Optimalization of Poly(neutral red) Coated-wire Electrode for Determination of Citrate in Soft Drinks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Electrode preparation
2.3. Potentiometric measurements
2.4. Potentiometric determination of citrate in soft drinks
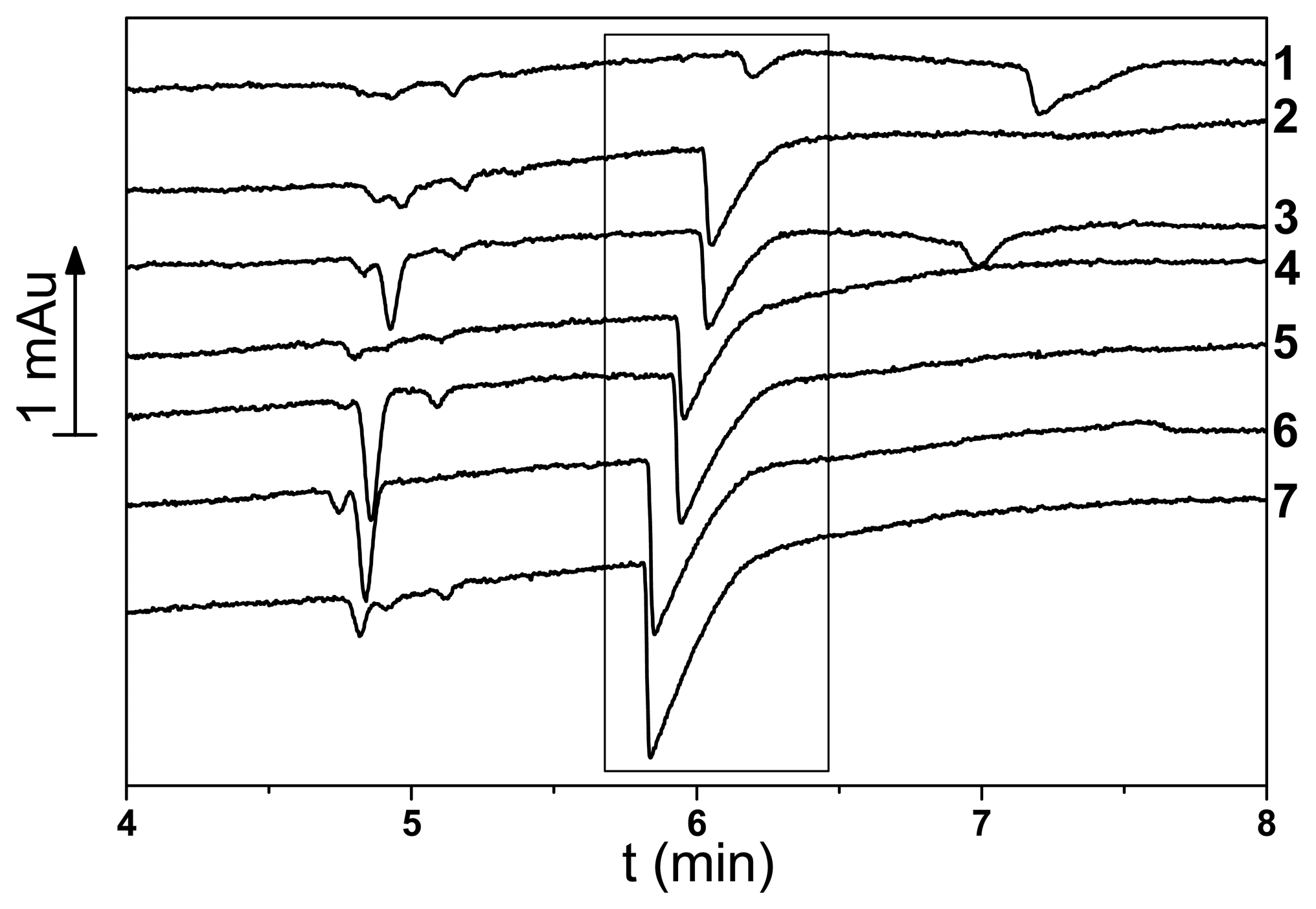
2.5. Determination of citrate content of soft drinks by capillary electrophoresis
3. Results and Discussion
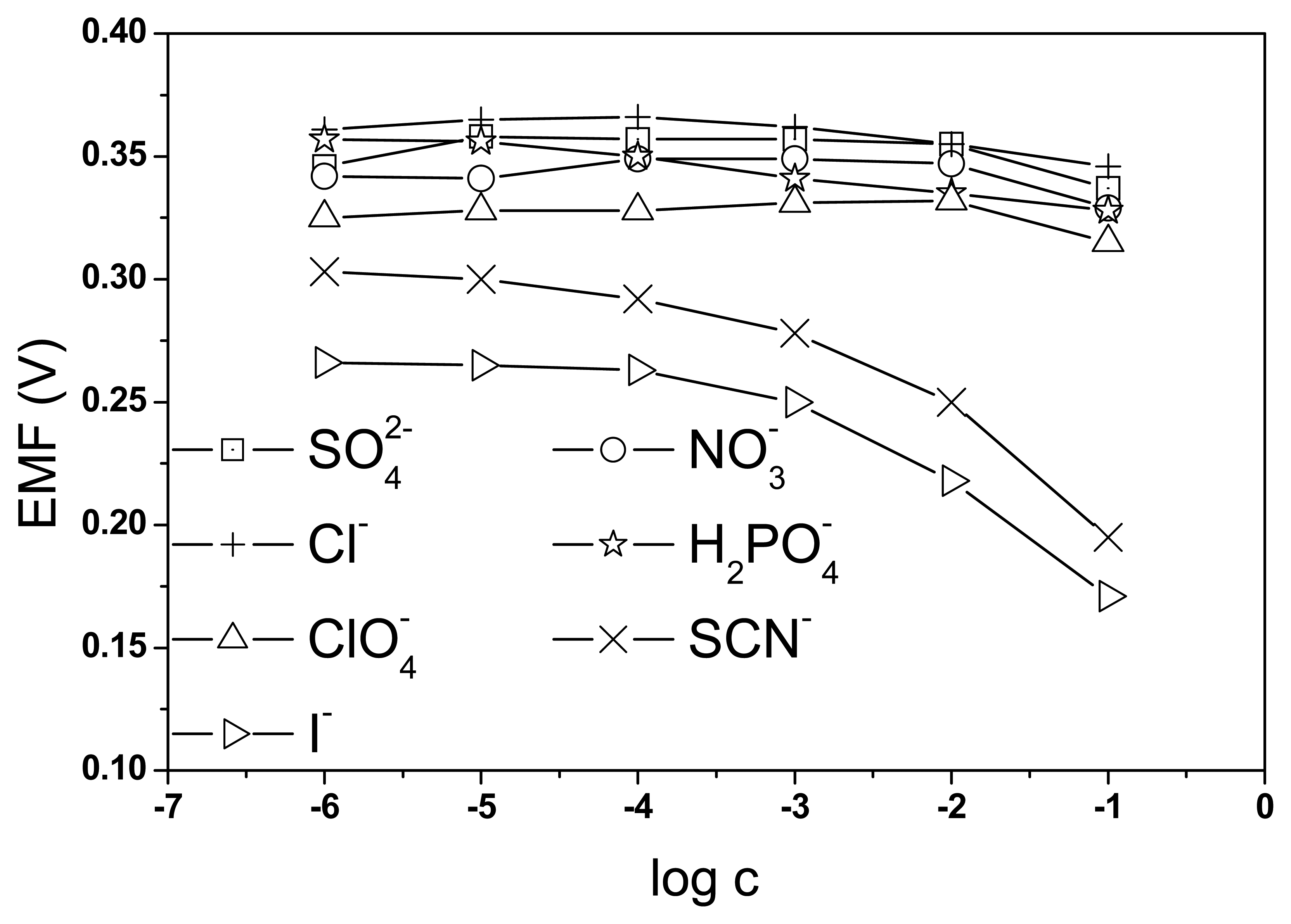
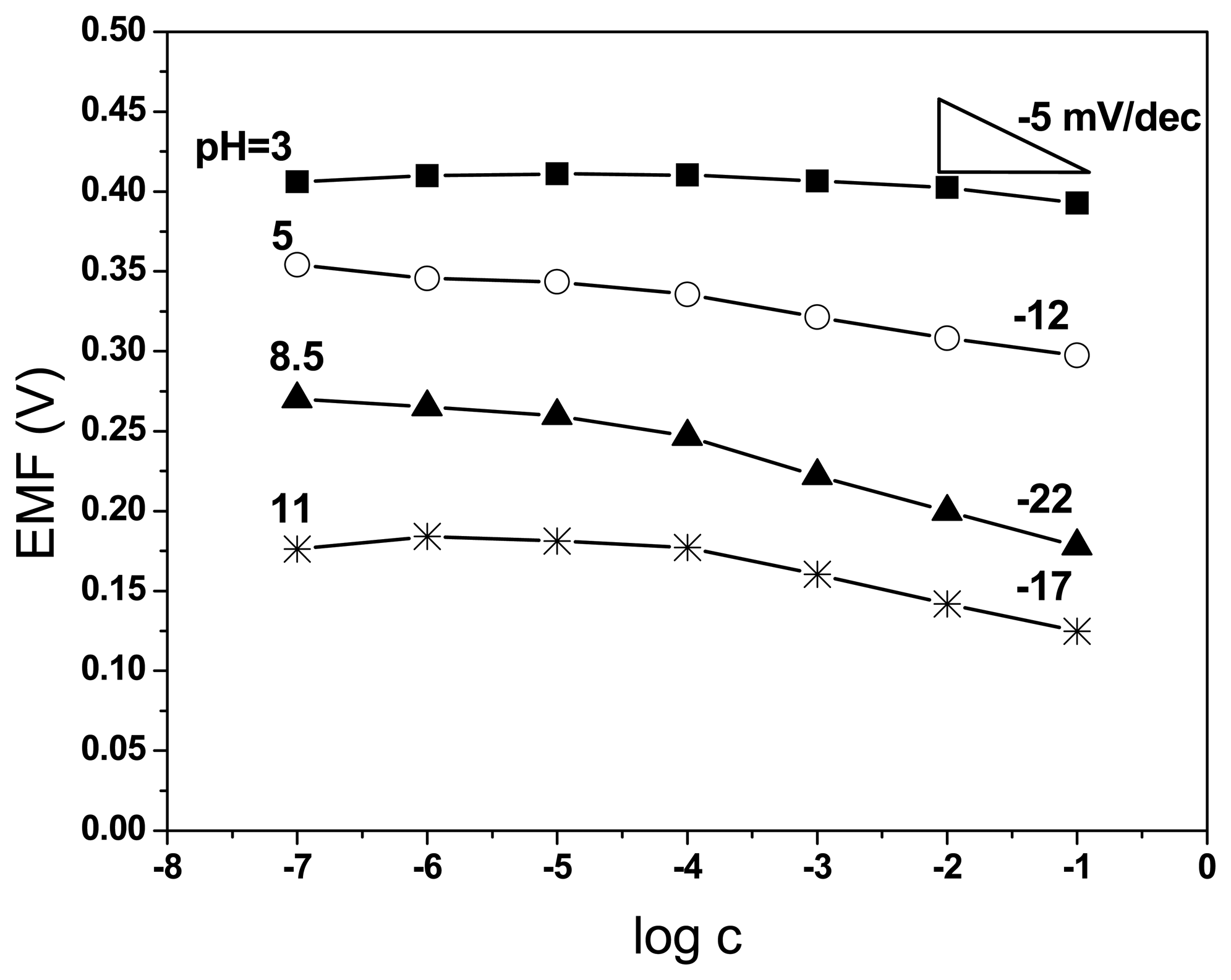
3.1. The choice of appropriate medium for response testing with poly(neutral red) electrodes
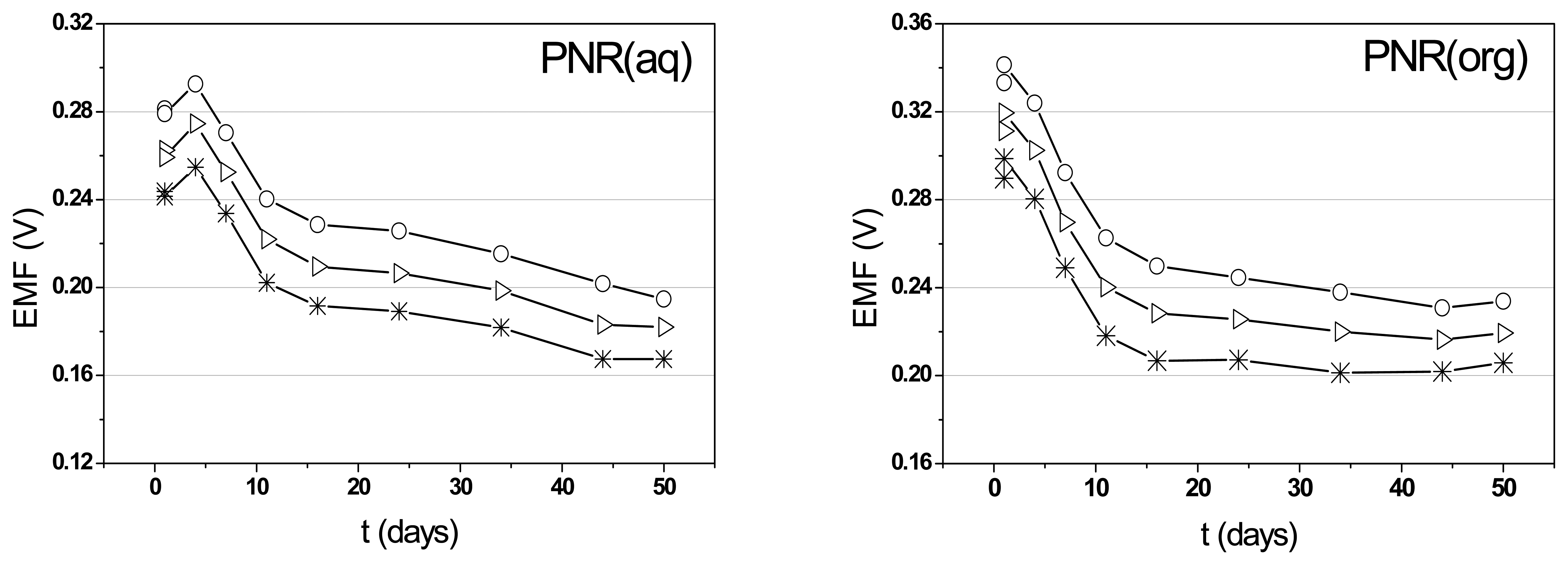
3.2. The characteristic parameters of poly(neutral red) electrodes
3.3. Determination of citrate content of soft drinks with poly(neutral red) electrodes by potentiometric and electrophoresis methods
4. Conclusion
Acknowledgments
References and Notes
- Trojanowicz, M. Application of conducting polymers in chemical analysis. Microchimica Acta. 2003, 143, 75–91. [Google Scholar]
- Skotheim, T. A.; Elsenbaumer, R. L.; Reynolds, J. R. Handbook of Conducting Polymers, Second Edition ed; Marcel Dekker: New York, 1998. [Google Scholar]
- Cadogan, A.; Lewenstam, A.; Ivaska, A. Anionic responses of electrochemically synthesized polypyrrole film. Talanta 1992, 39(6), 617–620. [Google Scholar]
- Bobacka, J.; Ivaska, A.; Lewenstam, A. Potentiometric Ion Sensor Based on Conducting Polymers. Electroanalysis 2003, 15(5-6), 366–374. [Google Scholar]
- Migdalski, J.; Blaz, T.; Lewenstam, A. Electrochemical deposition and properties of polypyrrole films doped with calcion ligands. Anal. Chim. Acta. 1999, 395, 35. [Google Scholar]
