Imaging In Mice With Fluorescent Proteins: From Macro To Subcellular
Abstract
:1. Introduction
2. Noninvasive Imaging
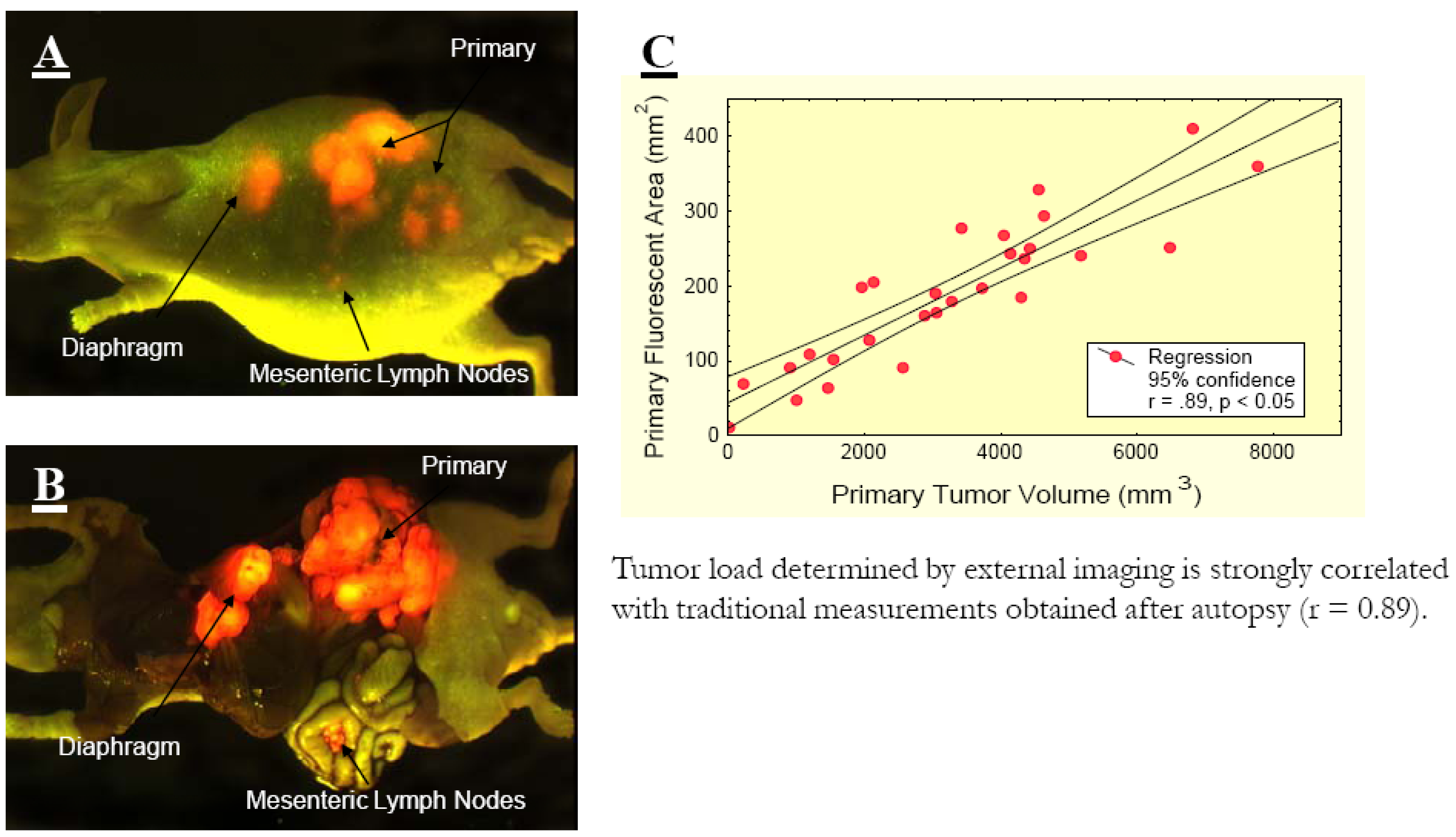
2.1 Non-Invasive Cellular Imaging of Cancer Cell-Stromal Cell Interaction [10]
2.2 Method of Choice for Whole-Body Imaging
3. Subcellular Imaging of Cancer Cell Trafficking In Vivo
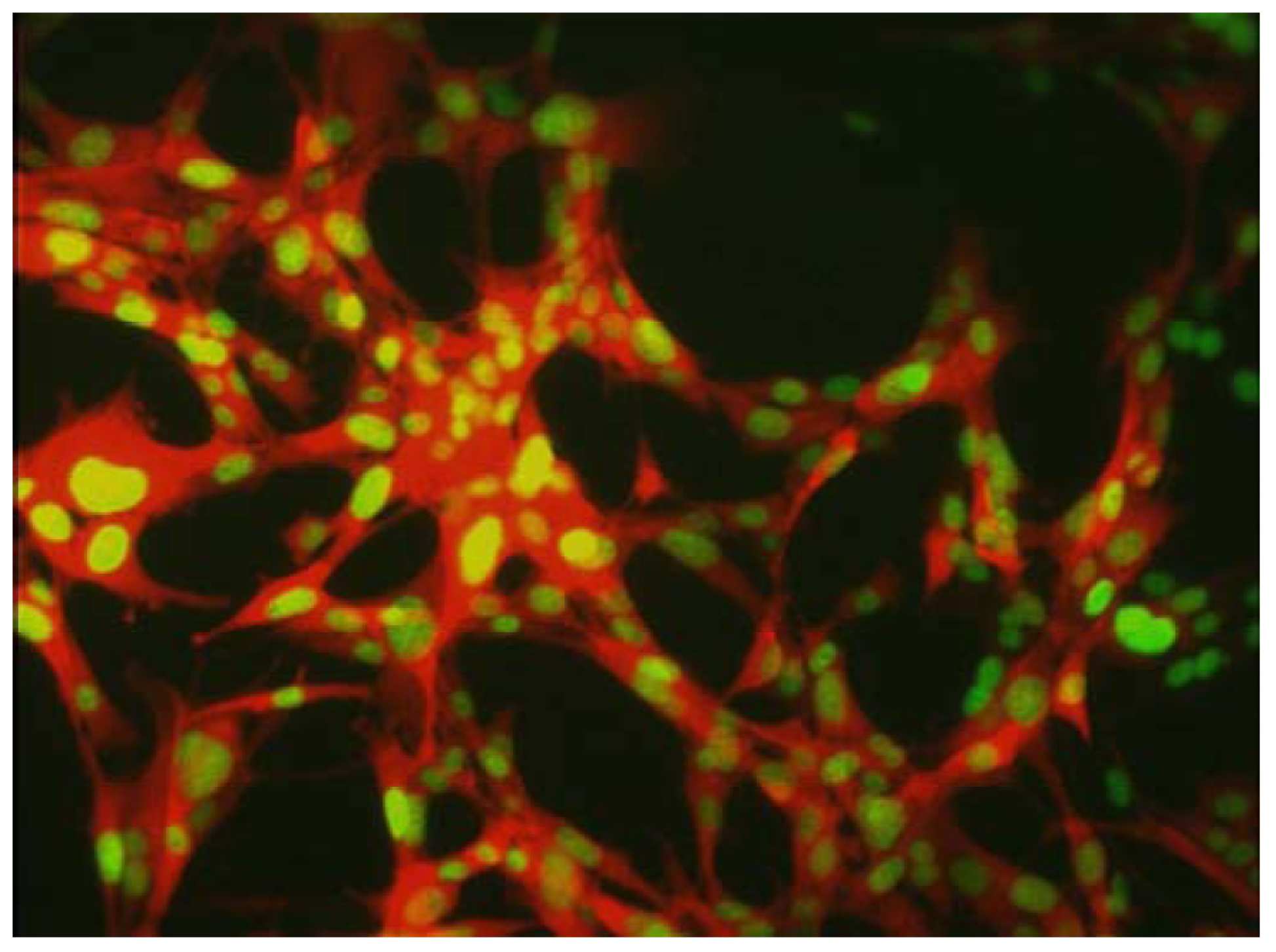
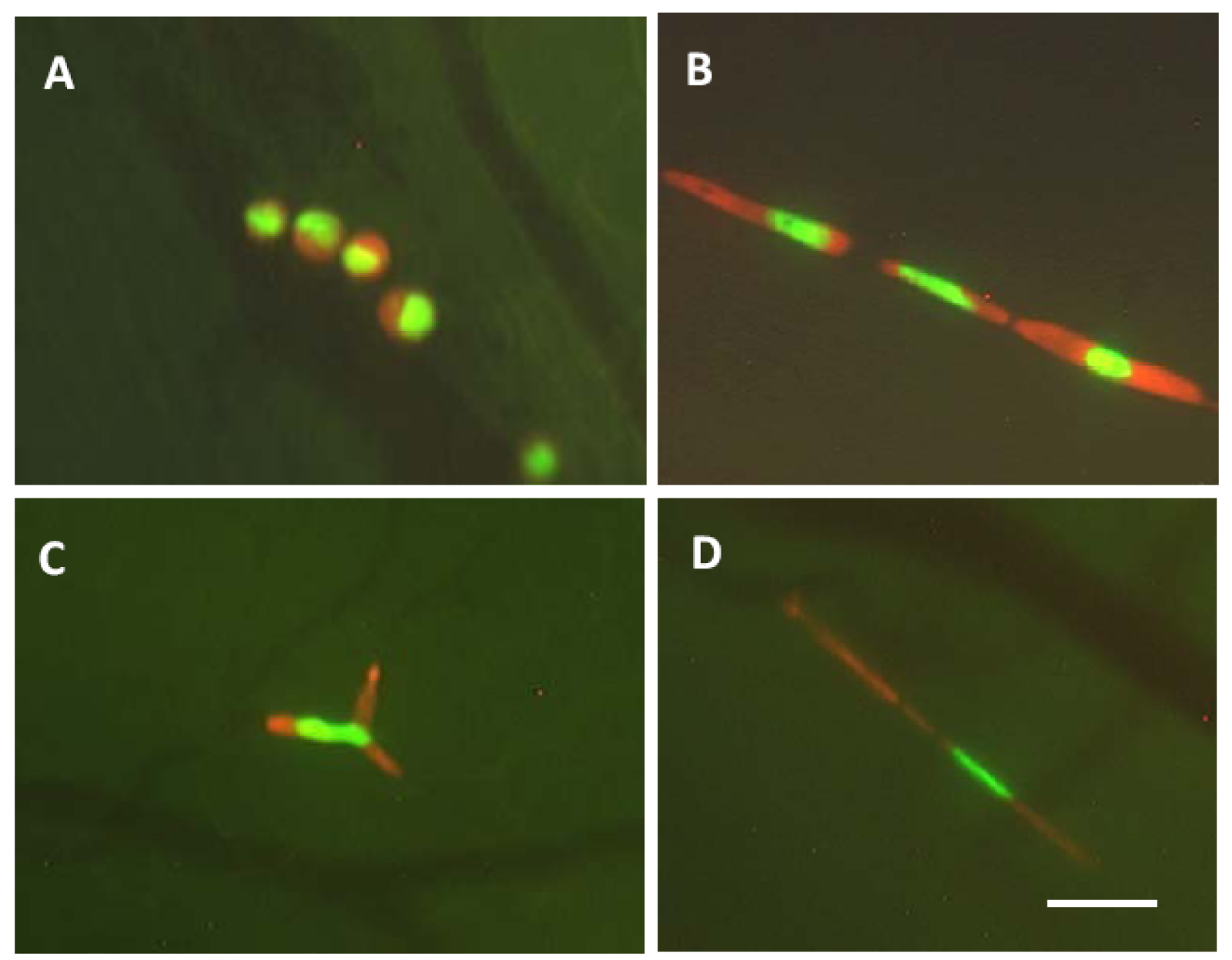
3.1 Real Time Imaging of Nuclear-Cytoplasmic Dynamics of Trafficking and Extravasating Cancer Cells
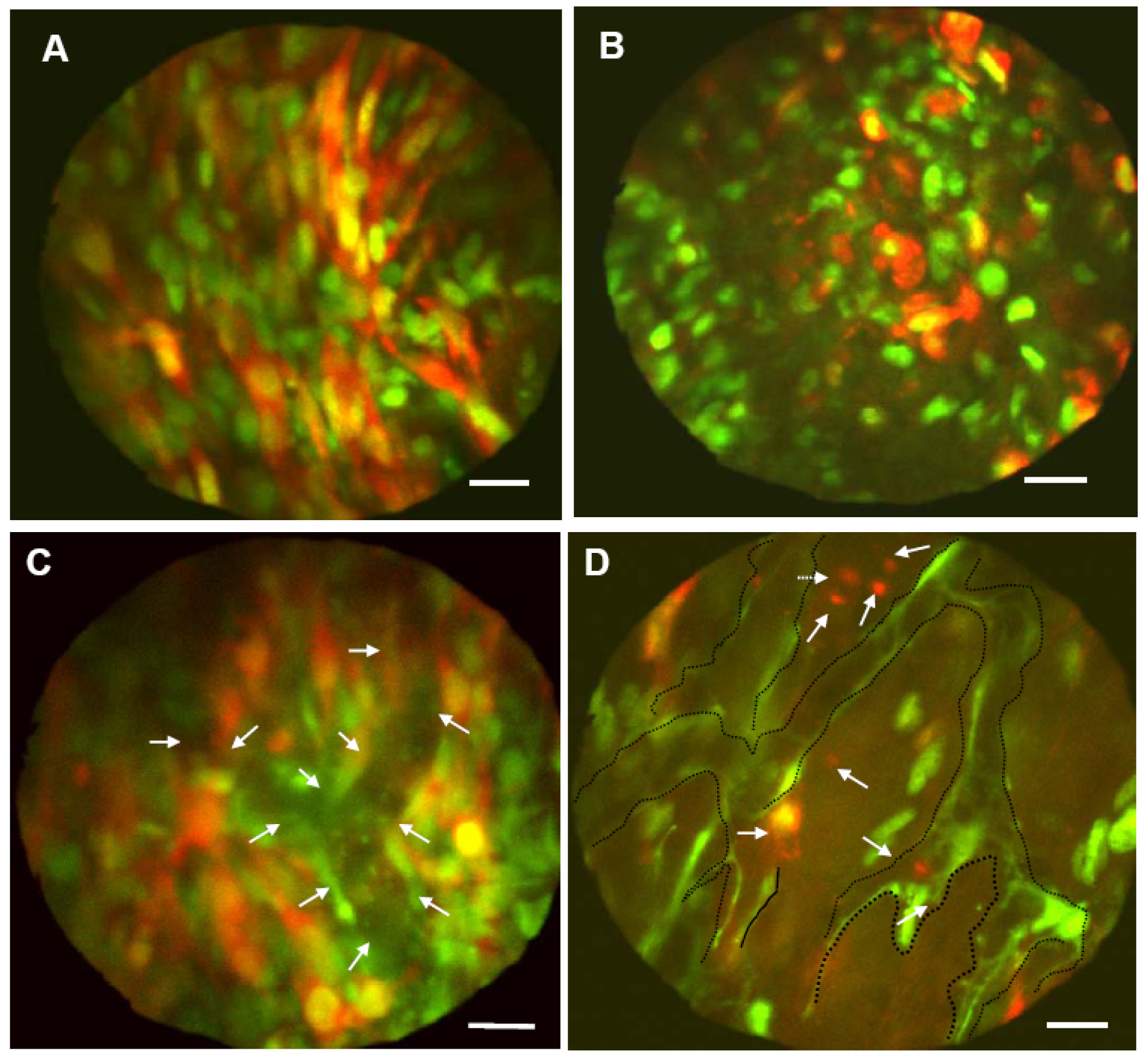
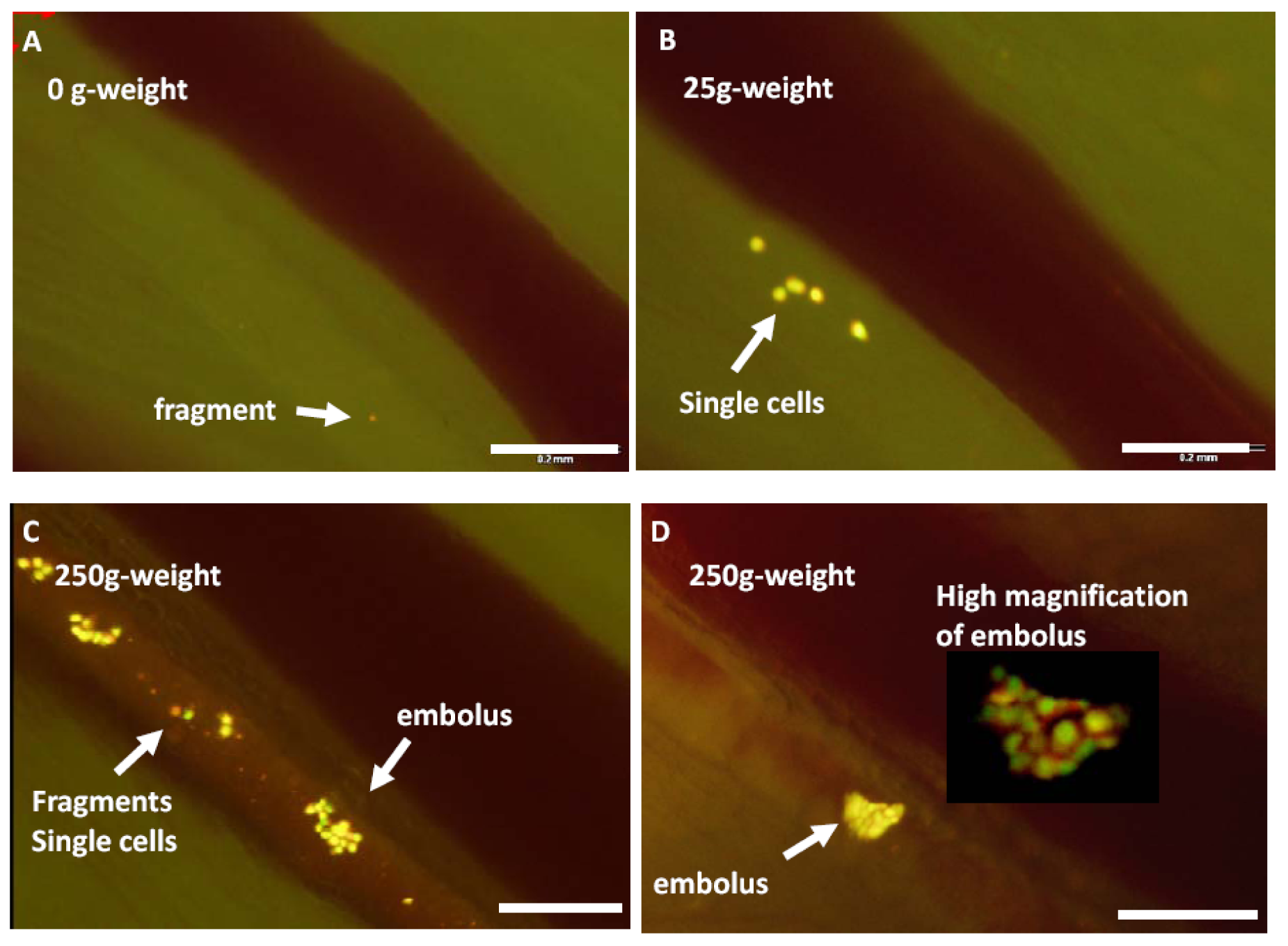
3.2 Imaging Quiescence, Proliferation, and Cell Death in Blood Vessels
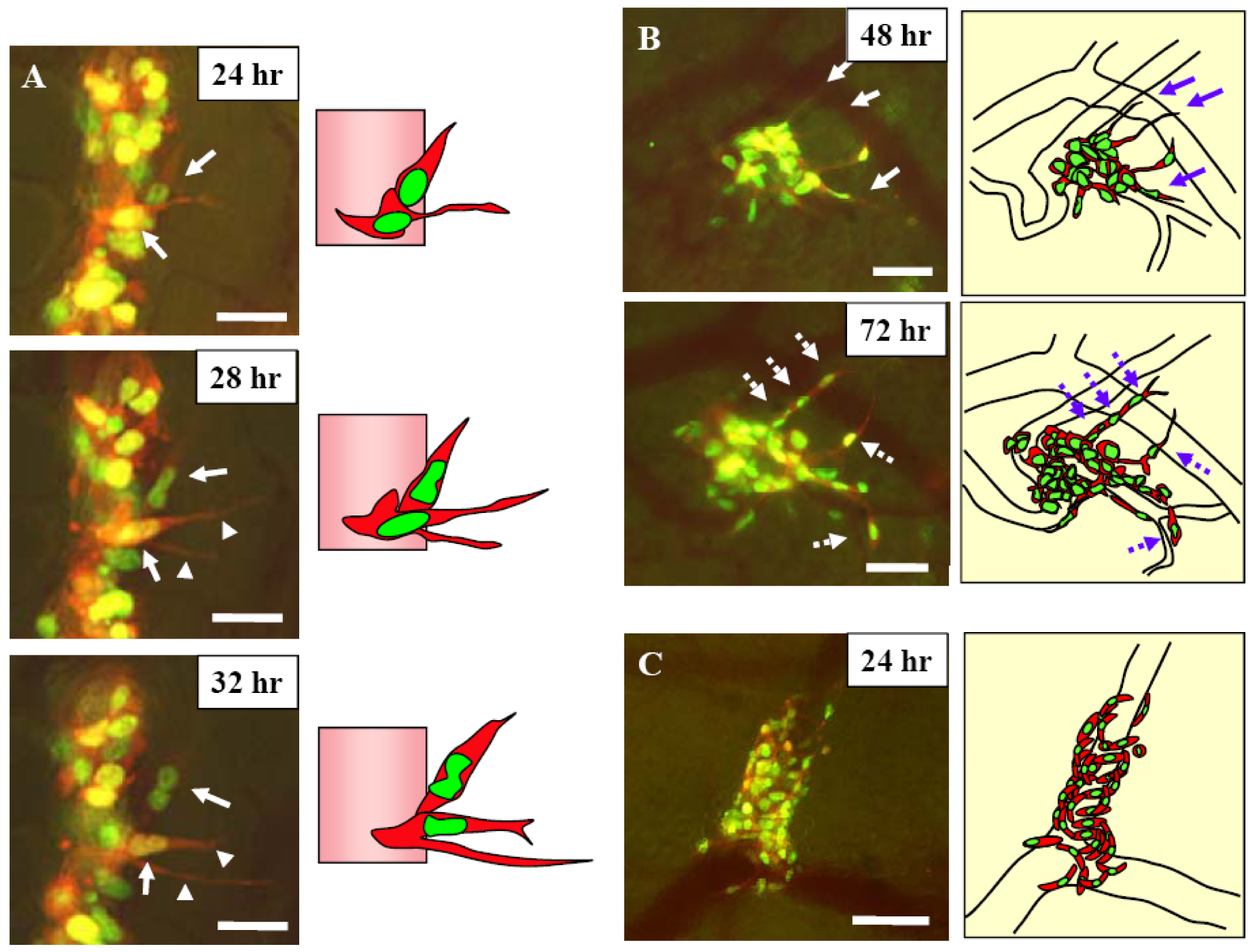
3.3 Imaging of Nuclear-Cytoplasmic Dynamics, Proliferation, and Cell Death of Cancer Cells in the Portal Vein Area
3.4 Imaging trafficking of cancer cells in lymphatic vessels
4. Conclusions
References
- Hoffman, R.M. The multiple uses of fluorescent proteins to visualize cancer in vivo. Nat. Rev. Cancer 2005, 5, 796–806. [Google Scholar]
- Chishima, T.; Miyagi, Y.; Wang, X.; Yamaoka, H.; Shimada, H.; Moossa, A.R.; Hoffman, R.M. Cancer invasion and micrometastasis visualized in live tissue by green fluorescent protein expression. Cancer Res. 1997, 57, 2042–2047. [Google Scholar]
