamine Fluctuations After Cocaine in the Brain of Behaving Rats. Proc. Natl Acad. Sci. USA 2005, 102, 10023–10028. [Google Scholar]
- Phillips, P.E.M.; Stuber, G.D.; Heien, M.L.A.V.; Wightman, R.M.; Carelli, R.M. Subsecond Dopamine Release Promotes Cocaine Seeking. Nature 2003, 422, 614–617. [Google Scholar]
- Cui, H.F.; Ye, J.S.; Chen, Y.; Chong, S.C.; Sheu, F.S. Microelectrode Array Biochip: Tool for in vitro Drug Screening Based on the Detection of a Drug Effect on Dopamine Release from PC12 Cells. Anal. Chem. 2006, 78, 6347–6355. [Google Scholar]
- Heien, M.L.A.V.; Phillips, P.E.M.; Stuber, G.D.; Seipel, A.T.; Wightman, R.M. Overoxidation of Carbon-fiber Microelectrodes Enhances Dopamine Adsorption and Increases Sensitivity. Analyst 2003, 128, 1413–1419. [Google Scholar]
- Yoo, J. -S.; Park, S.-M. Programmed Potential Sweep Voltammetry for Lower Detection Limits. Anal. Chem. 2005, 77, 3694–3699. [Google Scholar]
- Adams, R.N. Probing Brain Chemistry with Electroanalytical Techniques. Anal. Chem. 1976, 48, 1126A–1138A. [Google Scholar]
- Venton, B.J.; Troyer, K.P.; Wightman, R.M. Response Times of Carbon Fibre Microelectrodes to Dynamic Changes in Catecholamine Concentration. Anal. Chem. 2002, 74, 539–546. [Google Scholar]
- Troyer, K.P.; Heien, M.L.A.V.; Venton, B.J.; Wightman, R.M. Neurochemistry and Electroanalytical Probes. Curr. Opin. Chem. Biol. 2002, 6, 696–703. [Google Scholar]
- Justice, J.B. Quantitative Microdialysis of Neurotransmitters. J. Neurosci. Meth. 1993, 48, 263–276. [Google Scholar]
- O'Neill, R.D. Microvoltammetric Techniques and Sensors for Monitoring Neurochemical Dynamics in vivo: A Review. Analyst 1994, 119, 767–779. [Google Scholar]
- Cooper, J.M.; Foreman, P.L.; Glidle, A.; Ling, T.W.; Pritchard, D.J. Glutamate Oxidase Enzyme Electrodes: Microsensors for Neurotransmitter Determination Using Electrochemically Polymerized Permselective Films. J. Electroanal. Chem. 1995, 388, 143–149. [Google Scholar]
- Tse, D.C.S.; McCreery, R.L.; Adams, R.N. Potential Oxidative Pathways of Brain Catecholaminest. J. Med. Chem. 1976, 19, 37–40. [Google Scholar]
- Zhang, Y.; Cai, Y.; Su, S. Determination of Dopamine in the Presence of Ascorbic Acid by Poly(styrene sulfonic acid) Sodium Salt/Single Walled Carbon Nanotube Film Modified Glassy Carbon Electrode. Anal. BioChem. 2006, 350, 285–291. [Google Scholar]
- Chen, S.M.; Chzo, W.Y. Simultaneous Voltammetric Detection of Dopamine and Ascorbic Acid Using Didodecyldimethylammonium Bromide (DDAB) Film-Modified Electrodes. J. Electroanal. Chem. 2006, 587, 226–234. [Google Scholar]
- Kang, G.; Lin, X. RNA Modified Electrodes for Simultaneous Determination of Dopamine and Uric Acid in the Presence of High Amounts of Ascorbic Acid. Electroanal. 2006, 18, 2458–2466. [Google Scholar]
- Lin, X.; Li, Y. Simultaneous Electroanalysis of Dopamine, Ascorbic Acid and Uric Acid by Poly (vinyl alcohol) Covalently Modified Glassy Carbon Electrode. Sens. Actuat. B 2006, 115, 134–139. [Google Scholar]
- Balamurugan, A.; Chen, S.M. Poly(3, 4-ethylenedioxythiophene-co-(5-amino-2-naphthalenesulfonic acid)) (PEDOT-PANS) Film Modified Glassy Carbon Electrode for Selective Detection of Dopamine in the Presence of Ascorbic Acid and Uric Acid. Anal. Chim. Acta 2007, 596, 92–98. [Google Scholar]