- Maksymiuk, K.; Nybäck, A.-S.; Bobacka, J.; Ivaska, A.; Lewenstam, A. Metallic and non-metallic redox response of CPs. J. Electroanal. Chem. 1997, 430, 243–252. [Google Scholar]
- Daunert, S.; Wallace, S.; Florido, A.; Bachas, L.G. Anion-selective electrodes based on electropolymerized porphyrin films. Anal. Chem. 1991, 63(17), 1676–1679. [Google Scholar]
- Blair, T.L.; Allen, J. R.; Daunert, S.; Bachas, L. G. Potentiometric and fiber optic sensors for pH based on an electropolymerized cobalt porfyrin. Anal. Chem. 1993, 65, 2155–2158. [Google Scholar]
- Dong, S.; Sun, Z.; Lu, Z. Chloride chemical sensor based on an organic conducting polypyrrole polymer. Analyst 1988, 113, 1525–1528. [Google Scholar]
- Bobacka, J. Conducting Polymer-Based Solid-State Ion-Selective Electrodes. Electroanalysis 2006, 18(1), 7–18. [Google Scholar]
- Karami, H.; Mousavi, M.F. Dodecyl benzene sulfonate anion-selective electrode based on polyaniline-coated electrode. Talanta 2004, 63, 743–749. [Google Scholar]
- Luque-Peréz, E.; Ríos, A.; Valcárcel, M. Flow-injection spectrophotometric determination of citric acid in beverages based on a photochemical reaction. Anal. Chim. Acta. 1998, 366, 231–240. [Google Scholar]
- Moreno-Cid, A.; Yebra, M.C.; Santos, X. Flow injection determinations of citric acid: a review. Talanta 2004, 63, 509–514. [Google Scholar]
- Lima, J.L. F. C.; Delerue-Matos, C.; Vaz, M. C. V. F.; Silva, J. Determination of citric acid in soft drinks using flow injection with potentiometric detection. Fresenius J. Anal. Chem. 1999, 364, 266–269. [Google Scholar]