- Yang, M.; Baranov, E.; Jiang, P.; Sun, F-X.; Li, X-M.; Li, L.; Hasegawa, S.; Bouvet, M.; Al-Tuwaijri, M.; Chishima, T.; Shimada, H.; Moossa, A.R.; Penman, S.; Hoffman, R.M. Whole-body optical imaging of green fluorescent protein-expressing tumors and metastases. Proc. Natl. Acad. Sci. USA 2000, 97, 1206–1211. [Google Scholar]
- Yang, M.; Luiken, G.; Baranov, E.; Hoffman, R.M. Facile whole-body imaging of internal fluorescent tumors in mice with an LED flashlight. BioTechniques 2005, 39, 170–172. [Google Scholar]
- Matz, M.V.; Fradkov, A.F.; Labas, Y.A.; Savitsky, A.P.; Zaraisky, A.G.; Markelov, M.L.; Lukyanov, S.A. Fluorescent proteins from nonbioluminescent Anthozoa species. Nat. Biotechnol. 1999, 17, 969–973. [Google Scholar]
- Katz, M.H.; Li, L.; Tsuji, K.; Moossa, A.R.; Katsuoka, K.; Hoffman, R.M.; Bouvet, M. A novel red fluorescent protein orthotopic pancreatic cancer model for the preclinical evaluation of chemotherapeutics. J. Surg. Res. 2003, 113, 151–160. [Google Scholar]
