Absorbance Based Light Emitting Diode Optical Sensors and Sensing Devices
Abstract
:1. Introduction
2. Detectors commonly employed with LEDs
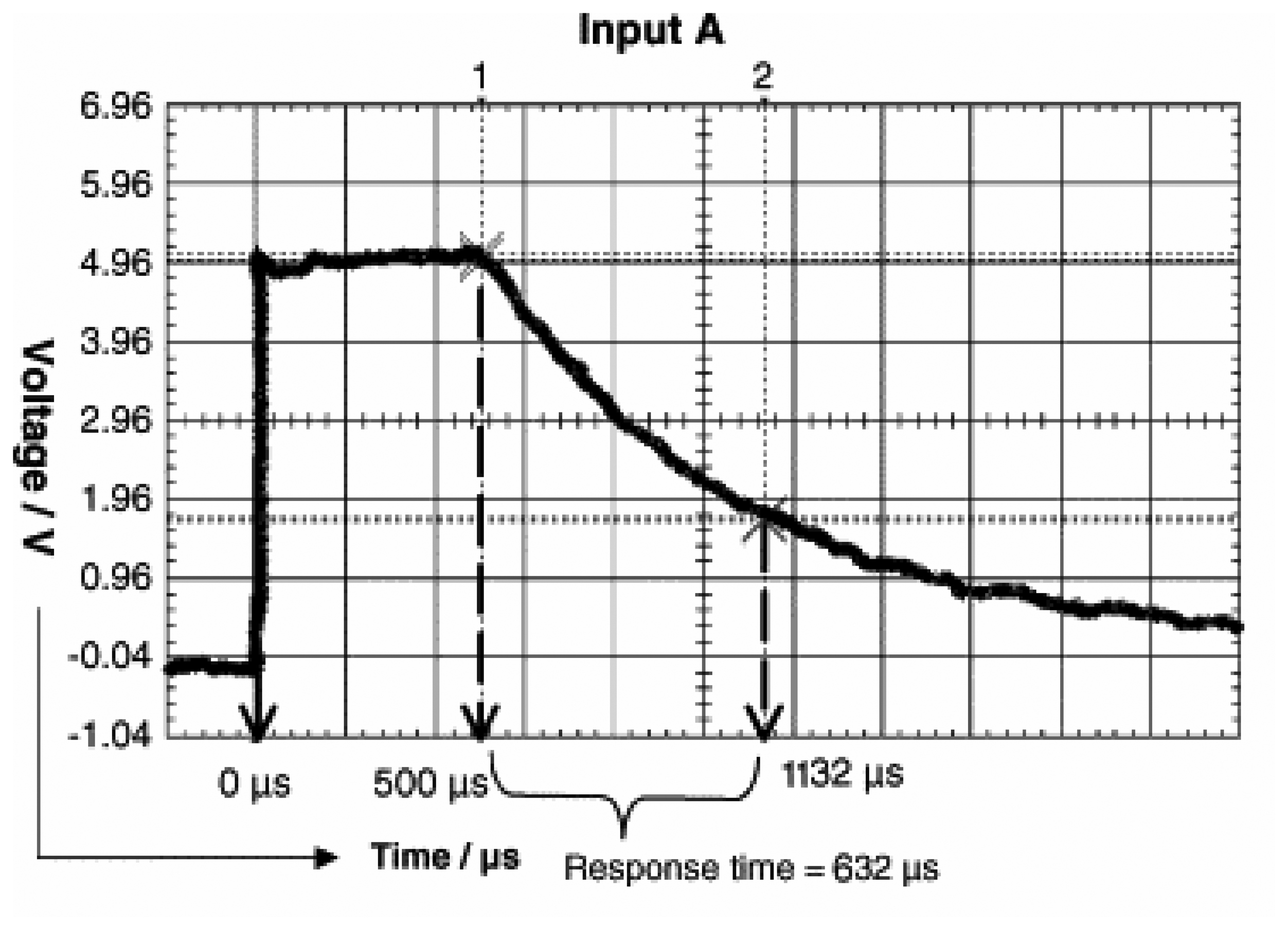
2.1 LEDs coupled with Phototransistors as a detector
2.2 LEDs coupled with Photodiodes as a detector
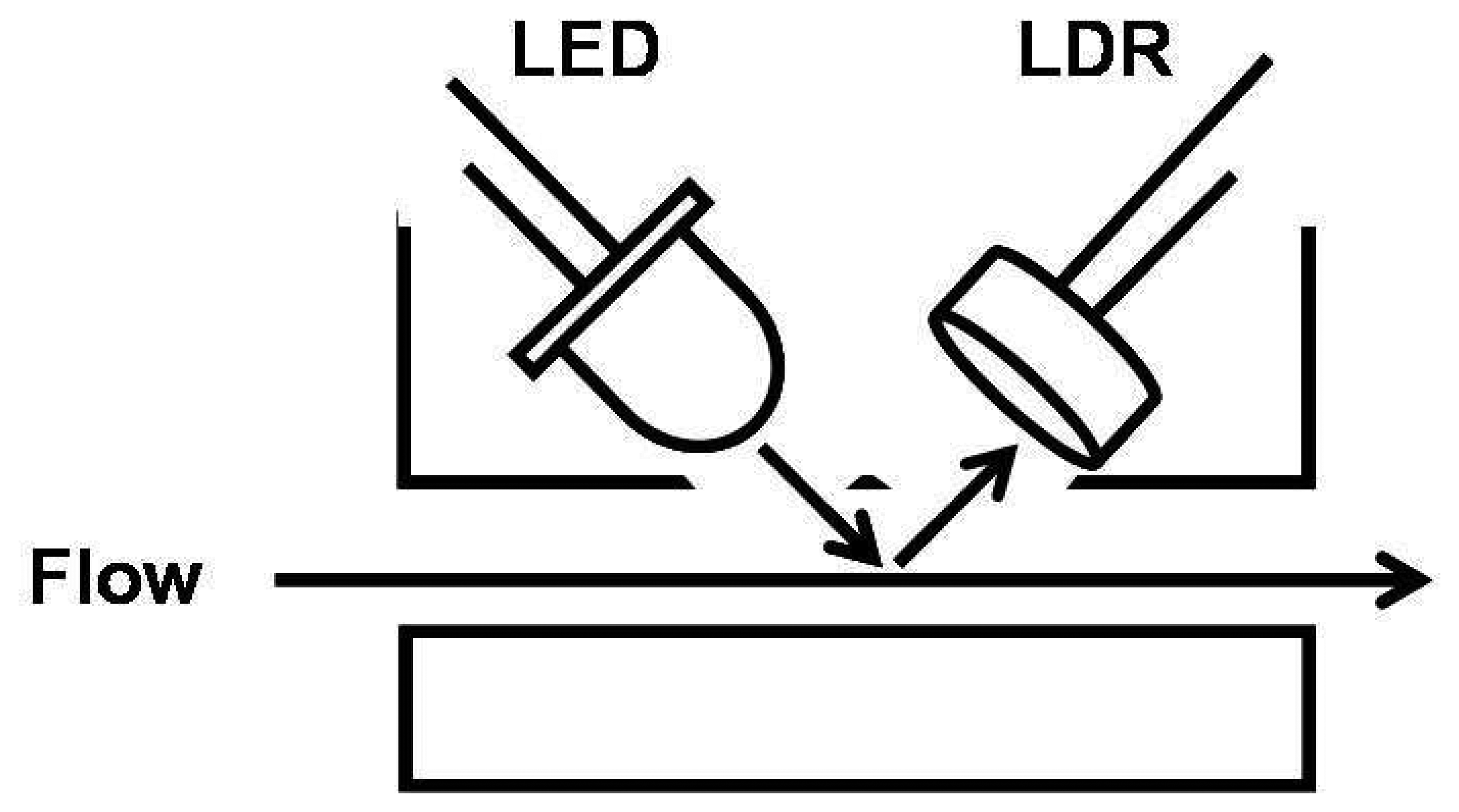
2.3 LEDs coupled with Light Dependent Resistors as a detector
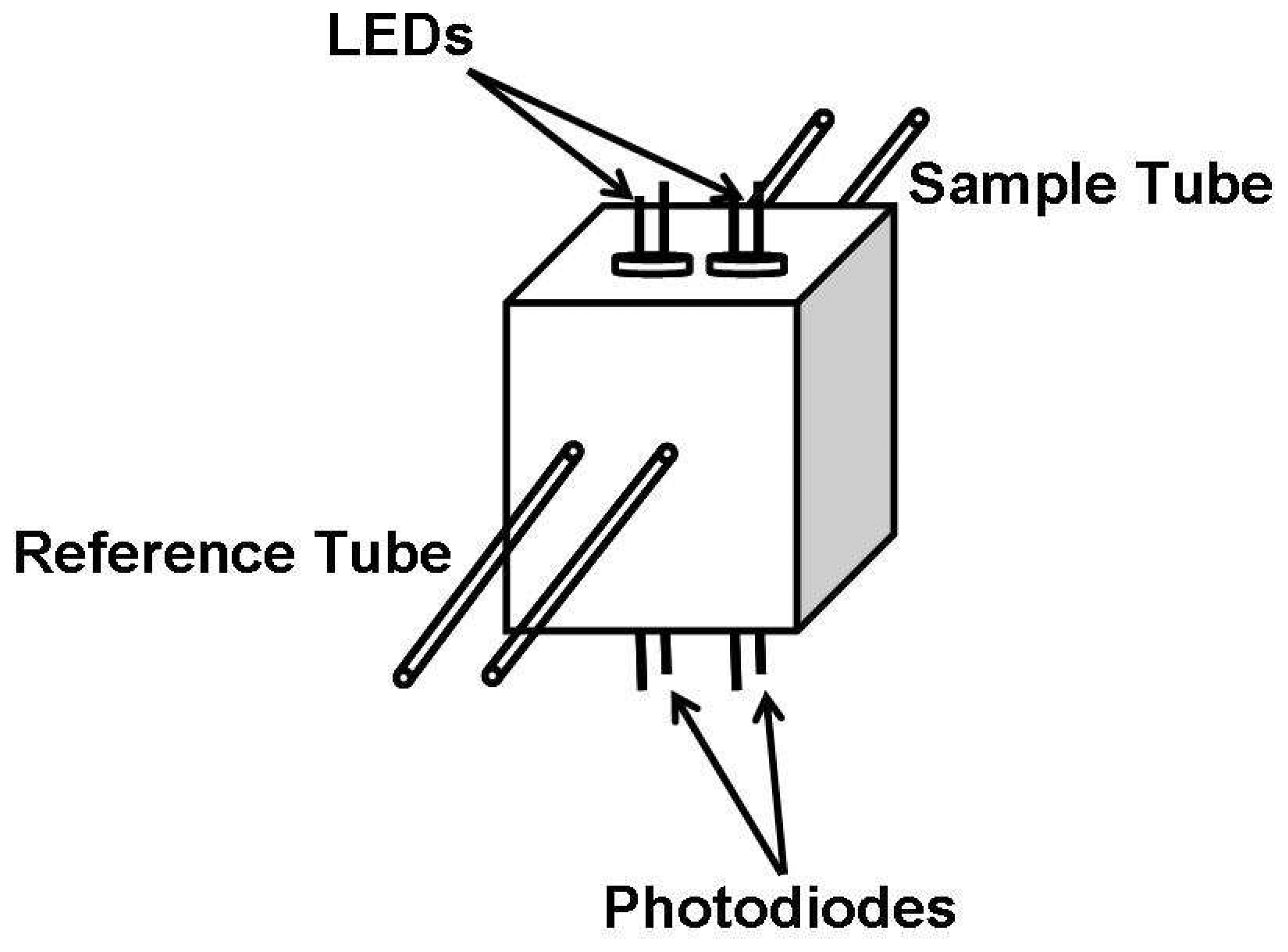
2.4 LEDs coupled with photodiode arrays as a detector
3. Configurations of LED Sensing Devices
3.1 Double Beam LED Optical Sensors
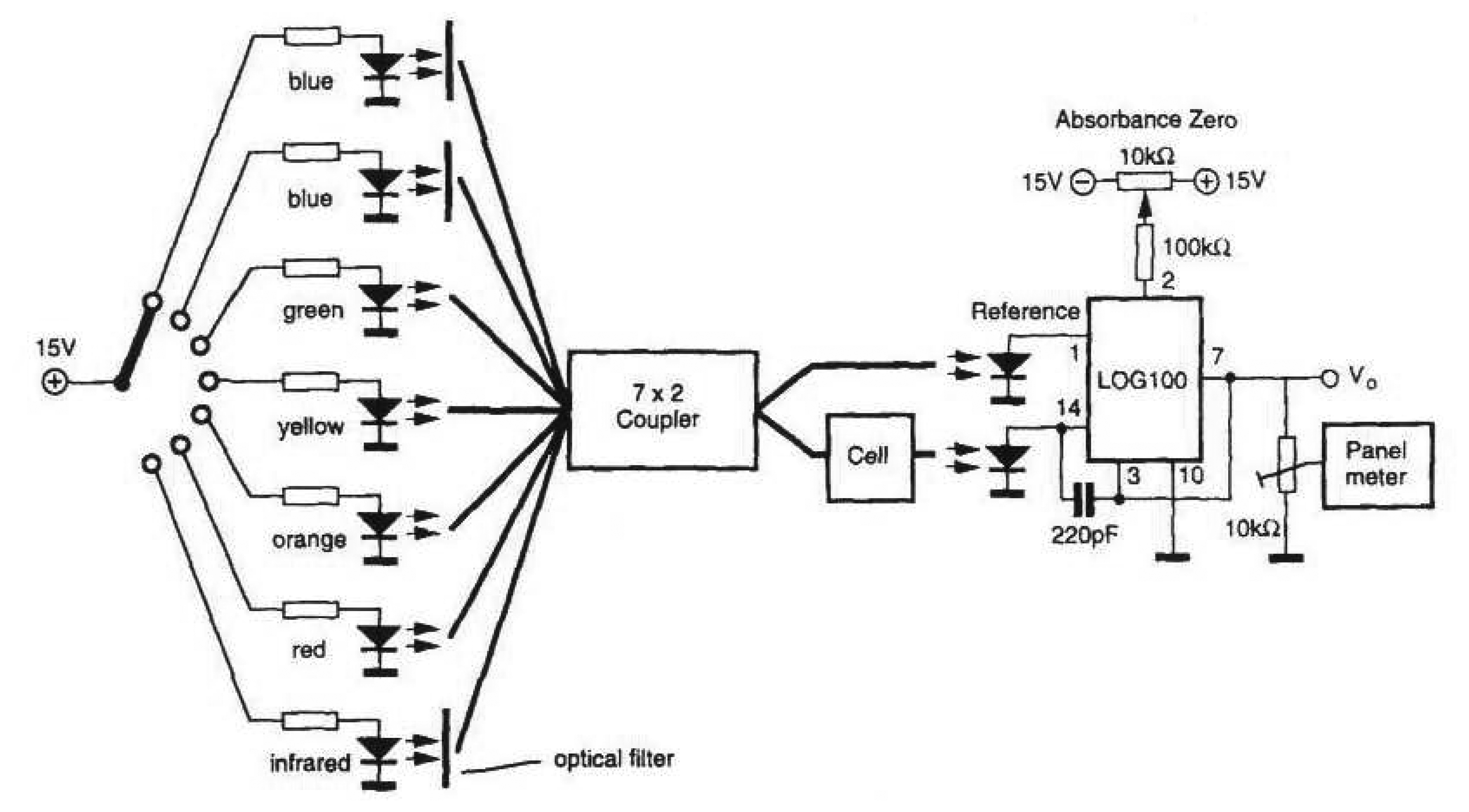
3.2 Multi - LEDs as a light source
3.3 Bi- / Tri- colour LEDs as a light source
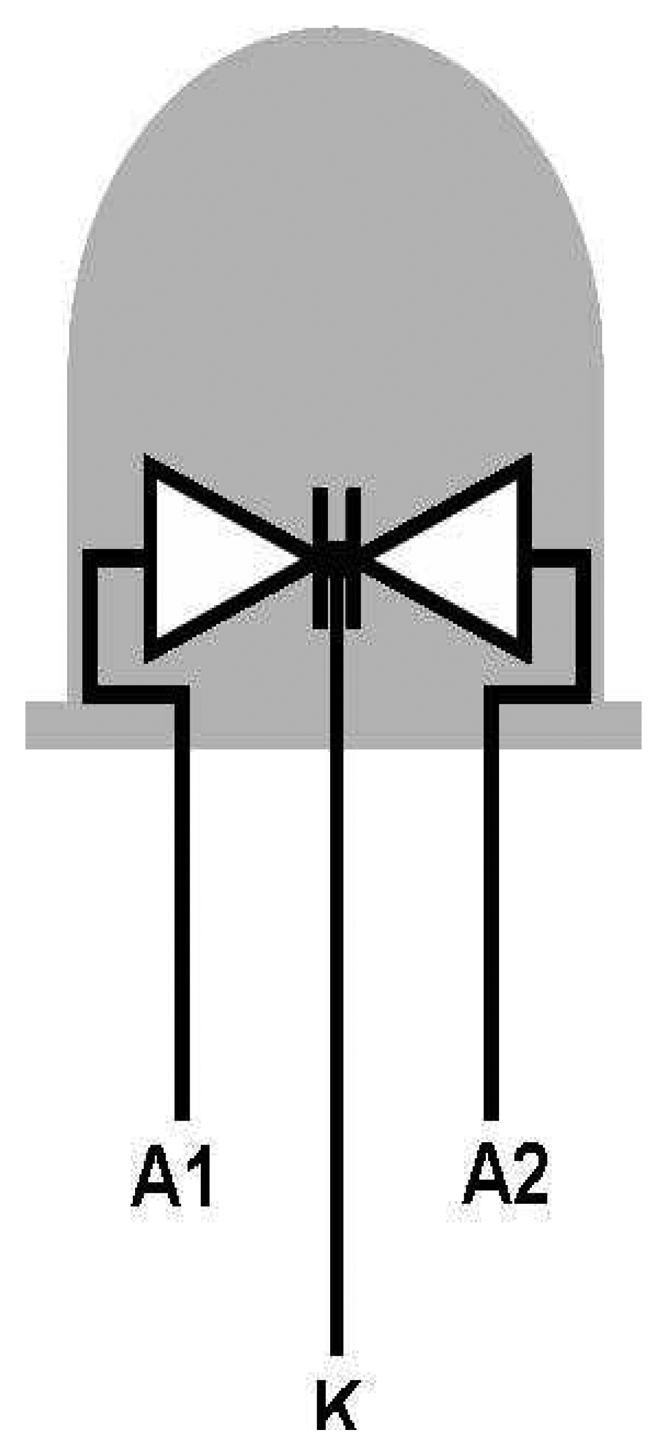
4. LEDs as Light Detectors
5. Applications of LED based chemical sensors
5.1 Health
5.2 Security
5.3 Environment
6. Conclusions
Acknowledgments
References
- Ho, C.K.; Robinson, A.; Miller, D.R.; Davis, M.J. Overview of sensors and needs for environmental monitoring. Sensors 2005, 5, 4–37. [Google Scholar]
- Marle, L.; Greenway, G.M. Microfluidic devices for environmental monitoring. Trends in Analytical Chemistry 2005, 24, 795–802. [Google Scholar]
- Sequeira, M.; Bowden, M.; Minogue, E.; Diamond, D. Towards autonomous environmental monitoring systems. Talanta 2002, 56, 355–363. [Google Scholar]
- Schawarz, M.A.; Hauser, P.C. Recent developments in detection methods for microfabricated analytical devices. Lab on a chip 2001, 1, 1–6. [Google Scholar]
- Bowden, M.; Diamond, D. The determination of phosphorus in a microfluidic manifold demonstrating long-term reagent lifetime and chemical stability utilising a colorimetric method. Sensors and Actuators B: Chemical 2003, 90, 170–174. [Google Scholar]
- Gotz, S.; Karst, U. Recent developments in optical detection methods for microchip separations. Analytical Bioanalytical Chemistry 2007, 387, 183–192. [Google Scholar]
