Sensitive Detection of Haloperidol and Hydroxyzine at Multi-Walled Carbon Nanotubes-Modified Glassy Carbon Electrodes
Abstract
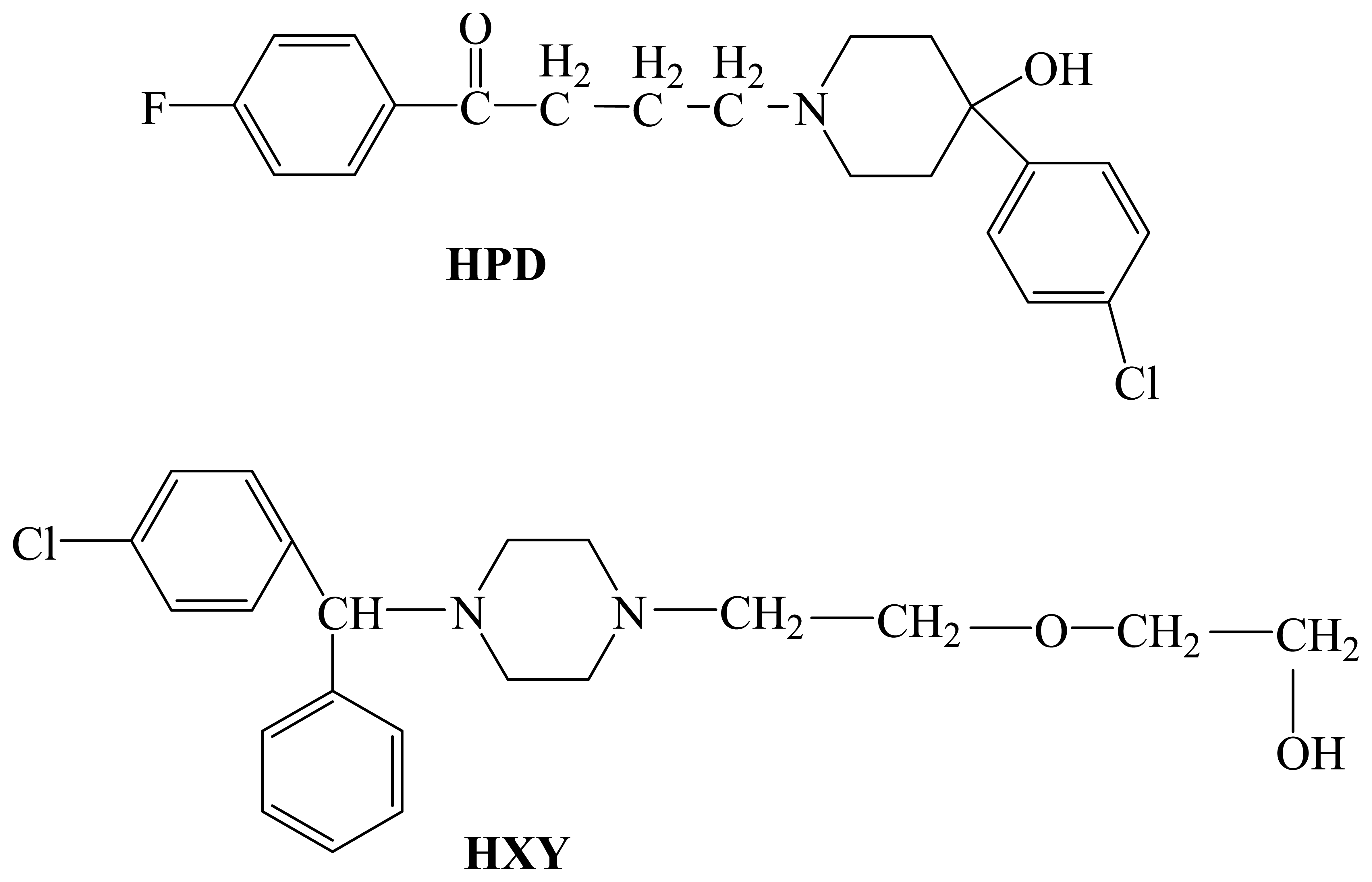
:1. Introduction
2. Experimental
2.1 Reagents
2.2 Apparatus
2.3 Fabrication of MWNTs/GC modified electrode
2.4 Procedure
3. Results and Discussion
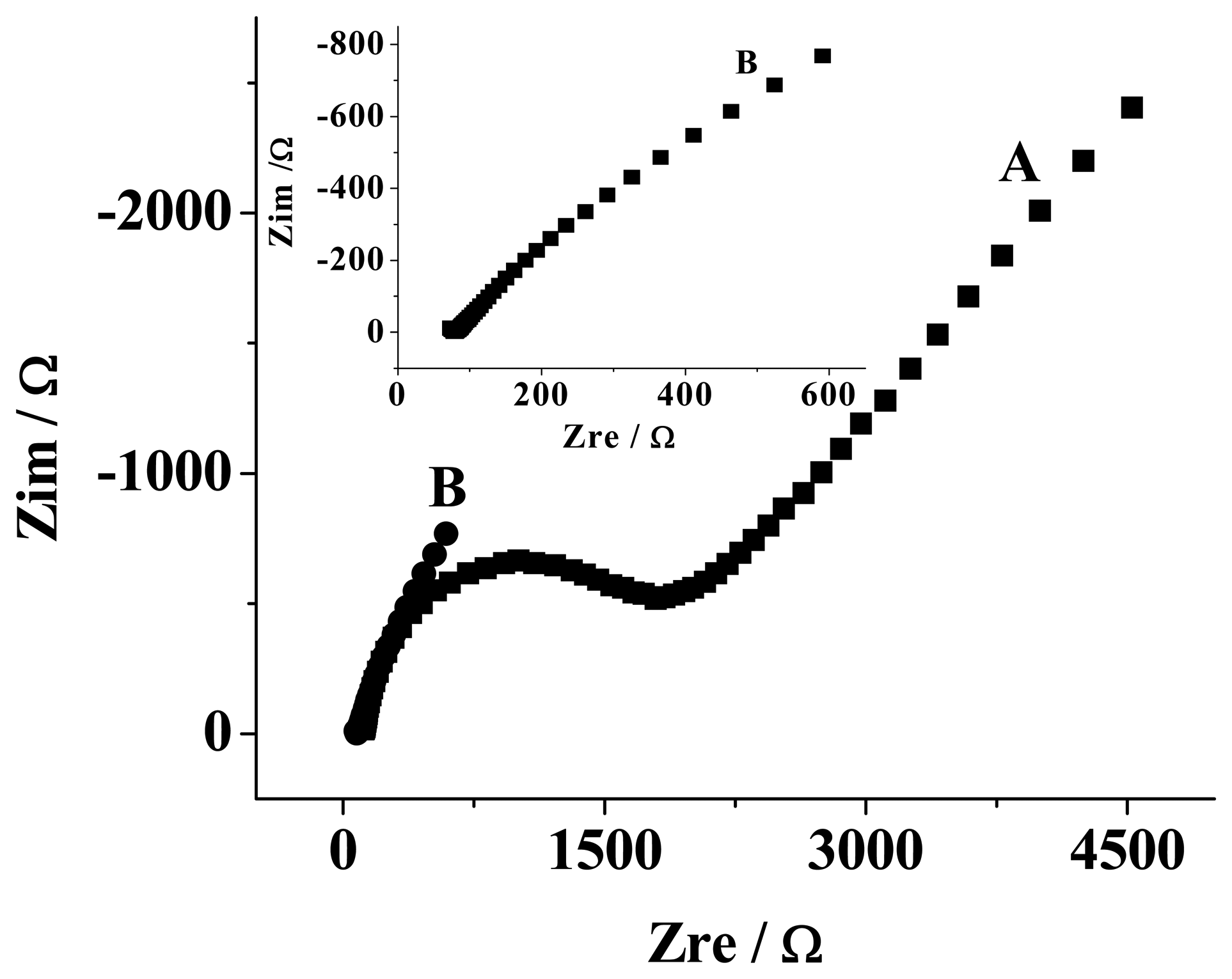
3.1 Electrochemical characterization of the MWNTs/GC modified electrode
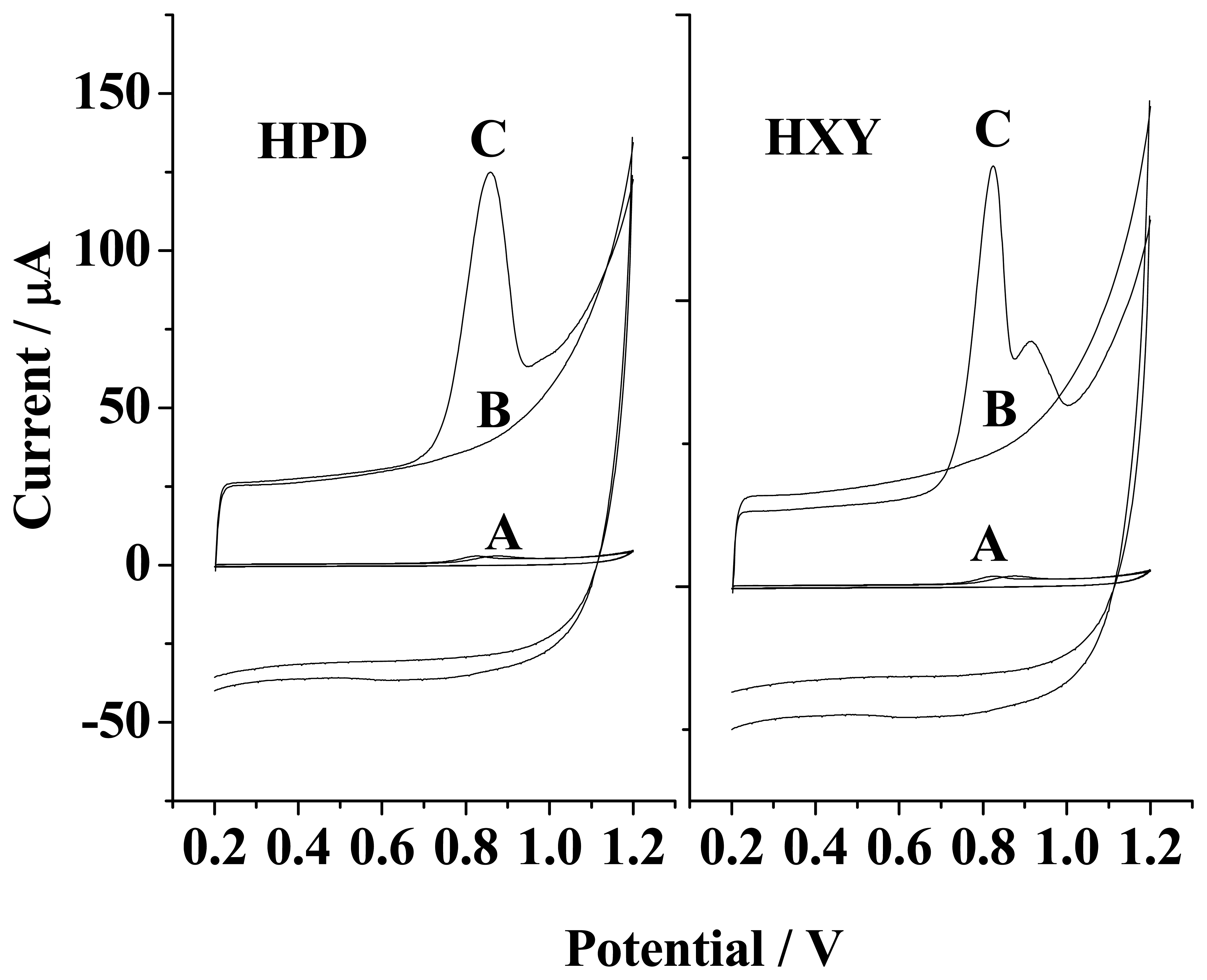
Cyclic voltammograms of HPD and HXY at MWNTs/GC modified electrode
3.2 Effect of scan rate
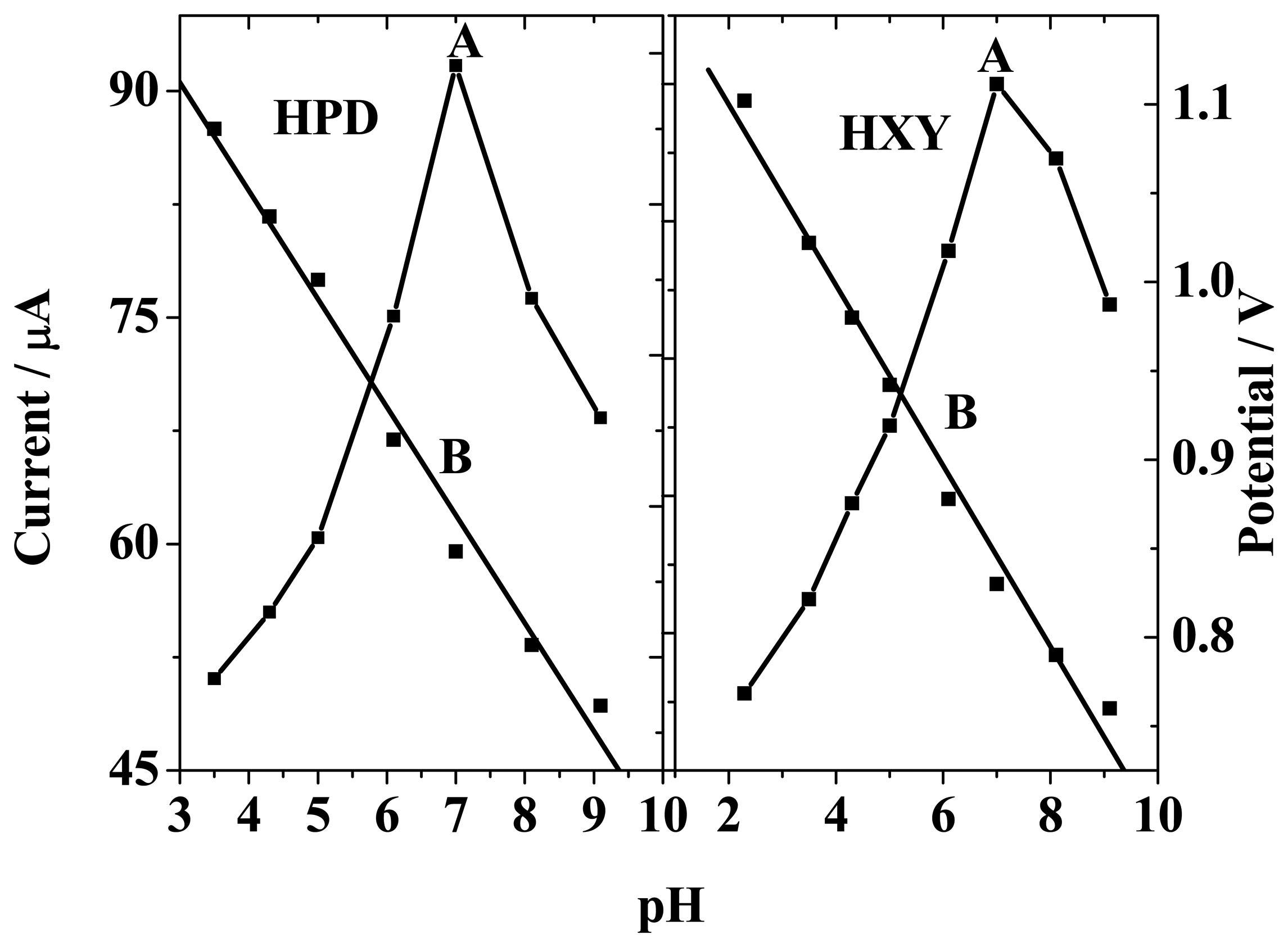
3.3 Optimization of the analytical conditions. Influence of pH and buffer solution
3.4 Influence of accumulation time and potential
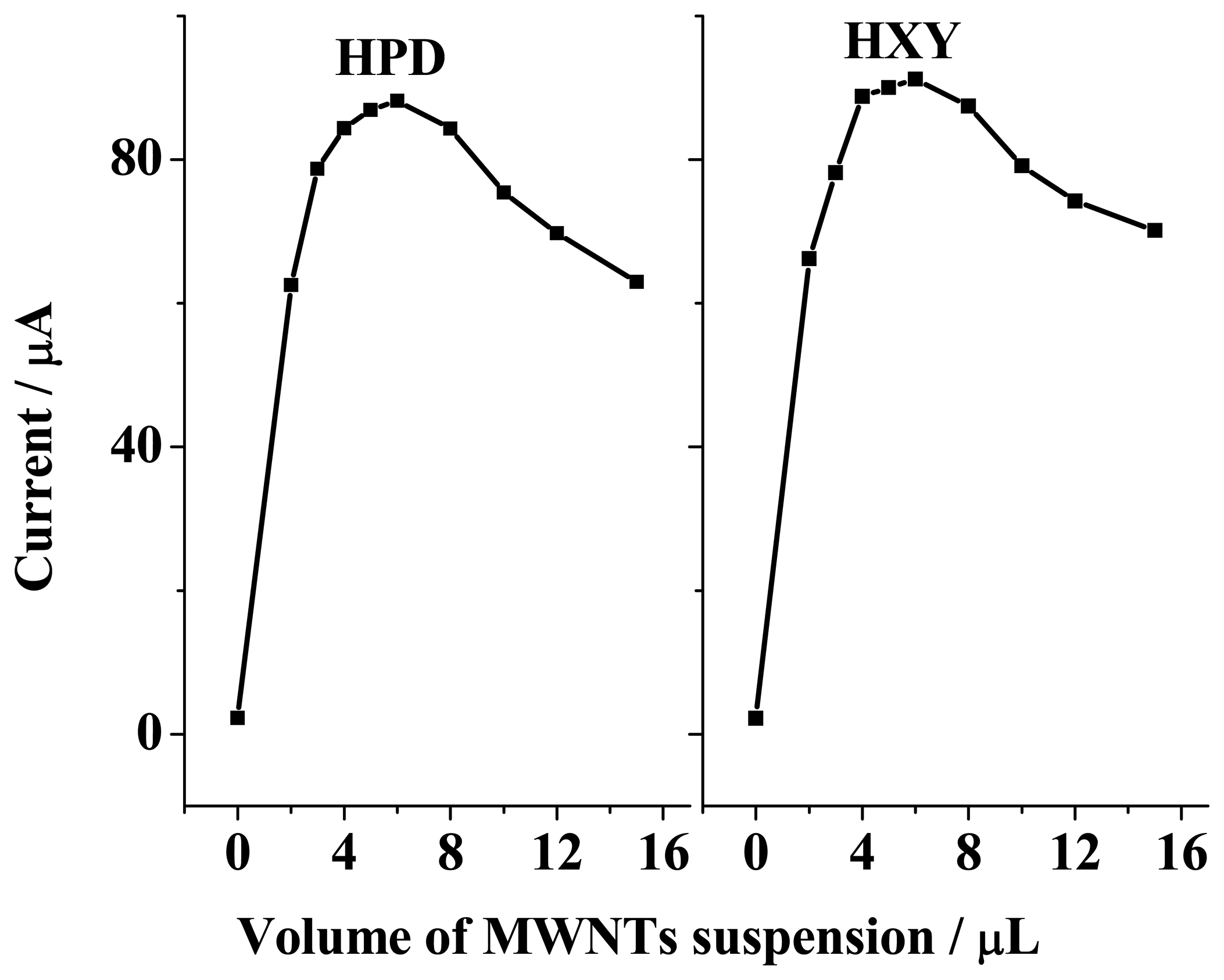
3.5 Influence of amount of MWNTs suspension
3.6 Calibration curve
3.7 Regeneration, reproducibility, stability and selectivity of MWNTs/GC modified electrode
