Ground Based Ultraviolet Remote Sensing of Volcanic Gas Plumes
Abstract
:1. Introduction
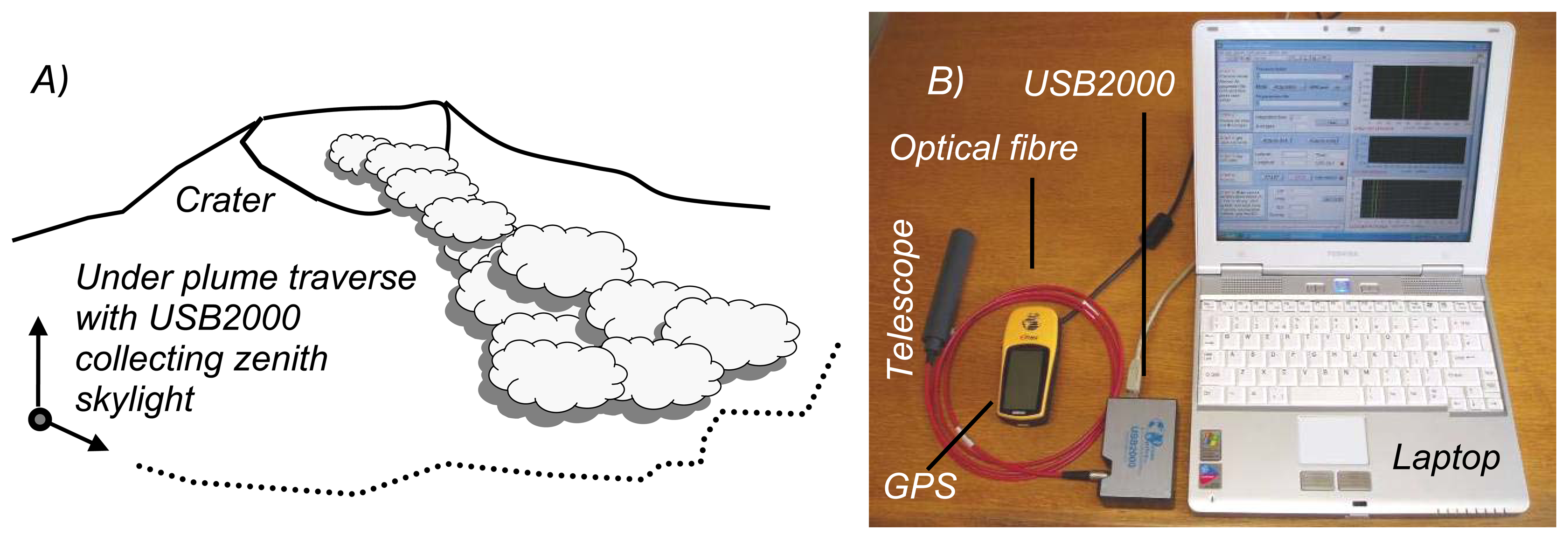
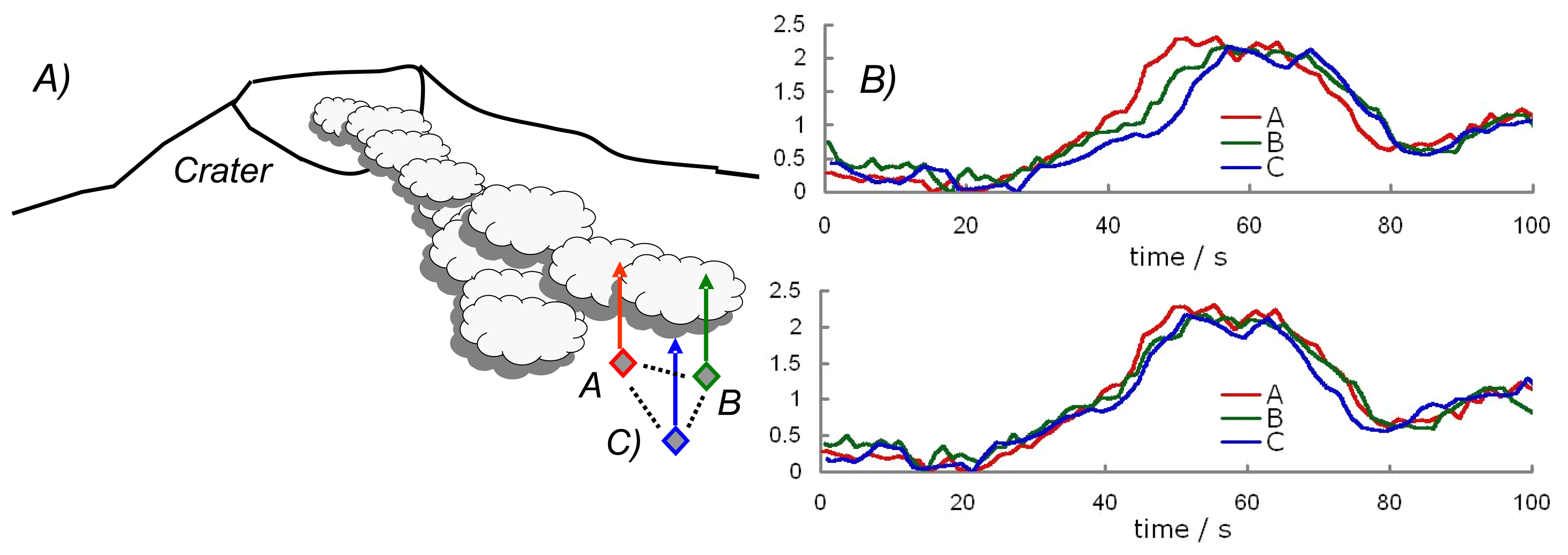
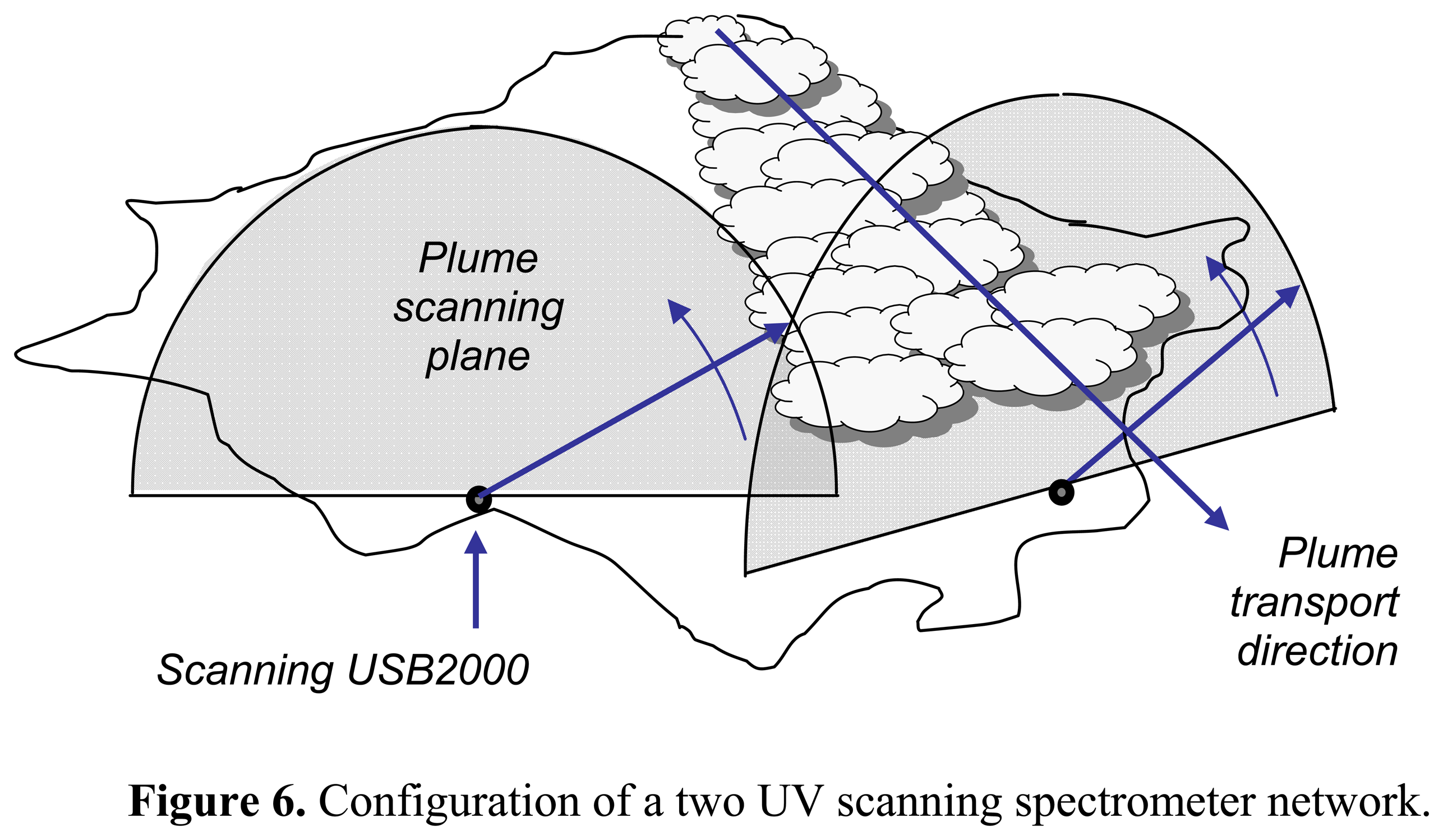
2. Ground based volcano remote sensing
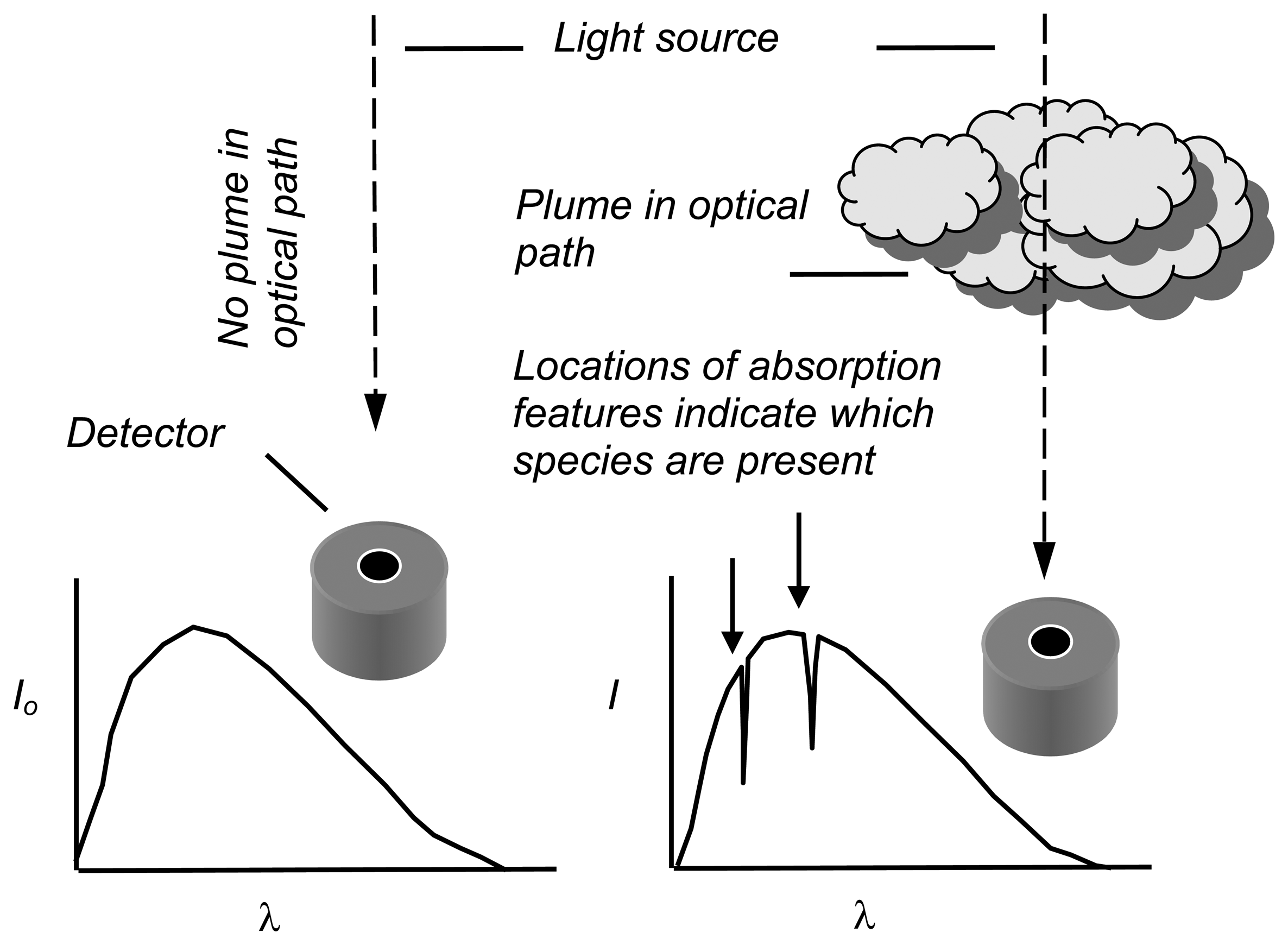
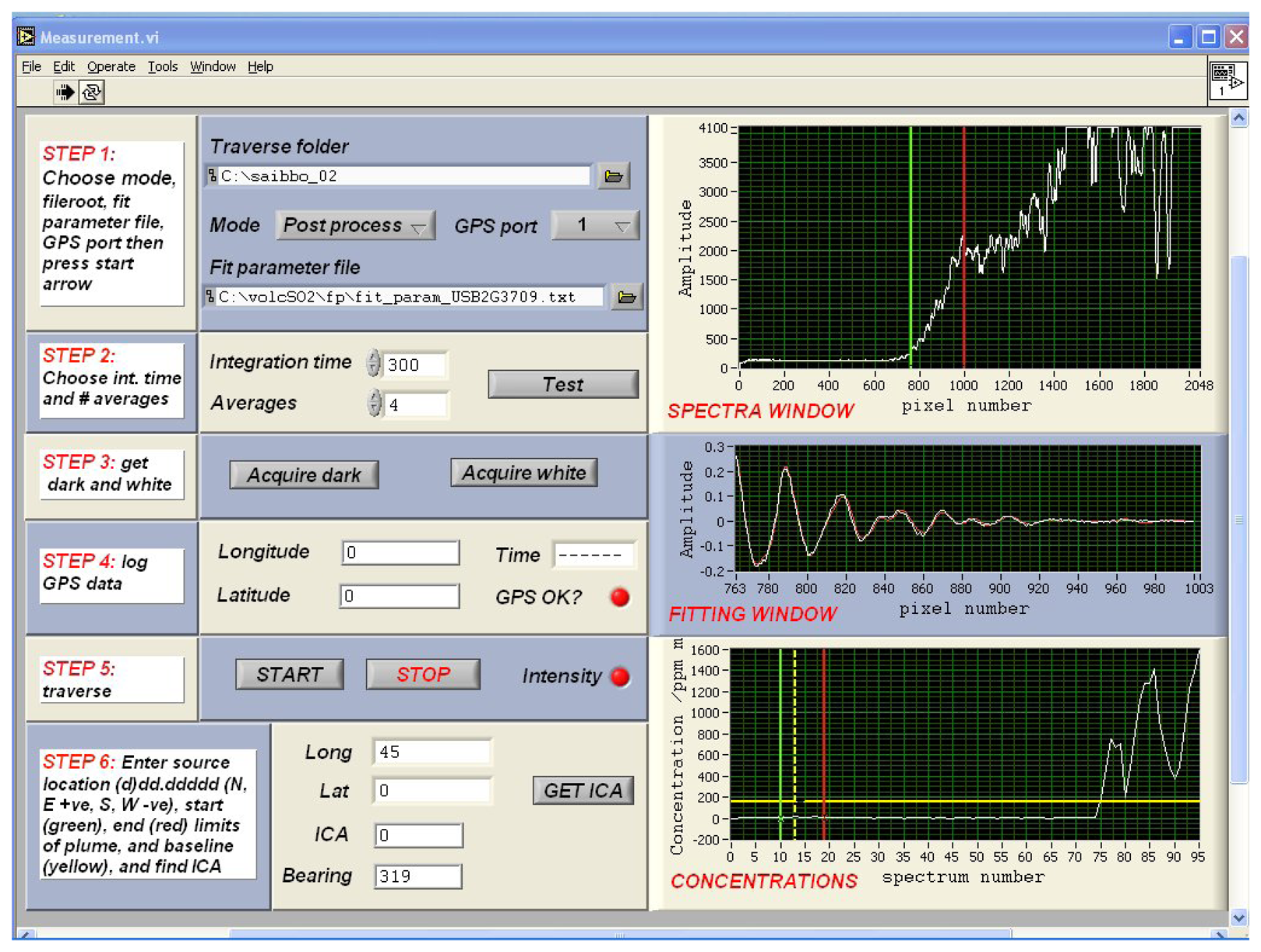
3. Ultraviolet spectroscopy of volcanic plumes
4. Concluding remarks
Acknowledgments
References and Notes
- Aiuppa, A.; Federico, C.; Giudice, G.; Gurrieri, S. Anomalous magmatic degassing prior to the 5th April 2003 paroxysm on Stromboli. Geophys. Res. Lett. 2004, 31, L14607. [Google Scholar]
- Aiuppa, A.; Federico, C.; Franco, A.; Giudice, G.; Gurrieri, S.; Inguaggiato, S.; Liuzzo, M.; McGonigle, A.J.S.; Valenza, M. Emission of bromine and iodine from Mount Etna volcano. Geochem. Geophys. Geosyst. 2005a, 6, Q08008. [Google Scholar]
- Aiuppa, A.; Inguaggiato, S.; McGonigle, A.J.S.; O'Dwyer, M.; Oppenheimer, C.; Padgett, M.J.; Rouwet, D.; Valenza, M. H2S fluxes from Mt. Etna, Stromboli, and Vulcano (Italy) and implications for the sulfur budget at volcanoes. Geochim. Cosmochim. Acta 2005b, 69, 1861–1871. [Google Scholar]
- Aiuppa, A.; Federico, C.; Giudice, G.; Gurrieri, S. Chemical mapping of a fumarolic field: La Fossa Crater, Vulcano Island (Aeolian Islands, Italy). Geophys. Res. Lett. 2005c, 32, L13309. [Google Scholar]
- Aiuppa, A.; Federico, C.; Giudice, G.; Gurrieri, S.; Liuzzo, M.; Shinohara, H.; Favara, R.; Valenza, M. Rates of carbon dioxide plume degassing from Mount Etna volcano. J. Geophys. Res. 2006, 111, B09207. [Google Scholar]
- Aiuppa, A.; Moretti, R.; Federico, C.; Giudice, G.; Gurrieri, S.; Liuzzo, M.; Papale, P.; Shinohara, H.; Valenza, M. Forecasting Etna eruptions by real-time observation of volcanic gas composition. Geology 2007, 35, 1115–1118. [Google Scholar]
- Allard, P.; Burton, M.; Muré, F. Spectroscopic evidence for a lava fountain driven by previously accumulated magmatic gas. Nature 2005, 433, 407–410. [Google Scholar]