- Sequeira, M.; Diamond, D.; Daridon, A.; Lichtenberg, J.; Verpoorte, S.; Rooij, N.F.D. Progress in the realisation of an autonomous environmental monitoring device for ammonia. Trends in Analytical Chemistry 2002, 21, 816–827. [Google Scholar]
- Diamond, D. Internet-Scale Sensing. Analytical Chemistry 2004, 76, 279A–286A. [Google Scholar]
- Holonyak, N., Jr.; Bevaqua, S.F. Coherent (visible) light emission from Ga(As1-xPx) junctions. Applied Physics Letters 1962, 1, 82–83. [Google Scholar]
- Trojanowicz, M.; Worsfold, P.J.; Clinch, J.R. Solid-state photometric detectors for flow injection analysis. Trends in Analytical Chemistry 1988, 7, 301–305. [Google Scholar]
- Liu, H.; Dasgupta, P.K.; Zheng, H.J. High performance optical absorbance detectors based on low noise switched integrators. Talanta 1993, 40, 1331–1338. [Google Scholar]
- Dasgupta, P.K.; Bellamy, H.S.; Liu, H.; Lopez, J.L.; Loree, E.L.; Morris, K.; Petersen, K.; Mir, K.A. Light emitting diode based flow-through optical absorption detectors. Talanta 1993, 40, 53–74. [Google Scholar]
- Taib, M.N.; Narayanaswamy, R. Solid-state Instruments for Optical Fibre Chemical Sensors. The Analyst 1995, 120, 1617–1625. [Google Scholar]
- Dasgupta, P.K.; Eom, I.-Y.; Morris, K.J.; Li, J. Light emitting diode-based detectors: Absorbance, fluorescence and spectroelectrochemical measurements in a planar flow-through cell. Analytica Chimica Acta 2003, 500, 337–364. [Google Scholar]
- Kovac, J.; Peternai, L.; Lengyel, O. Advanced light emitting diodes structures for optoelectronic applications. Thin Solid Films 2003, 433, 22–26. [Google Scholar]
- Zukauskas, A.; Shur, M.S.; Gaska, R. Introduction to Solid-State Lighting; John Wiley & Sons, Inc., 2002. [Google Scholar]