3.8 Applications
4. Conclusions
Acknowledgments
References
- Peng, T.Z.; Yang, Z.P.; Lu, R.S. Voltammetric Measurement of Haloperidol Following Adsorptive Accumulation at Glassy Carbon Electrodes. Talanta 1991, 38, 741–745. [Google Scholar]
- Beltagi, A.M.; Abdallah, O.M.; Ghoneim, M.M. Development of a Voltammetric Procedure for Assay of the Antihistamine Drug Hydroxyzine at a Glassy Carbon Electrode: Quantification and Pharmacokinetic Studies. Talanta 2007, in press. [Google Scholar]
- Chinese Pharmacopoeia Commission. Chinese Pharmacopoeia; Chemical Industry Press: Beijing, 2005; Volume 469. [Google Scholar]
- Walter, S.; Baue, S.; Roots, J.; Brockmoller, J. Quantification of the Antipsychotics Flupentioxol and Haloperidol in Human Serum by High–Performance Liquid Chromatography with Ultraviolet Detection. J. Chromatogr. B. 1998, 720, 231–237. [Google Scholar]
- Boberic-Borojevic, D.; Radulovic, D.; Ivanovic, D.; Ristic, P. Simultaneous Assay of Ephedrine Hydrochloride, Theophylline, Papaverine Hydrochloride and Hydrozyzine Hydrochloride in Tablets Using RP-LC. J. Pharm. Biomed. Anal. 1999, 21, 15–22. [Google Scholar]
- Ho, Y.H.; Wu, H.L.; Wu, S.M.; Chen, S.H.; Kou, H.S. Quantitative Enantiometric Analysis of Chlorcyclizine, Hydroxyzine, and Meclizine by Capillary Electrophoresis. Anal. Bioanal. Chem. 2003, 376, 859–863. [Google Scholar]
- Fujii, T.; Hatanaka, K.; Sato, G.; Yasui, Y.; Arimoto, H.; Mitsutsuka, Y. Selective Determination of Haloperidol in Hurman Serum: Surface Ionization Mass Spectrometry and Gas Chromatography with Surface Ionization Detection. J. Chromatogr. B 1996, 687, 395–403. [Google Scholar]
- Hopkala, H. Potentiometric Determination of the Quaternary Normal-Methiodides Obtained from Some Neuroleptic Drugs (Haloperidol, Trifluperidol, Penfluridol and Pimozide). Chem. Anal. 1990, 35, 647–652. [Google Scholar]