- Xiao, Y.; Guo, C.; Li, C.M.; Li, Y.; Zhang, J.; Xue, R.; Zhang, S. Highly Sensitive and Selective Method to Detect Dopamine in the Presence of Ascorbic Acid by a New Polymeric Composite Film. Anal. Biochem. 2007, 371, 229–237. [Google Scholar]
- Yin, T.; Wei, W.; Zeng, J. Selective Detection of Dopamine in the Presence of Ascorbic Acid by Use of Glassy-Carbon Electrode Modified with both Polyaniline Film and Multi-walled Carbon Nanotubes with Incorporated cyclodextrin. Anal. Bioanal. Chem. 2006, 386, 2087–2094. [Google Scholar]
- Bustos, E.B.; Jimenez, M.G.G.; Diaz-Sanchez, B.R.; Juaristi, E.; Chapman, T.W.; Godinez, L.A. Glassy Carbon Electrodes Modified with Composites of Starburst-PAMAM Dendrimers Containing Metal Nanoparticles for Amperometric Detection of Dopamine in Urine. Talanta 2007, 72, 1586–1592. [Google Scholar]
- Raoof, J.B.; Ojani, R.; Rashid-Nadimi, S. Voltammetric Determination of Ascorbic Acid and Dopamine in the Same Sample at the Surface of a Carbon Paste Electrode Modified with Polypyrrole/Ferrocyanide Films. Electrochim. Acta 2005, 50, 4694–4698. [Google Scholar]
- Shahrokhian, S.; Zare-Mehrjardi, H.R. Application of Thionine-Nafion Supported on Multi-Walled Carbon Nanotube for Preparation of a Modified Electrode in Simultaneous Voltammetric Detection of Dopamine and Ascorbic Acid. Electrochim. Acta 2007, 52, 6310–6317. [Google Scholar]
- Gopalan, A.I.; Lee, K.P.; Manesh, K.M.; Santhosh, P.; Kim, J.H.; Kang, J.S. Electrochemical Determination of Dopamine and Ascorbic Acid at a Novel Gold Nanoparticles Distributed Poly(4-aminothiophenol) Modified Electrode. Talanta 2007, 71, 1774–1781. [Google Scholar]
- Liu, A.; Honma, I.; Zhou, H. Simultaneous Voltammetric Detection of Dopamine and Uric Acid at Their Physiological Level in the Presence of Ascorbic Acid Using Poly(acrylic acid)-Multiwalled Carbon Nanotube Composite-Covered Glassy-Carbon Electrode. Biosens. Bioelectron. 2007, 23, 74–80. [Google Scholar]
- Boo, H.; Jeong, R.A.; Park, S.; Kim, K.S.; An, K.H.; Lee, Y.H.; Han, J.H.; Kim, H.C.; Chung, T.D. Electrochemical Nanoneedle Biosensor Based on Multiwall Carbon Nanotube. Anal. Chem. 2006, 78, 617–620. [Google Scholar]
- Suzuki, A.; Ivandini, T.A.; Yoshimi, K.; Fujishima, A.; Oyama, G.; Nakazato, T.; Hattori, N.; Kitazawa, S.; Einaga, Y. Fabrication, Characterization, and Application of Boron-Doped Diamond Microelectrodes for in vivo Dopamine Detection. Anal. Chem. 2007, 79, 8608–8615. [Google Scholar]
- Forzani, E.S.; Li, X.L.; Tao, N.J. Hybrid Amperometric and Conductometric Chemical Sensor Based on Conducting Polymer Nanojunctions. Anal. Chem. 2007, 79, 5217–5224. [Google Scholar]
- Heien, M.L.A.V.; Khan, A.S.; Ariansen, J.L.C.; J., F.; Phillips, P.E.M.; Wassum, K.M.; Wightman, R.M. Real-Time Measurement of Dopamine Fluctuations After Cocaine in the Brain of Behaving Rats. Proc. Natl. Acad. Sci. 2005, 102, 10023–10028. [Google Scholar]
- Heien, M.L.A.V.; Johnson, M.A.; Wightman, R.M. Resolving Neurotransmitter Detected by Fast-Scan Cyclic Voltammetry. Anal. Chem. 2004, 76, 5697–5704. [Google Scholar]