- Morales, M.L.; Ferreira, R.; González, A. G.; Troncoso, A. M. Simultaneous determination of organic acids and sweeteners in soft drinks by ion-exclusion HPLC. J. Sep. Sci. 2001, 24, 879–884. [Google Scholar]
- Matsumo, K.; Tsukatani, T. Simultaneous quantitation of citrate and isocitrate in citrus juice by a flow-injection method based on the use of enzyme reactors. Anal. Chim. Acta. 1996, 321, 157–164. [Google Scholar]
- Hattori, H.; Hoshino, M.; Wakii, T.; Yuchi, A. Effects of Two-Phase Reactions on Composition and Potential Response of Zirconium(IV)-Tetraphenylporphyrin Complexes as Carrier for Citrate-Selective Electrode. Anal. Chem. 2004, 76, 5056–5062. [Google Scholar]
- Broncová, G.; Shishkanova, T.V.; Matějka, P.; Volf, R.; Král, V. Citrate selectivity of poly(neutral red; electropolymerized films. Anal. Chim. Acta. 2004, 511, 197–205. [Google Scholar]
- Breznová, H.; Volf, R.; Král, V.; Sessler, J.L.; Try, A. C.; Shishkanova, T. V. Monomer and polymer quinoxaline derivatives for cationic recognition. Anal. Bioanal. Chem. 2003, 375, 1193–1198. [Google Scholar]
- Koryta, J.; Štulík, K. Iontově-selektivní elektrody; Academia: Prague, 1984. [Google Scholar]
- Tsagatakis, J.K.; Chaniotakis, N. A.; Jurkschat, K. 190. Multiorganyltin Compounds. Designing a novel phosphate-selective carrier. Helv. Chim. Acta. 1994, 77, 2191–2196. [Google Scholar]
- Chaniotakis, N.A.; Park, S. B.; Meyerhoff, M. E. Salicylate-Selective Membrane Electrode Based on Tin(IV) Tetrafeylporphyrin. Anal. Chem. 1989, 61, 566–570. [Google Scholar]
