Conformational Mobility of GOx Coenzyme Complex on Single-Wall Carbon Nanotubes
Abstract
:1. Introduction
2. Results and Discussion
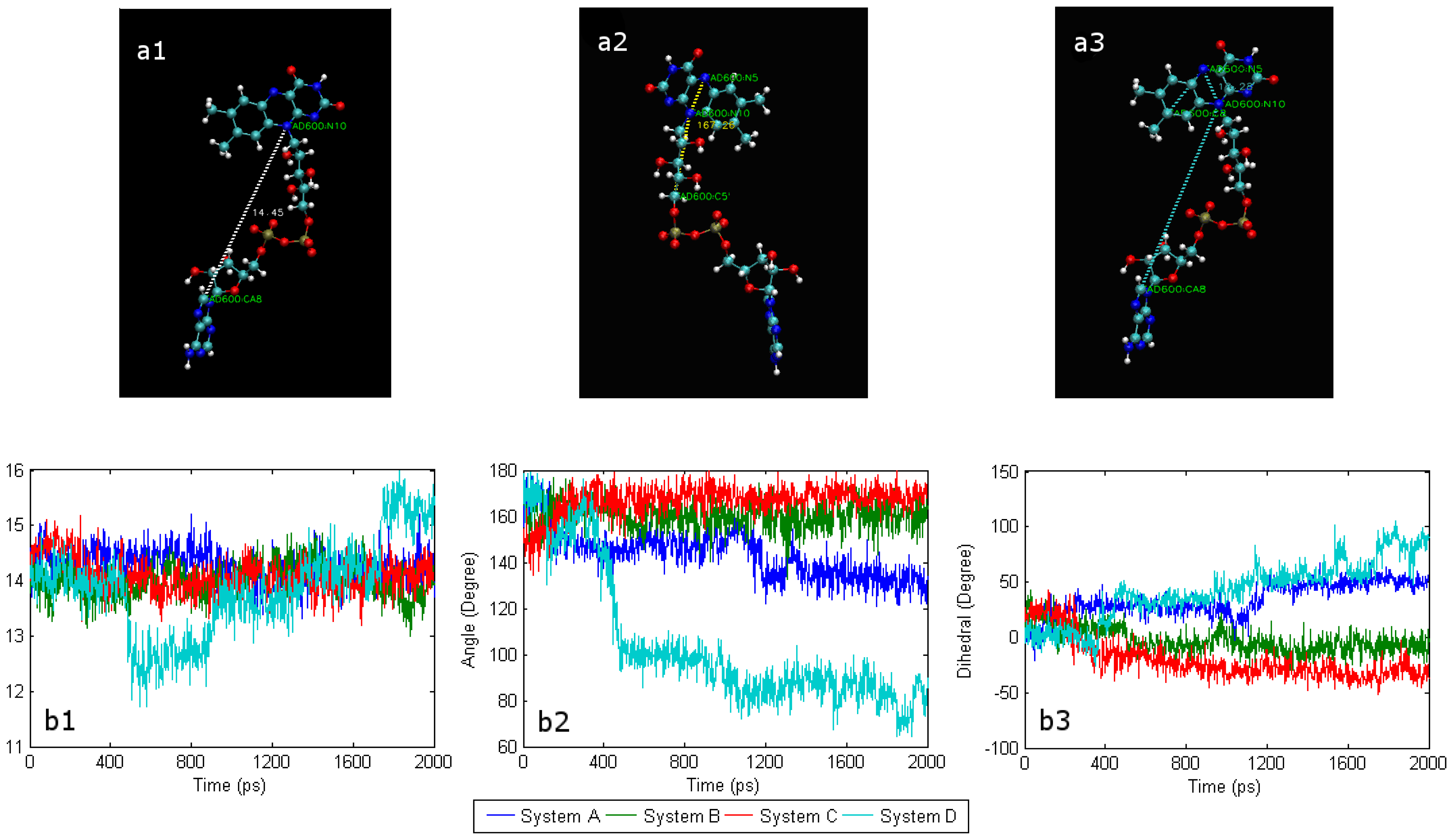
2.1. The conformational change of FAD
2.2. The interaction of FAD with SWCNT and apo-GOx
2.3. The interaction energy with water molecules
2.4. Conformational changes and the effect of water
3. Experimental Section
4. Conclusions
Acknowledgments
References and Notes
- Wilson, R.; Turner, A.P.F. Glucose oxidase: an ideal enzyme. Biosens. Bioelectron. 1992, 7, 165–185. [Google Scholar]
- Xiao, Y.; Patolsky, F.; Katz, E.; Hainfeld, J.F.; Willner, I. “Plugging into enzymes”: nanowiring ofredoxenzymes by a gold nanoparticle. Science 2003, 299, 1877–1881. [Google Scholar]
- Ramanavicius, A.; Kausaite, A.; Ramanaviciene, A. Biofuel cell based on direct bioelectrocatalysis. Biosens. Bioelectron. 2005, 20, 1962–1967. [Google Scholar]
- Ivnitski, D.; Branch, B.; Atanassov, P.; Apblett, C. Glucose oxidase anode for biofuel cell based on direct electron transfer. Electrochem. Commun. 2006, 8, 1204–1210. [Google Scholar]
- Rivas, G.A.; Rubianes, M.D.; Rodríguez, M.C.; Ferreyra, N.F.; Luque, G.L.; Pedano, M.L.; Miscoria, S.A.; Parrado, C. Carbon nanotubes for electrochemical biosensing. Talanta 2007, 74, 291–307. [Google Scholar]
- Davis, F.; Higson, S.P.J. Biofuel cells-Recent advances and applications. Biosens. Bioelectron. 2007, 22, 1224–1235. [Google Scholar]
- Frew, J.E.; Hill, H.A.O. Direct and indirect electron transfer between electrodes and redox proteins. Eur. J. Biochem. 1988, 172, 261–269. [Google Scholar]
- Hess, C.R.; Juda, G.A.; Dooley, D.M.; Amii, R.N.; Hill, M.G.; Winkler, J.R.; Gray, H.B. Gold electrodes wired for coupling with the deeply buried active site of arthrobacter globiformis amine oxidase. J. Am. Chem. Soc. 2003, 125, 7156–7157. [Google Scholar]
- Aguey-Zinsou, K.F.; Bernhardt, P.V.; Kappler, U.; McEwan, A.G. Direct electrochemistry of a bacterial sulfite dehydrogenase. J. Am. Chem. Soc. 2003, 125, 530–535. [Google Scholar]
- Lyons, M.E.G.; Keeley, G.P. The redox behaviour of randomly dispersed single walled carbon nanotubes both in the absence and in the presence of adsorbed glucose oxidase. Sensors 2006, 6, 1791–1826. [Google Scholar]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar]