- Lane, R.F.; Hubbard, A.T. Differential Double Pulse Voltammetry at Chemically Modified Platium Electrodes for in vivo Determination of Catecholamines. Anal. Chem. 1976, 48, 1287–1293. [Google Scholar]
- Bath, B.D.; Martin, H.B.; Wightman, R.M.; Anderson, M.R. Dopamine Adsorption at Surface Modified Carbon-Fiber Electrodes. Langmuir 2001, 17, 7032–7039. [Google Scholar]
- Downard, A.J.; Roddick, A.D.; Bond, A.M. Covalent Modification of Carbon Electrodes for Voltammetric Differentiation of Dopamine and Ascorbic Acid. Analy. Chim. Acta 1995, 317, 303–310. [Google Scholar]
- Hermans, A.; Seipel, A.T.; Miller, C.E.; Wightman, R.M. Carbon-Fiber Microelectrodes Modified iwth 4-Sulfobenzene Have Increased Sensitivity and Selectivity for Catechlamines. Langmuir 2006, 22, 1964–1969. [Google Scholar]
- Howell, J.O.; Kuhr, W.G.; Ensman, R.E.; Wightman, R.M. Background Substraction for Rapid Scan Voltammetry. J. Electroanal. Chem. 1986, 209, 77–90. [Google Scholar]
- Hermans, A.; Keithley, R.B.; Kita, J.M.; Sombers, L.A.; Wightman, R.M. Dopamine Detection with Fast-Scan Cyclic Voltammetry Used with Analog Background Subtraction. Anal. Chem. 2008, 80, 4040–4048. [Google Scholar]
- Beni, V.; Ghita, M.; Arrigan, D.W.M. Cyclic and Pulse Voltammetric Study of Dopamine at the Interface between Two Immiscible Electrolyte Solutions. Biosens. Bioelectron. 2005, 20, 2097–2103. [Google Scholar]
- Strawbridge, S.M.; Green, S.J.; Tucker, J.H.R. Electrochemical Detection of Catechol and Dopamine as Their Phenylboronate Ester Derivatives. Chem. Commun. 2000, 2393–2394. [Google Scholar]
- Wu, W.; Zhu, H.; Fan, L.; Liu, D.; Renneberga, R.; Yang, S. Sensitive Dopamine Recognition by Boronic Acid Functionalized Multiwalled Carbon Nanotubes. Chem. Comm. 2007, 2345–2347. [Google Scholar]
- Fabre, B.; Taillebois, L. Poly(aniline boronic acid)-based conductimetric sensor of dopamine. Chem. Commun. 2003, 2982–2983. [Google Scholar]
- Zheng, M.; Jagota, A.; Strano, M.S.; Santos, A.P.; Barone, P.; Chou, S.G.; Diner, B.A.; Dresselhaus, M.S.; Mclean, R.S.; Onoa, G.B.; Samsonidze, G.G.; Semke, E.D.; Usrey, M.; Walls, D.J. Structure-Based Carbon Nanotube Sorting by Sequence-Dependent DNA Assembly. Science 2003, 302, 1545–1548. [Google Scholar]
- Zheng, M.; Jagota, A.; Semke, E.D.; Diner, B.A.; Mclean, R.S.; Lustig, S.R.; Richardson, R.E.; Tassi, N.G. DNA-assisted Dispersion and Separation of Carbon Nanotubes. Nat. Mater. 2003, 2, 338–342. [Google Scholar]
- Ma, Y.F.; Ali, S.R.; Dodoo, A.S.; He, H.X. Enhanced Sensitivity for Biosensors: Multiple Functions of DNA Wrapped Single Walled Carbon Nanotubes in Self-Doped Polyaniline Nanocomposites. J. Phys. Chem. B 2006, 110, 16359–16365. [Google Scholar]
- Ma, Y.F.; Ali, S.R.; Wang, L.; Chiu, P.L.; Mendelsohn, R.; He, H.X. In-situ Fabrication of A Water-Soluble, Self-Doped Polyaniline Nanocomposite: the Unique Role of DNA Functionalized Single-Walled Carbon Nanotubes. J. Am. Chem. Soc. 2006, 128, 12064–12065. [Google Scholar]
- Ali, S.R.; Ma, Y.F.; Parajuli, R.R.; Balogan, Y.; Lai, W.Y.-C.; He, H.X. A Non-Oxidative Sensor Based on a Self-Doped Polyaniline/Carbon Nanotube Composite for Highly Sensitive and Selective Detection of the Neurotransmitter Dopamine. Anal. Chem. 2007, 79, 2583–2587. [Google Scholar]