- Shahrokhian, S.; Hamzehloei, A.; Bagherzadeh, M. Chromium(III) Porphyrin as a Selective Ionophore in a Salicylate-Selective Membrane Electrode. Anal. Chem. 2002, 74, 3312–3320. [Google Scholar]
- Lingenfelter, P. T. Electronic and ionic sensitivity of bilayers of poly(3,4-ethylenedioxythiophene) and poly(dibenzo-18-crown-6). Thesis, Laboratory of Analytical Chemistry, Abo Akademi University, Turku, Finland, 2000. [Google Scholar]
- Mousavi, Z.; Bobacka, J.; Lewenstam, A.; Ivaska, A. Response mechanism of potentiometric Ag+ sensor based on PEDOT doped with silver hexabromocarborane. J. Electroanal. Chem. 2006, 593, 219–226. [Google Scholar]
- Koziel, K.; Lapkowiski, M.; Lefrant, S. Spectroelectrochemical investigation of the memory effect in polyaniline. Synthetic Metals 1995, 69, 217–218. [Google Scholar]
- Odin, C.; Nechtschein, M. Memory effect on conducting polymers; Electrochemical and ESP studies on polyaniline. Synthetic Metals 1991, 41-43, 2943–2946. [Google Scholar]
- Bobacka, J.; Ivaska, A.; Grzeszczuk, M. Electrochemical study of poly(3-octylthiophene) film electrodes. I. Electrolyte effects on the voltammetric characteristics of the polymer. Synthetic Metals 1991, 44(1), 9–19. [Google Scholar]
- Dong, S.; Sun, Z.; Lu, Z. A new kind of chemical sensor based on CP film. J. Chem. Soc., Chem. Commun. 1988, 993–995. [Google Scholar]
- Wiskur, S.L.; Ait-Haddou, H.; Lavigne, J. J.; Anslyn, E. V. Teaching old indicators new tricks. Acc. Chem. Res. 2001, 34, 963–972. [Google Scholar]