- Balasubramanian, K.; Burghard, M. Biosensors based on carbon nanotubes. Anal. Bioanal. Chem. 2006, 385, 452–468. [Google Scholar]
- Guiseppi-Elie, A.; Lei, C.; Baughman, R.H. Direct electron transfer of glucose oxidase on carbon nanotubes. Nanotechnology 2002, 13, 559–564. [Google Scholar]
- Cai, C.; Chen, J. Direct electron transfer of glucose oxidase promoted by carbon nanotubes. Anal. Biochem. 2004, 332, 75–83. [Google Scholar]
- Tatke, S.S.; Renugopalakrishnan, V.; Prabhakaran, M. Interfacing biological macromolecules with carbon nanotubes and silicon surfaces: a computer modelling and dynamic simulation study. Nanotechnology 2004, 15, S684–S690. [Google Scholar]
- Shen, J.W.; Wu, T.; Wang, Q.; Kang, Y. Induced stepwise conformational change of human serum albumin on carbon nanotube surfaces. Biomaterials 2008, 29, 3847–3855. [Google Scholar]
- Shen, J.W.; Wu, T.; Wang, Q.; Pan, H.H. Molecular simulation of protein adsorption and desorption on hydroxyapatite surfaces. Biomaterials 2008, 29, 513–532. [Google Scholar]
- Chen, X.; Wu, T.; Wang, Q.; Shen, J.W. Shield effect of silicate on adsorption of protein onto silicon-doped hydroxyapatite (100) surface. Biomaterials 2008, 29, 2423–2432. [Google Scholar]
- Zhou, H.; Wu, T.; Dong, X.; Wang, Q.; Shen, J.W. Adsorption mechanism of BMP-7 on hydroxyapatite (001) surfaces. Biochem. Biophys. Res. Commun. 2007, 361, 91–96. [Google Scholar]
- Lin, C.S.; Zhang, R.Q.; Niehaus, T.A.; Frauenheim, T. Geometric and electronic structures of carbon nanotubes adsorbed with flavin adenine: a theoretical study. J. Phys. Chem. C 2007, 111, 4069–4073. [Google Scholar]
- Hecht, H.J.; Kalisz, H.M.; Hendle, J.; Schmid, R.D.; Schomburg, D. Crystal structure of glucose oxidase from Aspergillus niger refined at 2.3 Å resolution. J. Mol. Biol. 1993, 229, 153–172. [Google Scholar]
- van den Berg, P.A.W.; Feenstra, K.A.; Mark, A.E.; Berendsen, J.C.; Visser, J.W.G. Dynamic conformations of flavin adenine dinucleotide: simulated molecular dynamics of the flavin cofactor related to the time-resolved fluorescence characteristics. J. Phys. Chem. B 2002, 106, 8858–8869. [Google Scholar]