- Persaud, K.C.; Pelosi, P. Sensor Arrays Using Conducting Polymers for an Artificial Nose; Kluwer Academic Publishers: Boston, 1992. [Google Scholar]
- Ma, Y.F.; Zhang, J.M.; Zhang, G.J.; He, H.X. Polyaniline Nanowires on Si surfaces Fabricated with DNA Templates. J. Am. Chem. Soc. 2004, 126, 7097–7101. [Google Scholar]
- Paul, E.W.; Ricco, A.J.; Wrighton, M.S. Resistance of Polyaniline Films as A Function of Electrochemical Potential and theFabrication of Polyaniline-Based Microelectronic Devices. J. Phys. Chem. 1985, 89, 1441–1447. [Google Scholar]
- Virji, S.; Huang, J.X.; Kaner, R.B.; Weiller, B.H. Polyaniline Nanofiber Gas Sensors: Examination of Responce Mechanisms. Nano Lett. 2004, 4, 491–496. [Google Scholar]
- Sukeerthi, S.; Contractor, A.Q. Molecular Sensors and Sensor Arrays Based on Polyaniline Microtubules. Anal. Chem. 1999, 71, 2231–2236. [Google Scholar]
- Bartlett, P.N.; Simon, E. Poly(aniline)-Poly(acrylate) Composite Films as Modified Electrodes for the Oxidation of NADH. Phys. Chem. Chem. Phys. 2000, 2, 2599–2606. [Google Scholar]
- Bartlett, P.N.; Wang, J.H. Electroactivity, Stability and Application in an Enzyme Switch at pH 7 of Poly(aniline)-Poly(styrenesulfonate) Composite Films. J. Chem. Soc. Faraday Trans. 1996, 92, 4137–4143. [Google Scholar]
- Yue, J.; Wang, Z.H.; Cromack, K.R.; Epstein, A.J.; MacDiarmid, A.G. Effect of Sulfonic Acid Group on Polyaniline Backbone. J. Am. Chem. Soc. 1991, 113, 2665–2671. [Google Scholar]
- Chan, H.S.O.; Ho, P.K.H.; Ng, S.C.; Tan, B.T.G.; Tan, K.L. A New Water-Solube, Self-Doping Conducting Polyaniline from Poly(o-aminobenzylphosphonic acid) and Its Sodium Salts: Synthesis and Characterization. J. Am. Chem. Soc. 1995, 117, 8517–8523. [Google Scholar]
- Wei, X.-L.; Wang, Y.Z.; Long, S.M.; Bobeczko, C.; Epstein, A.J. Synthesis and Physical Properties of Highly Sulfonated Polyaniline. J. Am. Chem. Soc. 1996, 118, 2545–2555. [Google Scholar]
- Deore, B.A.; Hachey, S.; Freund, M.S. Electroactivity of Electrochemically Synthesized Poly(Aniline Boronic Acid) as a Function of pH: Role of Self-Doping. Chem. Mater. 2004, 16, 1427–1432. [Google Scholar]
- Bartlett, P.N.; Astier, Y. Microelectrochemical Enzyme Transistors. Chem. Commun. 2000, 105–112. [Google Scholar]
- Zengin, H.; Zhou, W.; Jin, J.; Czerw, R.; Smith, J.D.W.; Echegoyen, L.; Carroll, D.L.; Foulger, S.H.; Ballato, J. Carbon Nanotube Doped Polyaniline. Adv. Mater. 2002, 14, 1480–1483. [Google Scholar]
- Cochet, M.; Maser, W.K.; Benito, A.M.; Callejas, M.A.; Martínez, M.T.; Benoit, J.-M.; Schreiber, J.; Chauvet, O. Synthesis of a New Polyaniline/Nanotube Composite: “in-situ” Polymerisation and Charge Transfer through Site-Selective Interaction. Chem. Comm. 2001, 1450–1451. [Google Scholar]
- Chen, G.Z.; Shaffer, M.S.P.; Coleby, D.; Dixon, G.; Zhou, W.; Fray, D.J.; Windle, A.H. Carbon Nanotube and Polypyrrole Composites: Coating and Doping. Adv. Mater. 2000, 12, 522–526. [Google Scholar]