- Yang, M.; Li, L.; Jiang, P.; Moossa, A.R.; Penman, S.; Hoffman, R.M. Dual-color fluorescence imaging distinguishes tumor cells from induced host angiogenic vessels and stromal cells. Proc. Natl. Acad. Sci. USA 2003, 100, 14259–14262. [Google Scholar]
- Shaner, N.C.; Campbell, R.E.; Steinbach, P.A.; Giepmans, B.N.; Palmer, A.E.; Tsien, R.Y. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 2004, 22, 1567–1572. [Google Scholar]
- Shcherbo, D.; Merzlyak, E.M.; Chepurnykh, T.V.; Fradkov, A.F.; Ermakova, G.V.; Solovieva, E.A.; Lukyanov, K.A.; Bogdanova, E.A.; Zaraisky, A.G.; Lukyanov, S.; Chudakov, D.M. Bright far-red fluorescent protein for whole-body imaging. Nat. Methods 2007, 4, 741–746. [Google Scholar]
- Yang, M.; Jiang, P.; Hoffman, R.M. Whole-body subcellular multicolor imaging of tumor-host interaction and drug response in real time. Cancer Res. 2007, 67, 5195–5200. [Google Scholar]
- Folkman, J. Angiogenesis and apoptosis. Semin Cancer Biol 2003, 13, 159–67. [Google Scholar]
- Paget, S. The distribution of secondary growths in cancer of the breast. Lancet 1889, 133, 571–3. [Google Scholar]
- Orimo, A.; Gupta, P.B.; Sgroi, D.C.; Arenzana-Seisdedos, F.; Delaunay, T.; Naeem, R.; Carey, V.J.; Richardson, A.L.; Weinberg, R.A. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 2005, 121, 335–48. [Google Scholar]