- Bouklouze, A.; Elbouzekraoui, M.; Cherrah, Y.; Hassar, M.; Kauffmann, J.M. Potentiometric Sensor for Hydroxyzine Determination. Electroanalysis 2002, 14, 1369–1374. [Google Scholar]
- Vire, J.C.; Fischer, M.; Patriarche, G.J. Electrochemical Behavior of Some Neurleptics: Haloperidol and Its Derivatives. Talanta 1981, 28, 313–317. [Google Scholar]
- El-Desoky, H.S.; Ghoneim, M.M. Assay of the Anti-Psychotic Drug Haloperidol in Bulk Form, Pharmaceutical Formulation and Biological Fluids Using Square Wave Adsorptive Stripping Voltammetry at a Mercury Electrode. J. Pharm. Biomed. Anal. 2005, 38, 543–550. [Google Scholar]
- Trojanowicz, M. Analytical Applications of Carbon Nanotubes: a Review. Trends. Anal. Chem. 2006, 25, 480–489. [Google Scholar]
- Balasubramanian, K.; Burghard, M. Biosensors Based on Carbon Nanotubes. Anal. Bioanal. Chem. 2006, 385, 452–468. [Google Scholar]
- Zeng, B.Z.; Huang, F. Electrochemical Behavior and Determination of Fluphenazine at Multi-Walled Carbon Nanotubes /(3-Mercaptopropyl)trimethoxysilane Bilayer Modified Gold Electrodes. Talanta 2004, 64, 380–386. [Google Scholar]
- Li, C.Y. Voltammetric Determination of 2-Chlorophenol Using a Glassy Carbon Electrode Coated with Multi-Wall Carbon Nanotube-Dicetyl Phosphate Film. Microchim. Acta 2007, 157, 21–26. [Google Scholar]
- Bollo, S.; Ferreyra, N.F.; Rivas, G.A. Electrooxidation of DNA at Glassy Carbon Electrodes Modified with Multiwall Carbon Nanotubes Dispersed in Chitosan. Electroanalysis 2007, 19, 833–840. [Google Scholar]
- Abbaspour, A.; Izadyar, A. Carbon Nanotube Composite Coated Platinum Electrode for Detection of Cr(III) in Real Samples. Talanta 2007, 71, 887–892. [Google Scholar]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications; New York; John Wiley & Sons, 1980; p. 595. [Google Scholar]