- Li, X.H.; Wu, B.; Huang, J.-E.; Zhang, J.; Liu, Z.F.; Li, H.L. Fabrication and Characterization of Well-Dispersed Single-Walled Carbon Nanotube/Polyaniline Composites. Carbon 2002, 411, 1670–1673. [Google Scholar]
- Lou, X.; Detrembleur, C.; Pagnoulle, C.; Jérôme, R.; Bocharova, V.; Kiriy, A.; Stamm, M. Surface Modification of Multiwalled Carbon Nanotubes by Poly(2-vinylpyridine): Dispersion, Selective Deposition, and Decoration of the Nanotubes. Adv. Mater. 2004, 16, 2123–2127. [Google Scholar]
- An, K.H.; Jeong, S.Y.; Hwang, H.R.; Lee, Y.H. Enhanced Sensitive of a Gas Sensor Incorporating Single-Walled Carbon Nanotube-Polypyrrole Nanocomposites. Adv. Mater. 2004, 16, 1005–1009. [Google Scholar]
- Zhao, B.; Hu, H.; Haddon, R.C. Synthesis and Properties of a Water-Soluble Single-Wall Carbon Nanotube-Poly(m-aminobenzene sulfonic acid) Graft Copolymer. Adv. Funct. Mater. 2004, 14, 71–76. [Google Scholar]
- Nyholm, L.; Peter, L.M. Wide pH Range Microelectrode Study of the Electrochemical Behaviour of Polyaniline Films in Buffered Solutions. J. Chem. Soc. Faraday Trans. 1994, 90, 149–154. [Google Scholar]
- Dinh, H.N.; Ding, J.; Xia, S.J.; Birss, V.I. Multi-technique Study of the Anodic Degradation of Polyaniline Films. J. Electroanal. Chem. 1998, 459, 45–56. [Google Scholar]
- Kobayashi, E.; Yoneyama, H.; Tamura, H. Polyaniline Film-Coated Electrodes as Electrochromic Display Devices. J. Electroanal. Chem. 1984, 161, 419–423. [Google Scholar]
- Orata, D.; Buttry, D.A. Determination of Ion Populations and Solvent Content as Functions of Redox State and pH in Polyaniline. J. Am. Chem. Soc. 1987, 109, 3574–3581. [Google Scholar]
- Zheng, M.; Diner, B.A. Solution Redox Chemistry of Carbon Nanotubes. J. Am. Chem. Soc. 2004, 126, 15490–15494. [Google Scholar]
- Napier, M.E.; Hull, D.O.; Thorp, H.H. Electrocatalytic Oxidation of DNA-Wrapped Carbon Nanotubes. J. Am. Chem. Soc. 2005, 127, 11952–11953. [Google Scholar]
- Shoji, E.; Freund, M.S. Potentiometric Saccharide Detection Based on the pKa Change of Poly(aniline boronic acid). J. Am. Chem. Soc. 2002, 124, 12486–12493. [Google Scholar]
- Ma, Y.F.; Chiu, P.L.; Chen, A.M.; Serrano, A.; Ali, S.R.; He, H.X. The Electronic Role of DNA Functionalized Carbon Nanotubes: Efficacy for in-situ Polymerization of Conducting Polymer Nanocomposite. J. Am. Chem. Soc. 2008, 130, 7921–7928. [Google Scholar]
- Ma, Y.F.; Cheung, W.; Wei, D.; Bogozi, A.; Chiu, P.L.; Wang, L.; Pontoriero, F.; Mendelsohn, R.; He, H.X. Improved Conductivity of Carbon Nanotube Networks by In Situ Polymerization of a Thin Skin of Conducting Polymer. ACS Nano 2008, 2, 1197–1204. [Google Scholar]
- Wang, J.; Dai, J.H.; Yarlagadda, T. Carbon Nanotube-Conducting Polymer Composite Nanowires. Langmuir 2005, 21, 9–12. [Google Scholar]
- Martin, C.R. Nanomaterials: A Membrane-Based Synthetic Approach. Science 1994, 266, 1961–1966. [Google Scholar]
- Van Dyke, L.S.; Martin, C.R. Electrochemical Investigation of Electronically Conductive Polymers: Controlling the Supermolecular Structure Allows Charge Transport Rates to Be Enhanced. Langmuir 1990, 6, 1118–1123. [Google Scholar]
- Parthasarathy, R.; Martin, C.R. Template-Synthesized Polyaniline Microtubules. Chem. Mater. 1994, 6, 1627–1632. [Google Scholar]