- Ferrara, N.; Kerbel, R.S. Angiogenesis as a therapeutic target. Nature 2005, 438, 967–74. [Google Scholar]
- Kerbel, R.S. A cancer therapy resistant to resistance. Nature 1997, 390, 335–6. [Google Scholar]
- Chen, H.X.; Mooney, M.; Boron, M.; Vena, D.; Mosby, K.; Grochow, L. Phase II multicenter trial of bevacizumab plus fluorouracil and leucovorin in patients with advanced refractory colorectal cancer: an NCI Treatment Referral Center Trial TRC-0301. J Clin Oncol 2006, 24, 3354–60. [Google Scholar]
- Ray, P.; De, A.; Min, J.J.; Tsien, R.Y. Gambhir SS Imaging tri-fusion multimodality reporter gene expression in living subjects. Cancer Res. 64, 1323–1330.
- Hoffman, R.M.; Yang, M. Whole-body imaging with fluorescent proteins. Nat. Protocols 2006, 1, 1429–1438. [Google Scholar]
- Lukyanov, K.A.; Chudakov, D.M.; Lukyanov, S.; Verkhusha, V.V. Innovation: photoactivatable fluorescent proteins. Nat. Rev. Mol. Cell. Biol. 2005, 6, 885–891. [Google Scholar]
- Ando, R.; Hama, H.; Yamamoto-Hino, M.; Mizuno, H.; Miyawaki, A. An optical marker based on the UV-induced green-to-red photoconversion of a fluorescent protein. Proc. Natl. Acad. Sci. USA 2002, 99, 12651–12656. [Google Scholar]