- Andres, R.J.; Kasgnoc, A.D. A time averaged inventory of subaerial volcanic sulfur emissions. J. Geophys. Res. 1998, 103(D19), 25251–25261. [Google Scholar]
- Andres, R.J.; Schmid, J.W. The effects of volcanic ash on COSPEC measurements. J. Volcanol. Geotherm Res. 2001, 108, 237–244. [Google Scholar]
- Bagnato, E.; Aiuppa, A.; Parello, F.; Calabrese, C.; D'Alessandro, W.; Mather, T.A.; McGonigle, A.J.S.; Pyle, D.M.; Wängberg, I. Degassing of gaseous (elemental and reactive) and particulate mercury from Mount Etna volcano (Southern Italy). Atmos. Environ. 2007, 41, 7377–7388. [Google Scholar]
- Bluth, G.J.S.; Schnetzler, C.C.; Krueger, A.J.; Walter, L.S. The contribution of explosive volcanism to global atmospheric sulfur-dioxide concentrations. Nature 1993, 366, 327–329. [Google Scholar]
- Bluth, G.J.S.; Shannon, J.M.; Watson, I.M.; Prata, A.J.; Realmuto, V.J. Development of an ultraviolet digital camera for volcanic SO2 imaging. J. Volcanol. Geotherm. Res. 2007, 161, 47–56. [Google Scholar]
- Bobrowski, N.; Hönninger, G.; Galle, B.; Platt, U. Detection of bromine monoxide in a volcanic plume. Nature 2003, 423, 273–276. [Google Scholar]
- Bobrowski, N.; Hönninger; Lohberger, F.; Platt, U. IDOAS: A new monitoring technique to study the 2D distribution of volcanic gas emissions. J. Volcanol. Geotherm. Res. 2006, 150, 329–338. [Google Scholar]
- Bobrowski, N.; von Glasow, R.; Aiuppa, A.; Inguaggiato, S.; Louban, I.; Ibrahim, O.W.; Platt, U. Reactive halogen chemistry in volcanic plumes. J. Geophys. Res. 2007, 112, D06311. [Google Scholar]
- Burton, M.; Allard, P.; Muré, F.; La Spina, A. Magmatic gas composition reveals the source depth of slug-driven strombolian explosive activity. Science 2007, 317, 227–230. [Google Scholar]
- Caltabiano, T.; Romano, R.; Budetta, G. SO2 flux measurements at Mount Etna (Sicily). J. Geophys. Res. 1984, 99. [Google Scholar]
- Casadevall, T.J. The 1989– 1990 eruption of Redoubt Volcano, Alaska, impacts on aircraft operations. J. Volcanol. Geotherm. Res. 1994, 62, 301–316. [Google Scholar]