- Liu, W.; Kumar, J.; Tripathy, S.; Senecal, K.J.; Samuelson, L. Enzymatically Synthesized Conducting Polyaniline. J. Am. Chem. Soc. 1999, 121, 71–78. [Google Scholar]
- Sun, Y.; Wilson, S.R.; Schuster, D.I. High Dissolution and Strong Light Emission of Carbon Nanotubes in Aromatic Amine Solvents. J. Am. Chem. Soc. 2001, 123, 5348–5349. [Google Scholar]
- Nicolas, M.; Fabre, B.; Marchand, G.; Simonet, J. New Boronic-Acid- and Boronate-Substituted Aromatic Compounds as Precursors of Fluoride-Responsive Conjugated Polymer Films. Eur. J. Org. Chem. 2000, 1703–1710. [Google Scholar]
- Pringsheim, E.; Terpetschnig, E.; Piletsky, S.A.; Wolfbeis, O.S. A PANI with Near-Infrared Optical Response to Saccharides. Adv. Mater. 1999, 11, 865–868. [Google Scholar]
- Nicolas, M.; Fabre, B.; Simonet, J. Electrochemical sensing of fluoride and sugars with a boronic acid-substituted bipyridine Fe(II) complex in solution and attached onto an electrode surface. Electrochim. Acta 2001, 46, 1179–1190. [Google Scholar]
- Shoji, E.; Freund, M.S. Potentiometric Saccharide Detection Based on the pKa Changes of Poly(aniline boronic acid). J. Am. Chem. Soc. 2002, 124, 12486–12493. [Google Scholar]
- Shoji, E.; Freund, M.S. Potentiometric Sensors Based on the Inductive Effect on the pKa of Poly(aniline): A Nonenzymatic Glucose Sensor. J. Am. Chem. Soc. 2001, 123, 3383–3384. [Google Scholar]
- He, H.X.; Zhu, J.S.; Tao, N.J.; Amlani, I.; Nagahara, L.A.; Tsui, R. A Conducting Polymer Nanojunction Switch. J. Am. Chem. Soc. 2001, 123, 7730–7731. [Google Scholar]
- Ofer, D.; Crooks, R.M.; Wrighton, M.S. Potential Dependence of the Conductivity of Highly Oxidized Polythiophenes, Polypyrroles, and Polyanilines: Finite Windows of High Conductivity. J. Am. Chem. Soc. 1990, 112, 7869. [Google Scholar]
- Huang, J.; Wrighton, M.S. Microelectrochemical Multitransistor Devices Based on Electrostatic Binding of Electroactive Anionic Metal Complexes in Protonated Poly(4-vinylpyridine): Devices That Can Detect and Distinguish up to Three Species Simultaneously. Anal. Chem. 1993, 65, 2740. [Google Scholar]
- Allen, J.B.; Larry, R.F. Electrochemical Methods: Fundamentals and Applications, 2nd Ed. ed; Wiley: New York, 2001. [Google Scholar]
- Arrigan, D.W.M.; Ghita, M.; Beni, V. Selective voltammetric detection of dopamine in the presence of ascorbate. Chem. Commun. 2004, 732–733. [Google Scholar]
- Beni, V.; Ghita, M.; Arrigan, D.W.M. Cyclic and pulse voltammetry study of dopamine at the interface between two immiscible electrolyte solutions. Biosens. Bioelectron. 2005, 20, 2097–2103. [Google Scholar]
- Ali, S.K.; Parajuli, R.R.; Ma, Y.F.; Balogan, Y.; Lai, W.Y.-C.; He, H.X. The Interference of Ascorbic Acid in Dopamine Detection by a Non-oxidative Approach. J. Phy. Chem. B 2007, 111, 12275–12281. [Google Scholar]
- Rahman, M.A.; Kwon, N.H.; Won, M.S.; Choe, E.S.; Shim, Y.B. Functionalized Conducting Polymer as an Enzyme-Immobilizing Substrate: An Amperometric Glutamate Microbiosensor for in Vivo Measurements. Anal. Chem. 2005, 77, 4854–4860. [Google Scholar]