- Taniyasu, Y.; Kasu, M.; Makimoto, T. An aluminium nitride light-emitting diode with a wavelength of 210 nanometres. Nature 2006, 441, 325–328. [Google Scholar]
- Kuo, J.S.; Kuyper, C.L.; Allen, P.B.; Fiorini, G.S.; Chiu, D.T. High-power blue/UV light-emitting diodes as excitation sources for sensitive detection. Electrophoresis 2004, 25, 3796–3804. [Google Scholar]
- http://www.roithner-laser.com/
- http://www.s-et.com/
- Flaschka, H.; McKeithan, C.; Barnes, R. Light emitting diodes and phototransistors in photometric modules. Analytical Letters 1973, 6, 585–594. [Google Scholar]
- Anfalt, T.; Graneli, A.; Strandberg, M. Probe photometer based on optoelectronic components for the determination of total alkalinity in seawater. Analytical Chemistry 1976, 48, 357–360. [Google Scholar]
- Betteridge, D.; Dagless, E.L.; Fields, B.; Graves, N.F. A highly sensitive flow-through phototransducer for unsegmented continuous-flow analysis demonstrating high-speed spectrophotometry at the parts per 109 level and a new method of refractometric determinations. The Analyst 1978, 103, 897–908. [Google Scholar]
- Boring, C.B.; Dasgupta, P.K. An affordable high-performance optical absorbance detector for capillary systems. Analytica Chimica Acta 1997, 342, 123–132. [Google Scholar]
- Dasgupta, P.K.; Genfa, Z.; Poruthoor, S.K.; Caldwell, S.; Dong, S.; Liu, S.-Y. High-Sensitivity Gas Sensors Based on Gas-Permeable Liquid Core Waveguides and Long-Path Absorbance Detection. Analytical Chemistry 1998, 70, 4661–4669. [Google Scholar]
- Toda, K.; Yoshioka, K.-I.; Ohira, S.-I.; Li, J.; Dasgupta, P.K. Trace Gas Measurement with an Integrated Porous Tube Collector/Long-Path Absorbance Detector. Analytical Chemistry 2003, 75, 4050–4056. [Google Scholar]
- Galanis, S.; Dasgupta, P.K. Measurement of parts per million levels of potassium hydroxide in polyether polyol streams. Analytica Chimica Acta 2001, 429, 101–110. [Google Scholar]