- Courtillot, V.; Olson, P. Mantle plumes link magnetic superchrons to phanerozoic mass depletion events. Earth Planet Sci. Lett. 2007, 260, 495–504. [Google Scholar]
- Daag, A.S.; Tubianosa, B.S.; Newhall, C.G.; Tuňgol, N.M.; Javier, D.; Dolan, M.T.; Reyes, P.J.D.; Arboleda, R.A.; Martinez, M.L.; Regalado, T.M. Monitoring sulphur dioxide emission at Mount Pinatubo. In Fire and mud: eruptions and lahars of Mount Pinatubo Philippines; Newhall, C.G., Punongbayan, R.S., Eds.; University of Washington Press: Seattle, 1996; pp. 409–414. [Google Scholar]
- Delgado-Granados, H.; Cárdenas González, L.; Piedad Sánchez, N. Sulphur dioxide emissions from Popocatépetl volcano (Mexico): case study of a high-emission rate, passively degassing erupting volcano. J. Volcanol. Geotherm. Res. 2001, 108, 107–120. [Google Scholar]
- Delmelle, P.; Stix, J.; Baxter, P.J.; Garcia-Alvarez, J.; Barquero, J. Atmospheric dispersion, environmental effects and potential health hazard associated with the low-altitude gas plume of Masaya volcano, Nicaragua. Bull. Volcanol. 2002, 64, 423–434. [Google Scholar]
- Edmonds, M.; Oppenheimer, C.; Pyle, D.M.; Herd, R.A.; Thompson, G. SO2 emissions from Soufrière Hills volcano and their relationship to conduit permeability, hydrothermal interaction and degassing regime. J. Volcanol. Geotherm. Res. 2003a, 124, 23–43. [Google Scholar]
- Edmonds, M.; Herd, R.A.; Galle, B.; Oppenheimer, C.M. Automated, high time-resolution measurements of SO2 flux at Soufrière Hills Volcano, Montserrat. Bull. Volcanol. 2003b, 65, 578–586. [Google Scholar]
- Edmonds, M.; Herd, R.A. A volcanic degassing event at the explosive-effusive transition. Geophys. Res. Lett. 2007, 34, L21310. [Google Scholar]
- Farrar, C.D.; Sorey, M.L.; Evans, W.C.; Howle, J.F.; Kerr, B.D.; Kennedy, B.M.; King, C.Y.; Southon, J.R. Forest-killing diffuse CO2 emission at Mammoth Mountain as a sign of magmatic unrest. Nature 1995, 376, 675–678. [Google Scholar]