- Granot, E.; Katz, E.; Basnar, B.; Willner, I. Enhanced Bioelectrocatalysis Using Au-Nanoparticle/Polyaniline Hybrid Systems in Thin Films and Microstructured Rods Assembled on Electrodes. Chem. Mater. 2005, 17, 4600–4609. [Google Scholar]
- Springsteen, G.; Wang, B. Alizarin Red S. as a general reporter for studying the binding of boronic acids with carbohydrates. Chem. Commun. 2001, 1608–1609. [Google Scholar]
- Springsteen, G.; Wang, B. A detailed examination of boronic acid-diol complexation. Tetrahedron 2002, 58, 5291–5300. [Google Scholar]
- Benesi, H.A.; Hildebrand, J.H. A Spectrophotometric Investigation of the Interaction of Iodine with Aromatic Hydrocarbons. J. Am. Chem. Soc. 1949, 71, 2703. [Google Scholar]
- Connors, K. Binding Constants; Wiley: New York, 1987. [Google Scholar]
- James, T.D.; Samankumara Sandanayake, K.R.A.; Iguchi, R.; Shinkai, S. Novel Saccharide-Photoinduced Electron Transfer Sensors Based on the Interaction of Boronic Acid and Amine. J. Am. Chem. Soc. 1995, 117, 8982–8987. [Google Scholar]
- Dhariwal, K.R.; Hartzell, W.O.; Levine, M. Ascorbic Acid and dehydroascorbic acid measurements in human plasma and serum. Am. J. Clin. Nutr. 1991, 54, 712–716. [Google Scholar]
- Huang, J.; Agus, D.B.; Winfree, C.J.; Kiss, S.; Mack, W.J.; McTaggart, R.A.; Choudhri, T.F.; Kim, L.J.; Mocco, J.; Pinsky, D.J.; Fox, W.D.; Israe, R.J.; Boyd, T.A.; Golde, D.W., Jr.; Connolly, E.S. Dehydroascorbic acid, a blood–brain barrier transportable form of vitamin C, mediates potent cerebroprotection in experimental stroke. Proc. Natl. Acad. Sci. 2001, 98, 11720–11724. [Google Scholar]
- Palmisano, F.; Zambonin, P.G. Ascorbic Acid Interferences in Hydrogen Peroxide Detecting Biosensors Based on Electrochemically Immobilized Enzymes. Anal. Chem. 1993, 65, 2690–2692. [Google Scholar]
- Anzai, J.-I.; Takeshita, H.; Kobayashi, Y.; Osa, T.; Hoshi, T. Layer-by-layer construction of Enzyme Multilayers on an Electrode for the Preparation of Glucose and Lactose Sensors: Elimination of Ascorbate Interference by Means of an Ascorbate Oxidase Monolayer. Anal. Chem. 1998, 70, 811–817. [Google Scholar]
- Lisdat, F.; Wollenberger, U.; Makower, A.; Hortnagl, H.; Pfeiffer, D.; Scheller, F.W. Catecholamine detection using enzymatic amplification. Biosens. Bioelectron. 1997, 12, 1199–1211. [Google Scholar]
- Rahman, M.A.; Kwon, N.-H.; Won, M.-S.; Choe, E.S.; Shim, Y.-B. Functionalized Conducting Polymer as an Enzyme-Immobilizing Substrate: An Amperometric Glutamate Microbiosensor for in Vivo Measurements. Anal. Chem. 2005, 77, 4854–4860. [Google Scholar]
- Harrison, J.; Turner, R.F.B.; Baltes, H.P. Characterization of Perfluorosulfonic Acid Polymer Coated Enzyme Electrodes and a Miniaturized Integrated Potentiostat for Glucose Analysis in While Blood. Anal. Chem. 1988, 60, 2002–2006. [Google Scholar]
- Wang, J.; Tuzhi, P. Selectivity and Sensitivity Improvements at Perfluorinated Ionomer/Cellulose Acetate Bilayer Electrodes. Anal. Chem. 1986, 58, 3257–3261. [Google Scholar]
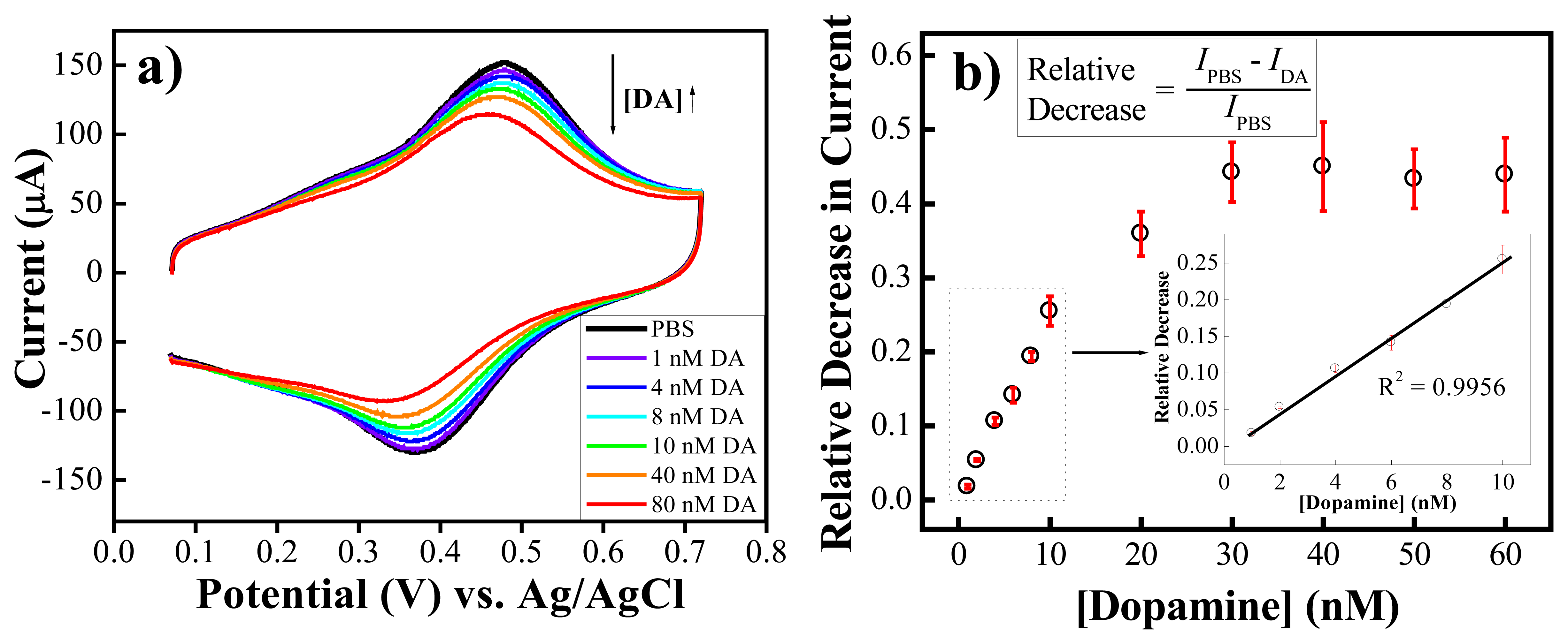
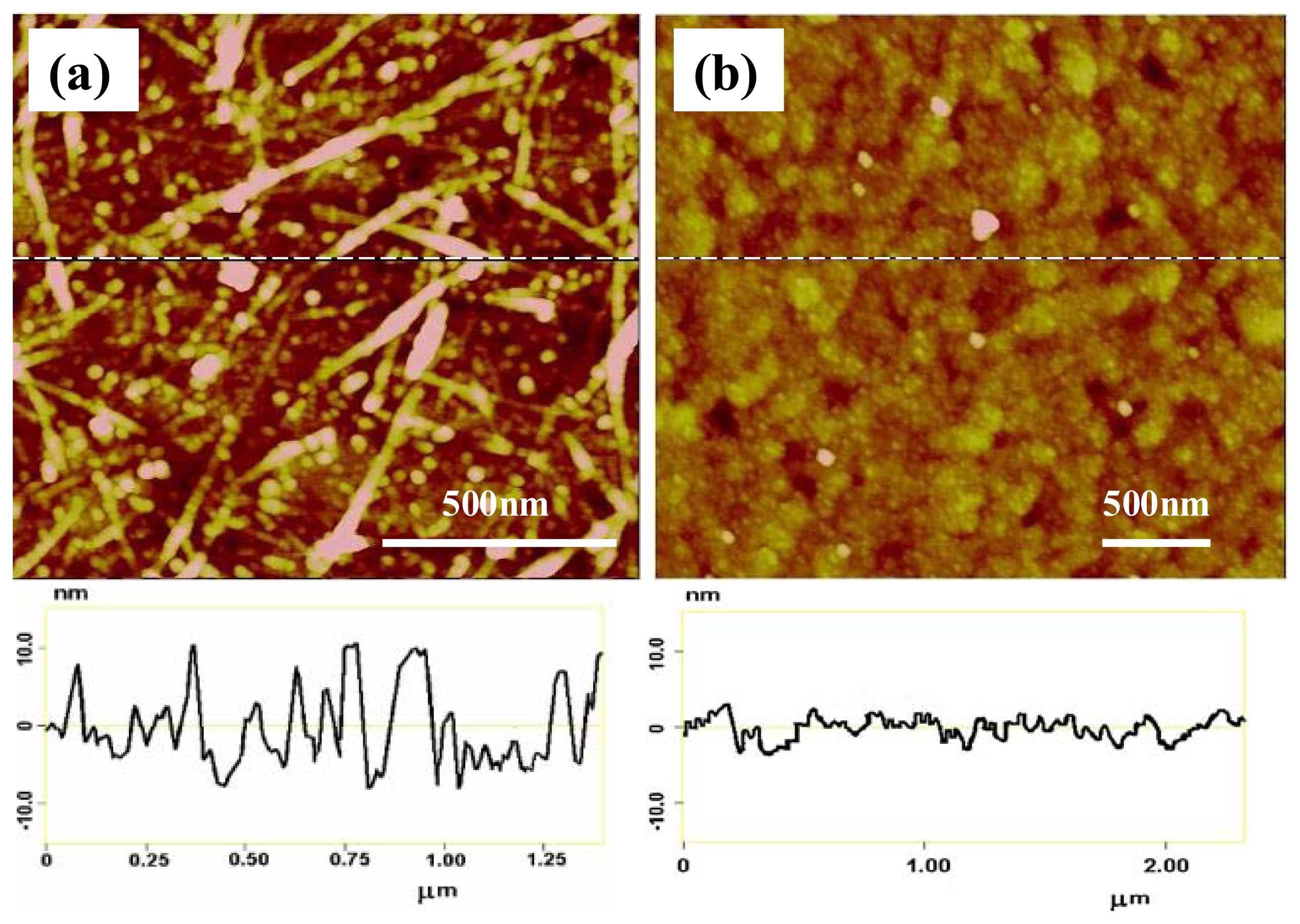
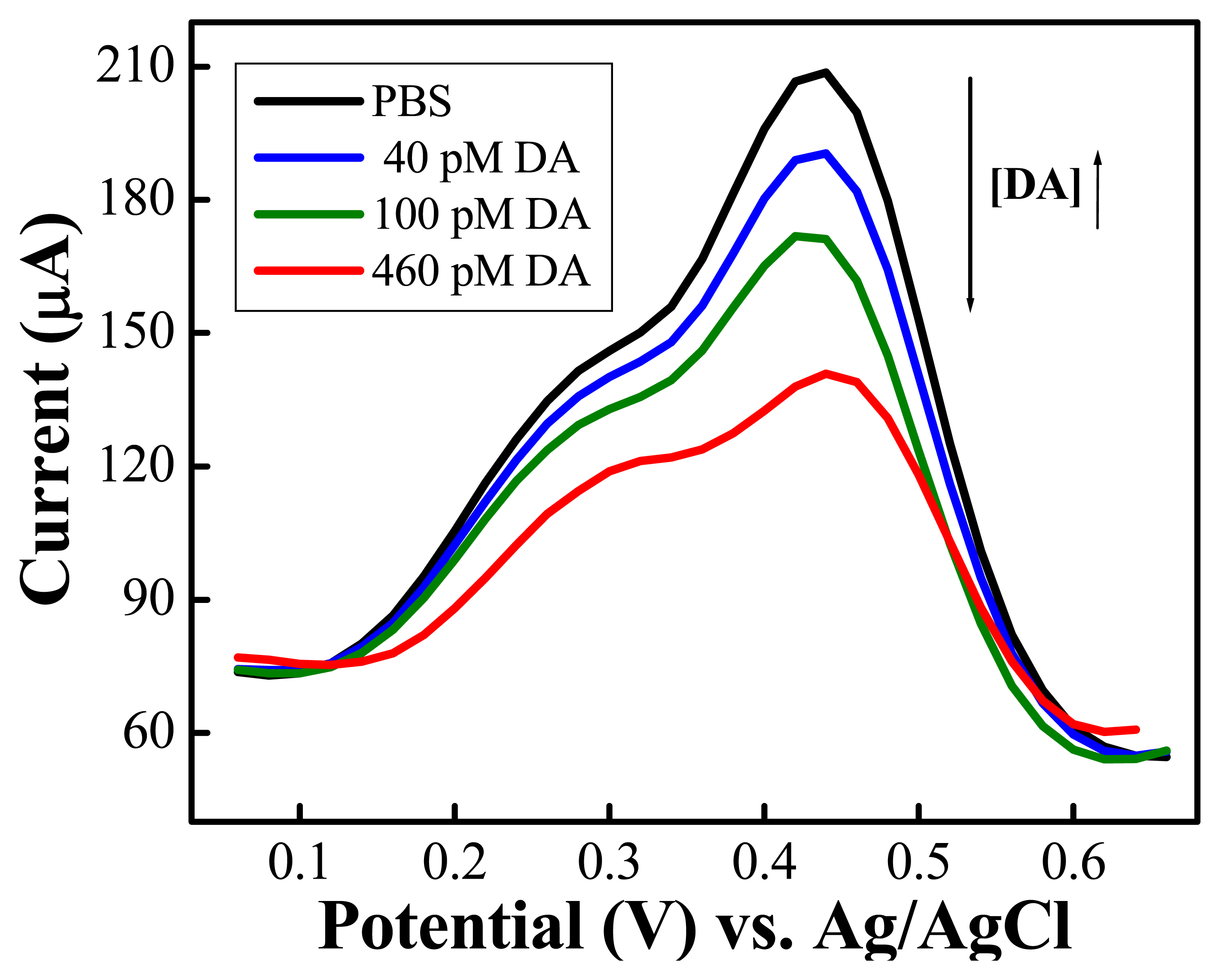
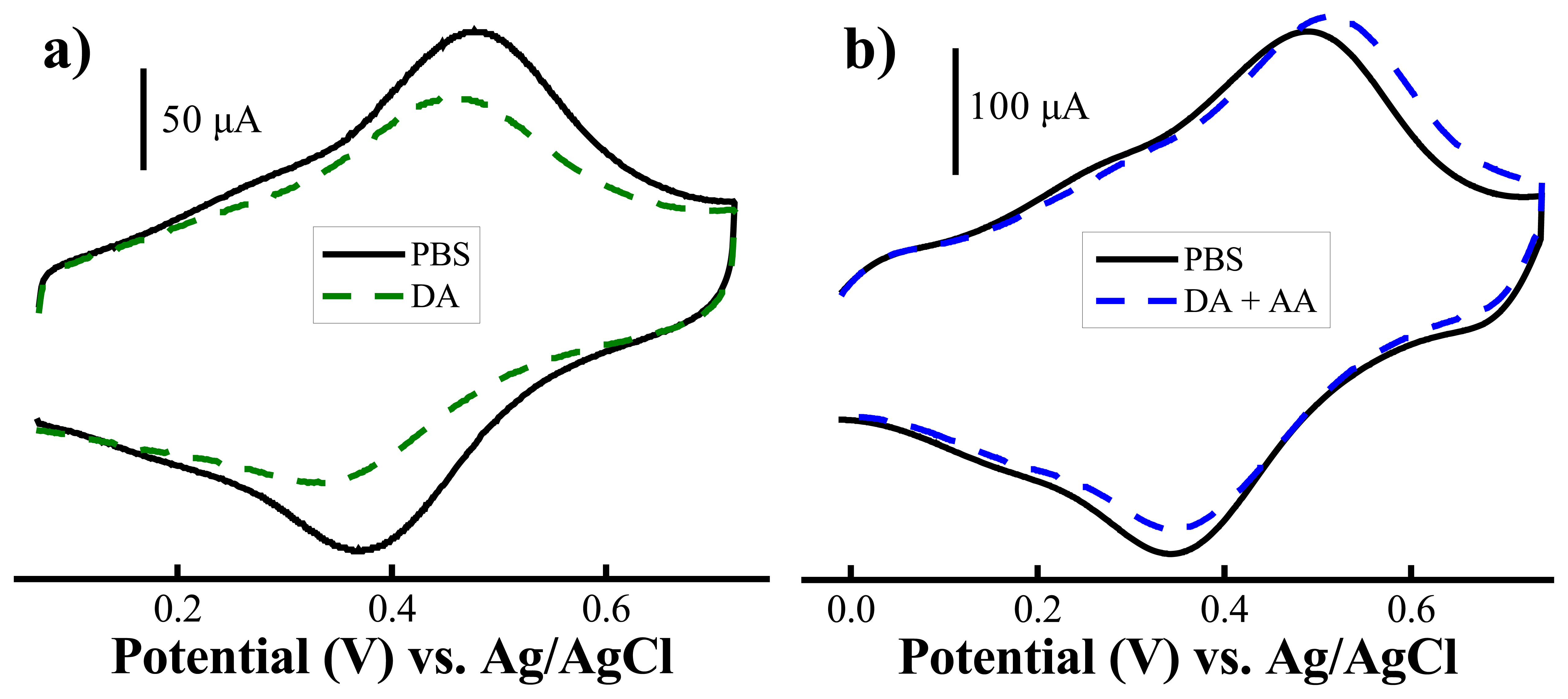
 ) of 0.15 mM AA (n=3) (Reproduced with permission from the American Chemical Society [48].)