- Yamauchi, K.; Yang, M.; Jiang, P.; Xu, M.; Yamamoto, N.; Tsuchiya, H.; Tomita, K.; Moossa, A.R.; Bouvet, M.; Hoffman, R.M. Development of real-time subcellular dynamic multicolor imaging of cancer cell trafficking in live mice with a variable-magnification whole-mouse imaging system. Cancer Res. 2006, 66, 4208–4214. [Google Scholar]
- Condeelis, J.; Segall, J.E. Intravital imaging of cell movement in tumours. Nat. Rev. Cancer 2003, 3, 921–930. [Google Scholar]
- Gross, S.; Piwnica-Worms, D. Spying on cancer: molecular imaging in vivo with genetically encoded reporters. Cancer Cell 2005, 7, 5–15. [Google Scholar]
- Weissleder, R.; Ntziachristos, V. Shedding light onto live molecular targets. Shedding light onto live molecular targets. Nat. Med. 2003, 9, 123–128. [Google Scholar]
- Ntziachristos, V.; Ripoll, J.; Wang, L.V.; Weissleder, R. Looking and listening to light: the evolution of whole-body photonic imaging. Nat. Biotechnol. 2005, 23, 313–320. [Google Scholar]
- Chambers, A.F.; Groom, A.C.; MacDonald, I.C. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer 2002, 2, 563–572. [Google Scholar]