- Fischer, T.P.; Morrissey, M.M.; Calvache, V.M.L.; Gòmez, M.D.; Torres, C.R.; Stix, J.; Williams, S.N. Correlations between SO2 flux and long period seismicity at Galeras volcano. Nature 1994, 368, 135–137. [Google Scholar]
- Francis, P.; Burton, M.R.; Oppenheimer, C. Remote measurements of volcanic gas compositions by solar occultation spectroscopy. Nature 1998, 396, 567–570. [Google Scholar]
- Galle, B.; Oppenheimer, C.; Geyer, A.; McGonigle, A.J.S.; Edmonds, M.; Horrocks, L.A. A miniaturised UV spectrometer for remote sensing of SO2 fluxes: a new tool for volcano surveillance. J. Volcanol. Geotherm. Res. 2003, 119, 241–254. [Google Scholar]
- Gerlach, T.M. Volcanic sources of tropospheric ozone-depleting trace gases. Geochem. Geophys. Geosyst. 2004, 5, Q09007. [Google Scholar]
- Graf, H.F.; Feichter, J.; Langmann, B. Volcanic sulfur emissions: estimates of source strength and its contribution to the global sulfate distribution. J. Geophys. Res. 1997, 102(D9), 10727–10738. [Google Scholar]
- Halmer, M.M.; Schmincke, H.U.; Graf, H.F. The annual volcanic as input to the atmosphere, in particular into the stratosphere: a global data set for the past 100 years. 2002, 115, 511–528. [Google Scholar]
- Hernández, P.A.; Notsu, K.; Salazar, J.M.; Mori, T.; Natale, G.; Okada, H.; Virgili, G.; Shimoike, Y.; Sato, M.; Pérez, N.M. Carbon dioxide degassing by advective flow from Usu volcano, Japan. Science 2001, 292, 83–86. [Google Scholar]
- Horton, K.A.; Williams-Jones, G.; Garbeil, H.; Elias, T.; Sutton, A.J.; Mouginis-Mark, P.; Porter, J.N.; Clegg, S. Real-time measurement of volcanic SO2 emissions: validation of a new UV correlation spectrometer (FLYSPEC). Bull. Volcanol. 2006, 68, 323–327. [Google Scholar]
- Iwashita, K.; Asaka, T.; Nishikawa, H.; Kondoh, T.; Tahara, T. Vegetation biomass change of the Bosoh Peninsula Impacted by the volcano fumes from the Miyakejima. Advances in Space Research 2006, 37, 734–740. [Google Scholar]
- Kazahaya, K.; Shinohara, H.; Uto, K.; Odai, M.; Nalkahori, Y.; Mori, H.; Iino, H.; Miyashita, M.; Hirabayashi, J. Gigantic SO2 emission from Miyakejima volcano, Japan, caused by caldera collapse. Geology 2004, 32, 425–428. [Google Scholar]