- Esteves, V.I.; Lima, S. S. F.; Lima, D. L. D.; Duarte, A. C. Using capillary electrophoresis for the determination of organic acids in Port wine. Anal. Chim. Acta. 2004, 513, 163–167. [Google Scholar]
- Horie, H.; Yamauchi, Y.; Kohota, K. Analysis of organic anions in tea infusions using capillary electrophoresis. J. Chromatogr. A. 1998, 817, 139–144. [Google Scholar]
- Saavedra, L.; García, A.; Barbas, C. Development and validation of a capillary electrophoresis method for direct measurement of isocitric, citric, tartaric and malic acids in orange juice. J. Chromatography A. 2000, 881, 395–401. [Google Scholar]


| Sample | Composition of soft drinks |
|---|---|
| Gatorade - orange | Water, glucose syrup, saccharose, citric acid, sodium citrate, NaCl, KH2PO4, emulsifier E414 and E445, MgCO3, aroma, dyes E104, E110 |
| Hello juice - apple | Drinking water, sugar, apple juice, apple aroma, citric acid, preservatives E202 and E211, dyes E104 and E133 |
| Joe's baby cola | Drinking water, sugar, phosphoric acid, citric acid, E211, natural aroma, dye caramel, caffeine, CO2 |
| Kofola | Water, syrup KOFO (syrup, sugar, water, caramel, dye E150d, citric acid, NaCl, herbal extract, licorice extract, E211, caffeine), CO2 |
| Mountain Dew lemon | - Water, sugar, citric acid, E211, caffeine, stabilizer E414 and E445, sodium citrate, aroma, ascorbic acid, dye E102 (tartrazine yellow), CO2 |
| Poděbradka - lemon | Mineral water (HCO3-, Cl-, Na+, Ca2+), sugar, citric acid, natural aromatic compounds, E211, CO2 |
| Zón - lemon | Drinking water; sugar, citric acid, ascorbic acid, E202, E211, CO2. |