- Samanta, G.; Boring, C.B.; Dasgupta, P.K. Continuous Automated Measurement of Hexavalent Chromium in Airborne Particulate Matter. Analytical Chemistry 2001, 73, 2034–2040. [Google Scholar]
- Greenway, G.M.; Haswell, S.J.; Petsul, P.H. Characterisation of a micro-total analytical system for the determination of nitrite with spectrophotometric detection. Analytica Chimica Acta 1999, 387, 1–10. [Google Scholar]
- Collins, G.E.; Lu, Q. Radionuclide and metal ion detection on a capillary electrophoresis microchip using LED absorbance detection. Sensors and Actuators B: Chemical 2001, 76, 244–249. [Google Scholar]
- Petsul, P.H.; Greenway, G.M.; Haswell, S.J. The development of an on-chip micro-flow injection analysis of nitrate with a cadmium reductor. Analytica Chimica Acta 2001, 428, 155–161. [Google Scholar]
- White, B.J.; Harmon, H.J. Optical solid-state detection of organophosphates using organophosphorus hydrolase. Biosensors and Bioelectronics 2005, 20, 1977–1983. [Google Scholar]
- Ferrer, L.; de Armas, G.; Miro, M.; Estela, J.M.; Cerda, V. A multisyringe flow injection method for the automated determination of sulfide in waters using a miniaturised optical fiber spectrophotometer. Talanta 2004, 64, 1119–1126. [Google Scholar]
- Collins, G.E.; Lu, Q. Microfabricated capillary electrophoresis sensor for uranium (VI). Analytica Chimica Acta 2001, 436, 181–189. [Google Scholar]
- Lu, Q.; Collins, G.E. Microchip separations of transition metal ions via LED absorbance detection of their PAR complexes. The Analyst 2001, 126, 429–432. [Google Scholar]
- Chen, S-J.; Chen, M.-J.; Chang, H.-T. Light-emitting diode-based indirect fluorescence detection for simultaneous determination of anions and cations in capillary electrophoresis. Journal of Chromatography A 2003, 1017, 215–224. [Google Scholar]