- Krotkov, N.A.; Carn, S.A.; Krueger, A.J.; Bhartia, P.K.; Yang, K. Band residual difference algorithm for retrieval of SO2 from the aura Ozone Monitoring Instrument (OMI). IEEE Trans. Geosci. Remote Sens. 2006, 44, 1259–1266. [Google Scholar]
- Krueger, A.J. Sighting of El Chichón sulphur dioxide clouds with the Nimbus 7 Total Ozone Mapping Spectrometer Science. Science 1983, 220, 1377–1379. [Google Scholar]
- Lee, C.; Kim, Y.J.; Tanimoto, H.; Bobrowski, N.; Platt, U.; Mori, T.; Yamamoto, K.; Hong, C.S. High ClO and ozone depletion observed in the plume of Sakurajima volcano, Japan. Geophys. Res. Lett. 2005, 32, L21809. [Google Scholar]
- Leonardi, S.; Gresta, S.; Mulargia, F. Searching for a significant correlation between volcanic tremor amplitude and SO2 emissions at Mount Etna volcano, Sicily. Geophys. J. Int. 2000, 141, 832–834. [Google Scholar]
- Malinconico, L.L. Fluctuations in SO2 emission during recent eruptions of Etna. Nature 1979, 278, 43–45. [Google Scholar]
- Mather, T.A.; Pyle, D.M.; Tsanev, V.I.; McGonigle, A.J.S.; Oppenheimer, C.; Allen, A.G. A reassessment of current volcanic emissions from the Central American arc with specific examples from Nicaragua. J. Volcanol. Geotherm. Res. 2006, 149, 297–311. [Google Scholar]
- McCormick, M.P.; Thomason, L.W.; Trepte, C.R. Atmospheric effects of the Mt. Pinatubo eruption. Nature 1995, 373, 399–404. [Google Scholar]
- McGee, K.A. The structure, dynamics and chemical composition of non-eruptive plumes from Mt. St. Helens, 1980-88. J. Volcanol. Geotherm. Res. 1992, 51, 269–282. [Google Scholar]
- McGonigle, A.J.S.; Oppenheimer, C.; Galle, B.; Mather, T.A.; Pyle, D.M. Walking traverse and scanning DOAS measurements of volcanic gas emission rates. Geophys. Res. Lett. 2002, 29(20), 1985. [Google Scholar]