- Yamauchi, K.; Yang, M.; Jiang, P.; Yamamoto, N.; Xu, M.; Amoh, Y.; Tsuji, K.; Bouvet, M.; Tsuchiya, H.; Tomita, K.; Moossa, A.R.; Hoffman, R.M. Real-time in vivo dual-color imaging of intracapillary cancer cell and nucleus deformation and migration. Cancer Res. 2005, 65, 4246–4252. [Google Scholar]
- Yamauchi, K.; Yang, M.; Hayashi, K.; Jiang, P.; Xu, M.; Yamamoto, N.; Tsuchiya, H.; Tomita, K.; Moossa, A.R.; Bouvet, M.; Hoffman, R.M. Induction of intravascular proliferation, extravasation, and colony formation of cancer cells by cyclophosphamide pretreatment of host mice: an opposite effect of chemotherapy. Cancer Res 2008, 65, 516–520. [Google Scholar]
- Tsuji, K.; Yamauchi, K.; Yang, M.; Jiang, P.; Bouvet, M.; Endo, H.; Kanai, Y.; Yamashita, K.; Moossa, A.R.; Hoffman, R.M. Dual-color imaging of nuclear-cytoplasmic dynamics, viability, and proliferation of cancer cells in the portal vein area. Cancer Res 2006, 66, 303–306. [Google Scholar]
- Nathanson, S.D. Insights into the mechanisms of lymph node metastasis. Cancer 2003, 98, 413–423. [Google Scholar]