- Collins, G.E.; Lu, Q.; Pereira, N.; Wu, P. Long pathlength, three-dimensional absorbance microchip. Talanta 2007, 72, 301–304. [Google Scholar]
- Schmidt, W. A high performance micro-dual-wavelength-spectrophotometer (MDWS). Journal of biochemical and biophysical methods 2004, 58, 15–24. [Google Scholar]
- Zhang, T.; Fang, Q.; Wang, S.-L.; Qin, L.-F.; Wang, P.; Wu, Z.-Y.; Fang, Z.-L. Enhancement of signal-to-noise level by synchronized dual wavelength modulation for light emitting diode fluorimetry in a liquid-core-waveguide microfluidic capillary electrophoresis system. Talanta 2005, 68, 19–24. [Google Scholar]
- Lu, Q.; Collins, G.E.; Smith, M.; Wang, J. Sensitive capillary electrophoresis microchip determination of trinitroaromatic explosives in nonaqueous electrolyte following solid phase extraction. Analytica Chimica Acta 2002, 469, 253–260. [Google Scholar]
- Deng, G.; Collins, G.E. Nonaqueous based microchip separation of toxic metal ions using 2-(5-bromo-2-pyridylazo)-5-(N-propyl-N-sulfopropylamino)phenol. Journal of Chromatography A 2003, 989, 311–316. [Google Scholar]
- Matias, F.A.A.; Vila, M.M.D.C.; Tubino, M. A simple device for quantitative colorimetric diffuse reflectance measurements. Sensors and Actuators B: Chemical 2003, 88, 60–66. [Google Scholar]
- Sombatsompop, N.; Intawong, N.-S.; Intawong, N.-T. Design and construction of photo-conductive light pressure sensor for highly viscous fluids. Sensors and Actuators A: Physical 2002, 102, 76–82. [Google Scholar]
- Schrodle, S.; Buchner, R.; Kunz, W. Automated apparatus for the rapid determination of liquid-liquid and solid-liquid phase transitions. Fluid Phase Equilibria 2004, 216, 175–182. [Google Scholar]
- Lau, K.-T.; Shepherd, R.; Diamond, D.; Diamond, D. Solid State pH Sensor Based on Light Emitting Diodes (LED) As Detector Platform. Sensors 2006, 6, 848–859. [Google Scholar]