- Frey, J.T.; Doren, D.J. TubeGen. University of Delaware: Newark, DE, 2003. Available online: http://deaddog.duch.udel.edu/~frey/research/tubegenonline.html.
- Phillips, J.C.; Braun, R.; Wang, W.; Gumbart, J.; Tajkhorshid, E.; Villa, E.; Chipot, C.; Skeel, R.D.; Kalé, L.; Schulten, K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005, 26, 1781–1802. [Google Scholar]
- MacKerell, A.D.; Bashford, D.; Bellott, M.; Dunbrack, R.L.; Evanseck, J.D.; Field, M.J.; Fischer, S.; Gao, J.; Guo, H.; Ha, S.; Joseph-McCarthy, D.; Kuchnir, L.; Kuczera, K.; Lau, F.T.K.; Mattos, C.; Michnick, S.; Ngo, T.; Nguyen, D.T.; Prodhom, B.; Reiher, W.E.; Roux, B.; Schlenkrich, M.; Smith, J.C.; Stote, R.; Straub, J.; Watanabe, M.; Wiórkiewicz-Kuczera, J.; Yin, D.; Karplus, M. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J. Phys. Chem. B 1998, 102, 3586–3616. [Google Scholar]
- Hirschfelder, J.O.; Curtiss, C.F.; Brid, R.B. Molecular theory of gases and liquids; Wiley: New York, NY, USA, 1954. [Google Scholar]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: visual molecular dynamics. J. Mol. Graphics 1996, 14, 33–38. [Google Scholar]
- Patolsky, F.; Weizmann, Y.; Willner, I. Long-range electrical contacting of redox enzymes by SWCNT connectors. Angew. Chem. Int. Ed. 2004, 43, 2113–2117. [Google Scholar]
- Wohlfahrt, G.; Trivic, S.; Zeremski, J.; Pericin, D.; Leskovac, V. The chemical mechanism of action of glucose oxidase from Aspergillus niger. Mol. Cell. Biochem. 2004, 160, 69–83. [Google Scholar]



© 2008 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liu, F.; Ye, X.-s.; Wu, T.; Wang, C.-T.; Shen, J.-w.; Kang, Y. Conformational Mobility of GOx Coenzyme Complex on Single-Wall Carbon Nanotubes. Sensors 2008, 8, 8453-8462. https://doi.org/10.3390/s8128453
Liu F, Ye X-s, Wu T, Wang C-T, Shen J-w, Kang Y. Conformational Mobility of GOx Coenzyme Complex on Single-Wall Carbon Nanotubes. Sensors. 2008; 8(12):8453-8462. https://doi.org/10.3390/s8128453
Chicago/Turabian StyleLiu, Feng, Xue-song Ye, Tao Wu, Chang-Tao Wang, Jia-wei Shen, and Yu Kang. 2008. "Conformational Mobility of GOx Coenzyme Complex on Single-Wall Carbon Nanotubes" Sensors 8, no. 12: 8453-8462. https://doi.org/10.3390/s8128453