- Jiang, P.; Yamauchi, K.; Yang, M.; Tsuji, K.; Xu, M.; Maitra, A.; Bouvet, M.; Hoffman, R.M. Tumor cells genetically labeled with GFP in the nucleus and RFP in the cytoplasm for imaging cellular dynamics. Cell Cycle 2006, 5, 1198–1201. [Google Scholar]
- Yamamoto, N.; Jiang, P.; Yang, M.; Xu, M.; Yamauchi, K.; Tsuchiya, H.; Tomita, K.; Wahl, G.M.; Moossa, A.R.; Hoffman, R.M. Cellular dynamics visualized in live cells in vitro and in vivo by differential dual-color nuclear-cytoplasmic fluorescent-protein expression. Cancer Research 2004, 64, 4251–4256. [Google Scholar]
- Okabe, M.; Ikawa, M.; Kominami, K.; Nakanishi, T.; Nishimune, Y. ′Green mice′ as a source of ubiquitous green cells. FEBS Ltrs. 1997, 407, 313–319. [Google Scholar]
- Vintersten, K.; Monetti, C.; Gertsenstein, M.; Zhang, P.; Laszlo, L.; Biechele, S.; Nagy, A. Mouse in red: red fluorescent protein expression in mouse ES cells, embryos, and adult animals. Genesis 2004, 40, 241–246. [Google Scholar]
- Yang, M.; Jiang, P.; Hoffman, R.M. Whole-body subcellular multicolor imaging of tumor-host interaction and drug response in real time. Cancer Res. 2006, 67, 5195–5200. [Google Scholar]