- Tubino, M.; de Souza, R.L. Determination of diclofenac in pharmaceutical preparations by diffuse reflectance photometry. Talanta 2006, 68, 776–780. [Google Scholar]
- Tubino, M.; Queiroz, C.A.R. Flow injection visible diffuse reflectance quantitative analysis of nickel. Analytica Chimica Acta 2006. In Press. [Google Scholar]
- Johnson, K.S.; Beehler, C.L.; Sakamoto-Arnold, C.M. A submersible flow analysis system. Analytica Chimica Acta 1986, 179, 245–257. [Google Scholar]
- Betteridge, D.; Cheng, W.C.; Dagless, E.L.; David, P.; Goad, T.B.; Deans, D.R.; Newton, D.A.; Pierce, T.B. An automated viscometer based on high-precision flow injection analysis. The Analyst 1983, 108, 1–16. [Google Scholar]
- Feres, M.A.; Reis, B.F. A downsized flow set up based on multicommutation for the sequential photometric determination of iron(II)/iron(III) and nitrite/nitrate in surface water. Talanta 2005, 68, 422–428. [Google Scholar]
- Tan, A.; Huang, J.; Geng, L.; Xu, J.; Zhao, X. A multi-channel photometric detector for multi-component analysis in flow injection analysis. Journal of Automatic Chemistry 1994, 16, 71–73. [Google Scholar]
- Trojanowicz, M.; Augustyniak, W.; Hulanicki, A. Photometric flow-injection measurements with flow-cell employing light emitting diodes. Microchimica Acta 1984, 83, 17–25. [Google Scholar]
- Santos, S.R.B.D.; Araujo, M.C.U.D.; Barbosa, R.A. An automated FIA system to determine alcoholic grade in beverages based on Schlieren effect measurements using an LED-photocolorimeter. The Analyst 2002, 127, 324–327. [Google Scholar]
- Schmidt, G.J.; W. Scott, R.P. Simple and sensitive ion chromatograph for trace metal determination. The Analyst 1984, 109, 997–1002. [Google Scholar]