- McGonigle, A.J.S.; Oppenheimer, C.; Hayes, A.R.; Galle, B.; Edmonds, M.; Caltabiano, T.; Salerno, G.; Burton, M.; Mather, T. A. Sulphur dioxide flux measurements at Mount Etna, Vulcano and Stromboli measured with an automated scanning static ultraviolet spectrometer. J. Geophys. Res. 2003, 108(B9), 2455. [Google Scholar]
- McGonigle, A.J.S.; Delmelle, P.; Oppenheimer, C.; Tsanev, V.I.; Delfosse, T.; Williams-Jones, G.; Horton, K.; Mather, T.A. SO2 depletion in tropospheric volcanic plumes. Geophys. Res. Lett. 2004, 31, L13201. [Google Scholar]
- McGonigle, A.J.S.; Hilton, D.R.; Fischer, T.P.; Oppenheimer, C. Plume velocity determination for volcanic SO2 flux measurements. Geophys. Res. Lett. 2005, 32, L11302. [Google Scholar]
- McGonigle, A.J.S. Measurement of volcanic SO2 fluxes with differential optical absorption spectroscopy. J. Volcanol. Geotherm. Res. 2007, 162, 111–122. [Google Scholar]
- McGonigle, A.J.S.; Aiuppa, A.; Giudice, G.; Tamburello, G.; Hodson, A.J.; Gurrieri, S. Unmanned aerial vehicle measurements of volcanic carbon dioxide fluxes. Geophys. Res. Lett. 2008, in press. [Google Scholar] [CrossRef]
- Mori, T.; Mori, T.; Kazahaya, K.; Ohwada, M.; Hirabayashi, J.; Yoshikawa, S. Effect of UV scattering on SO2 emission rate measurements. Geophys. Res. Lett. 2006, 33, L17315. [Google Scholar]
- Mori, T.; Burton, M. The SO2 camera: A simple, fast and cheap method for ground-based imaging of SO2 in volcanic plumes. Geophys. Res. Lett. 2006, 33, L24804. [Google Scholar]
- Notsu, K.; Mori, T.; Igarashi, G.; Tohjima, Y.; Wakita, H. Infrared spectral radiometer: A new tool for remote measurement of SO2 of volcanic gas. Geochemical Journal 1993, 27, 361–366. [Google Scholar]
- O'Dwyer, M.; Padgett, M.J.; McGonigle, A.J.S.; Oppenheimer, C.; Inguaggiato, S. Real-time measurement of volcanic H2S and SO2 concentrations by UV spectroscopy. Geophys. Res. Lett. 2003, 30(12), 1652. [Google Scholar]
- Oppenheimer, C.; Francis, P.; Stix, J. Depletion rates of sulfur dioxide in tropospheric volcanic plumes. Geophys. Res. Lett. 1998, 25, 2671–2674. [Google Scholar]