) of 0.15 mM AA (n=3) (Reproduced with permission from the American Chemical Society [48].)
 ) of 0.15 mM AA (n=3) (Reproduced with permission from the American Chemical Society [48].)
) of 0.15 mM AA (n=3) (Reproduced with permission from the American Chemical Society [48].)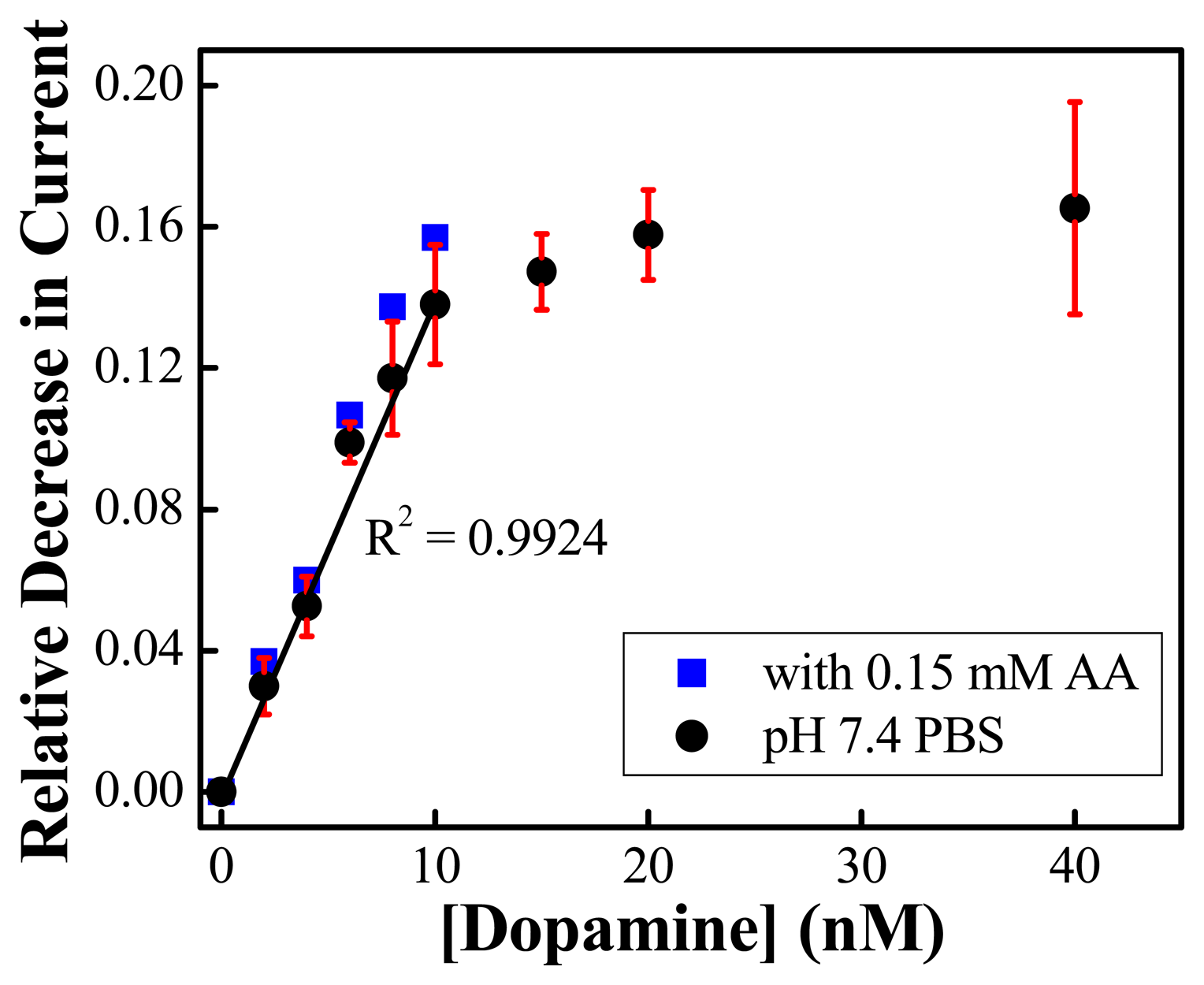


© 2008 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ali, S.R.; Parajuli, R.R.; Balogun, Y.; Ma, Y.; He, H. A Nonoxidative Electrochemical Sensor Based on a Self-Doped Polyaniline/Carbon Nanotube Composite for Sensitive and Selective Detection of the Neurotransmitter Dopamine: A Review. Sensors 2008, 8, 8423-8452. https://doi.org/10.3390/s8128423
Ali SR, Parajuli RR, Balogun Y, Ma Y, He H. A Nonoxidative Electrochemical Sensor Based on a Self-Doped Polyaniline/Carbon Nanotube Composite for Sensitive and Selective Detection of the Neurotransmitter Dopamine: A Review. Sensors. 2008; 8(12):8423-8452. https://doi.org/10.3390/s8128423
Chicago/Turabian StyleAli, Shah R., Rishi R. Parajuli, Yetunde Balogun, Yufeng Ma, and Huixin He. 2008. "A Nonoxidative Electrochemical Sensor Based on a Self-Doped Polyaniline/Carbon Nanotube Composite for Sensitive and Selective Detection of the Neurotransmitter Dopamine: A Review" Sensors 8, no. 12: 8423-8452. https://doi.org/10.3390/s8128423