| Electrode | PNR(aq) | PNR(org) |
|---|---|---|
| Slope S (mV decade-1) | -18.7 ± 3.0 | -20.1 ± 2.2 |
| Potential constant K (mV) | 244.6 ± 25.6 | 238.8 ± 12.4 |
| PDL (M) | (2 – 6) ×10-6 | (2 – 5) × 10-6 |
| Sample | PNR(aq) electrodes | PNR(org) electrodes | CE |
|---|---|---|---|
| Gatorade - orange | 3.26 ± 0.76 | 3.01 ± 0.25 | 3.43 ± 0.19 |
| Hello juice - apple | 4.77 ± 0.78 | 5.09 ± 0.55 | 3.93 ± 0.25 |
| Joe's baby cola | 0.48 ± 0.06 | 0.40 ± 0.04 | 0.38 ± 0.02 |
| Kofola | 1.22 ± 0.15 | 1.45 ± 0.11 | 1.51 ± 0.11 |
| Mountain Dew - lemon | 1.13 ± 0.23 | 1.74 ± 0.27 | 1.37 ± 0.08 |
| Poděbradka - lemon | 2.61 ± 0.40 | 2.50 ± 0.23 | 2.82 ± 0.15 |
| Zón - lemon | 1.56 ± 0.25 | 1.37 ± 0.15 | 1.60 ± 0.11 |
© 2008 by MDPI Reproduction is permitted for noncommercial purposes.
Share and Cite
Broncová, G.; Shishkanova, T.V.; Krondak, M.; Volf, R.; Král, V. Optimalization of Poly(neutral red) Coated-wire Electrode for Determination of Citrate in Soft Drinks. Sensors 2008, 8, 594-606. https://doi.org/10.3390/s8020594
Broncová G, Shishkanova TV, Krondak M, Volf R, Král V. Optimalization of Poly(neutral red) Coated-wire Electrode for Determination of Citrate in Soft Drinks. Sensors. 2008; 8(2):594-606. https://doi.org/10.3390/s8020594
Chicago/Turabian StyleBroncová, Gabriela, Tatiana V. Shishkanova, Martin Krondak, Radko Volf, and Vladimír Král. 2008. "Optimalization of Poly(neutral red) Coated-wire Electrode for Determination of Citrate in Soft Drinks" Sensors 8, no. 2: 594-606. https://doi.org/10.3390/s8020594