- Laviron, E. General Expression of the Linear Potential Sweep Voltammogram in the Case of Diffusionless Electrochemical Systems. J. Electroanal. Chem. 1979, 101, 19–28. [Google Scholar]
- Zheng, H. Medical Chemistry; People's Medical Publishing House: Beijing, 2001; p. 44. [Google Scholar]
- Wuhan University. Analytical Chemistry; Higher Education Press: Beijing, 2000; p. 322. [Google Scholar]
- Harvey, D. Modern Analytical Chemistry; McGraw-Hill: Higher Education, 2000; 95. [Google Scholar]

| HPD (2.66 μM) No. | Add (μM) | Found (μM) | Recovery % |
|---|---|---|---|
| 1 | 2.66 | 5.20 | 95.5 |
| 2 | 5.32 | 7.82 | 97.0 |
| 3 | 7.98 | 10.80 | 102.0 |
| 4 | 10.64 | 13.50 | 101.9 |
| HXY (2.79 μM) No. | Add (μM) | Found (μM) | Recovery % |
| 1 | 2.79 | 5.48 | 96.4 |
| 2 | 5.58 | 8.27 | 98.2 |
| 3 | 8.37 | 11.30 | 101.7 |
| 4 | 11.16 | 14.10 | 101.3 |
© 2008 by MDPI. Reproduction is permitted for noncommercial purposes.
Share and Cite
Huang, F.; Peng, Y.; Jin, G.; Zhang, S.; Kong, J. Sensitive Detection of Haloperidol and Hydroxyzine at Multi-Walled Carbon Nanotubes-Modified Glassy Carbon Electrodes. Sensors 2008, 8, 1879-1889. https://doi.org/10.3390/s8031879
Huang F, Peng Y, Jin G, Zhang S, Kong J. Sensitive Detection of Haloperidol and Hydroxyzine at Multi-Walled Carbon Nanotubes-Modified Glassy Carbon Electrodes. Sensors. 2008; 8(3):1879-1889. https://doi.org/10.3390/s8031879
Chicago/Turabian StyleHuang, Fei, Youyuan Peng, Guiying Jin, Song Zhang, and Jilie Kong. 2008. "Sensitive Detection of Haloperidol and Hydroxyzine at Multi-Walled Carbon Nanotubes-Modified Glassy Carbon Electrodes" Sensors 8, no. 3: 1879-1889. https://doi.org/10.3390/s8031879