- Clinch, J.R.; Worsfold, P.J.; Casey, H. An automated spectrophotometric field monitor for water quality parameters: Determination of nitrate. Analytica Chimica Acta 1987, 200, 523–531. [Google Scholar]
- Hauser, P.C.; Tan, S.S.; Cardwell, T.J.; Cattrall, R.W.; Hamilton, I.C. Versatile manifold for the simultaneous determination of ions in flow injection analysis. The Analyst 1988, 113, 1551–1555. [Google Scholar]
- Hauser, P.C.; Chiang, D.W.L. A photometric detector based on a blue light-emitting diode. Talanta 1993, 40, 1193–1200. [Google Scholar]
- Rainelli, A.; Stratz, R.; Schweizer, K.; Hauser, P.C. Miniature flow-injection analysis manifold created by micromilling. Talanta 2003, 61, 659–665. [Google Scholar]
- Trojanowicz, M.; Szpunar-Lobinska, J. Simultaneous flow-injection determination of aluminium and zinc using LED photometric detection. Analytica Chimica Acta 1990, 230, 125–130. [Google Scholar]
- Freeman, P.R.; McKelvie, I.D.; Hart, B.T.; Cardwell, T.J. Flow-injection technique for the determination of low levels of phosphorus in natural waters. Analytica Chimica Acta 1990, 234, 409–416. [Google Scholar]
- Chediak, J.A.; Luo, Z.; Seo, J.; Cheung, N.; Lee, L.P.; Sands, T.D. Heterogeneous integration of CdS filters with GaN LEDs for fluorescence detection microsystems. Sensors and Actuators A: Physical 2004, 111, 1–7. [Google Scholar]
- Yamada, A.; Sakuraba, M.; Murota, J. Integration of Si p-i-n diodes for light emitter and detector with optical waveguides. Materials Science in Semiconductor Processing 2005, 8, 435–438. [Google Scholar]
- Park, J.M.; Shon, O.J.; Hong, H.-G.; Kim, J.S.; Kim, Y.; Lim, H.B. Development of a microchip metal ion sensor using dinitro-azocalix[4]azacrown. Microchemical Journal 2005, 80, 139–144. [Google Scholar]
- Sonne, K.; Dasgupta, P.K. Simultaneous photometric flow-injection determination of sulfide, polysulfide, sulfite, thiosulfate, and sulfate. Analytical Chemistry 1991, 63, 427–432. [Google Scholar]