- Oppenheimer, C. Climatic, environmental and human consequences of the largest known historic eruption: Tambora volcano (Indonesia) 1815. Prog. Phys. Geogr. 2003, 27, 230–259. [Google Scholar]
- Oppenheimer, C.; Kyle, P.R.; Tsanev, V.I.; McGonigle, A.J.S.; Mather, T.A.; Sweeney, D. Mt. Erebus, the largest point source of NO2 in Antarctica. Atmos. Environ. 2005, 39, 6000–6006. [Google Scholar]
- Oppenheimer, C.; Tsanev, V.I.; Braban, C.F.; Cox, R.A.; Adams, J.W.; Aiuppa, A.; Bobrowski, N.; Delmelle, P.; Barclay, J.; McGonigle, A.J.S. BrO formation in volcanic plumes. Geochim. Cosmochim. Acta 2006a, 70, 2935–2941. [Google Scholar]
- Oppenheimer, C.; Bani, P.; Calkins, J.A.; Burton, M.R.; Sawyer, G.M. Rapid FTIR sensing of volcanic gases released by Strombolian explosions at Yasur volcano, Vanuatu. Appl. Phys. B 2006b, 85, 453–460. [Google Scholar]
- Robock, A. Volcanic eruptions and climate. Rev. Geophys. 2000, 38, 191–219. [Google Scholar]
- Shinohara, H. A new technique to estimate volcanic gas composition: plume measurements with a portable multi-sensor system. J. Volcanol. Geotherm. Res. 2005, 143, 319–333. [Google Scholar]
- Small, C.; Naumann, T. The global distribution of human population and recent volcanism. Environ. Hazards 2001, 3, 93–109. [Google Scholar]
- Stoiber, R.E.; Malinconico, L.L.; Williams, S.N. Use of the correlation spectrometer at volcanoes. In Forecasting Volcanic Events; Tazieff, H., Sabroux, J.C., Eds.; Elsevier: Amsterdam, 1983; pp. 425–444. [Google Scholar]
- Stoiber, R.E.; Williams, S.N.; Huebert, B. Annual contribution of sulfur-dioxide to the atmosphere by volcanos. J. Volcanol Geotherm. Res. 1987, 33, 1–8. [Google Scholar]
- Sutton, A.J.; Elias, T.; Gerlach, T.M.; Stokes, J.B. Implications for eruptive processes as indicated by sulfur dioxide emissions from Kilauea Volcano, Hawaii, 1979-1997. J. Volcanol. Geotherm. Res. 2001, 108, 283–302. [Google Scholar]
- Symonds, R.B.; Rose, W.I.; Bluth, G.J.S.; Gerlach, T.M. Volcanic gas studies—Methods, results, and applications. Rev. Mineral. 1994, 30, 1–66. [Google Scholar]