- Hayashi, K.; Jiang, P.; Yamauchi, K.; Yamamoto, N.; Tsuchiya, H.; Tomita, K.; Moossa, A.R.; Bouvet, M.; Hoffman, R.M. Real-time imaging of tumor-cell shedding and trafficking in lymphatic channels. Cancer Res. 2007, 67, 8223–8228. [Google Scholar]
- Sakaue-Sawano, A.; Kurokawa, H.; Morimura, T.; Hanyu, A.; Hama, H.; Osawa, H.; Kashiwagi, S.; Fukami, K.; Miyata, T.; Miyoshi, H.; Imamura, T.; Ogawa, M.; Masai, H.; Miyawaki, A. Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell 2008, 132, 487–498. [Google Scholar]
- Livet, J.; Weissman, T.A.; Kang, H.; Draft, R.W.; Lu, J.; Bennis, R.A.; Sanes, J.R.; Lichtman, J.W. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature 2007, 450, 56–62. [Google Scholar]


© 2008 by MDPI Reproduction is permitted for noncommercial purposes.
Share and Cite
Hoffman, R.M. Imaging In Mice With Fluorescent Proteins: From Macro To Subcellular. Sensors 2008, 8, 1157-1173. https://doi.org/10.3390/s8021157
Hoffman RM. Imaging In Mice With Fluorescent Proteins: From Macro To Subcellular. Sensors. 2008; 8(2):1157-1173. https://doi.org/10.3390/s8021157
Chicago/Turabian StyleHoffman, Robert M. 2008. "Imaging In Mice With Fluorescent Proteins: From Macro To Subcellular" Sensors 8, no. 2: 1157-1173. https://doi.org/10.3390/s8021157