- Thordarson, T.; Self, S.; Oskarsson, N.; Hulsebosch, T. Sulfur, chlorine, and fluorine degassing and atmospheric loading by the 1783-1784 AD Laki (Skaftár fires) eruption in Iceland. Bull. Volcanol. 1996, 58, 205–225. [Google Scholar]
- Wallace, P.J. Volcanic SO2 emissions and the abundance and distribution of exsolved gas in magma bodies. J. Volcanol. Geotherm Res. 2001, 108, 85–106. [Google Scholar]
- Watson, I.M.; Oppenheimer, C.; Voight, B.; Francis, P.W.; Clarke, A.; Stix, J.; Miller, A.; Pyle, D.M.; Burton, M.R.; Young, S.R.; Norton, G.; Loughlin, S.; Darroux, B. MVO Staff The relationship between degassing and ground deformation at Soufrière Hills Volcano, Montserrat. J. Volcanol. Geotherm. Res. 2000, 98, 117–126. [Google Scholar]
- Weibring, P.; Edner, H.; Svanberg, S.; Cecchi, G.; Pantani, L.; Ferrara, R.; Caltabiano, T. Monitoring of volcanic sulphur dioxide emissions using differential absorption lidar (DIAL), differential optical absorption spectroscopy (DOAS) and correlation spectroscopy (COSPEC). Appl. Phys. B 1998, 67, 419–426. [Google Scholar]
- Williams-Jones, G.; Horton, K.; Elias, T.; Garbeil, H.; Mouginis-Mark, P.J.; Sutton, A.J.; Harris, A.J.L. Accurately measuring volcanic plume velocity with multiple UV spectrometers. Bull. Volcanol. 2006, 68, 328–332. [Google Scholar]
- Witham, C.S. Volcanic disasters and incidents: A new database. J. Volcanol. Geotherm. Res. 2005, 148, 191–233. [Google Scholar]


© 2008 by MDPI Reproduction is permitted for noncommercial purposes.
Share and Cite
Kantzas, E.P.; McGonigle, A.J.S. Ground Based Ultraviolet Remote Sensing of Volcanic Gas Plumes. Sensors 2008, 8, 1559-1574. https://doi.org/10.3390/s8031559
Kantzas EP, McGonigle AJS. Ground Based Ultraviolet Remote Sensing of Volcanic Gas Plumes. Sensors. 2008; 8(3):1559-1574. https://doi.org/10.3390/s8031559
Chicago/Turabian StyleKantzas, Euripides P., and Andrew J. S. McGonigle. 2008. "Ground Based Ultraviolet Remote Sensing of Volcanic Gas Plumes" Sensors 8, no. 3: 1559-1574. https://doi.org/10.3390/s8031559