- Dong, S.; Dasgupta, P.K. Automated determination of total phosphorus in aqueous samples. Talanta 1991, 38, 133–137. [Google Scholar]
- Worsfold, P.J.; Clinch, J.R.; Casey, H. Spectrophotometric field monitor for water quality parameters: The Determination of Phosphate. Analytica Chimica Acta 1987, 197, 43–50. [Google Scholar]
- Liu, H.; Dasgupta, P.K. Dual-wavelength photometry with light emitting diodes. Compensation of refractive index and turbidity effects in flow-injection analysis. Analytica Chimica Acta 1994, 289, 347–353. [Google Scholar]
- Huang, J.; Liu, H.; Tan, A.; Xu, J.; Zhao, X. A dual-wavelength light-emitting diode based detector for flow-injection analysis process analysers. Talanta 1992, 39, 589–592. [Google Scholar]
- Gros, N. Spectrometer with microreaction chamber and tri-colour light emitting diode as a light source. Talanta 2004, 62, 143–150. [Google Scholar]
- Gros, N. A new type of a spectrometric microtitration set up. Talanta 2005, 65, 907–912. [Google Scholar]
- Hauser, P.C.; Rupasinghe, T.W.T.; Cates, N.E. A multi-wavelength photometer based on light-emitting diodes. Talanta 1995, 42, 605–612. [Google Scholar]
- Fonseca, A.; Raimundo, J.; Ivo, M. A multichannel photometer based on an array of light emitting diodes for use in multivariate calibration. Analytica Chimica Acta 2004, 522, 223–229. [Google Scholar]
- Suzuki, A.; Kondoh, J.; Matsui, Y.; Shiokawa, S.; Suzuki, K. Development of novel optical waveguide surface plasmon resonance (SPR) sensor with dual light emitting diodes. Sensors and Actuators B: Chemical 2005, 106, 383–387. [Google Scholar]
- Lau, K.T.; Baldwin, S.; Shepherd, R.L.; Dietz, P.H.; Yerzunis, W.S.; Diamond, D. Novel fused-LEDs devices as optical sensors for colorimetric analysis. Talanta 2004, 63, 167–173. [Google Scholar]
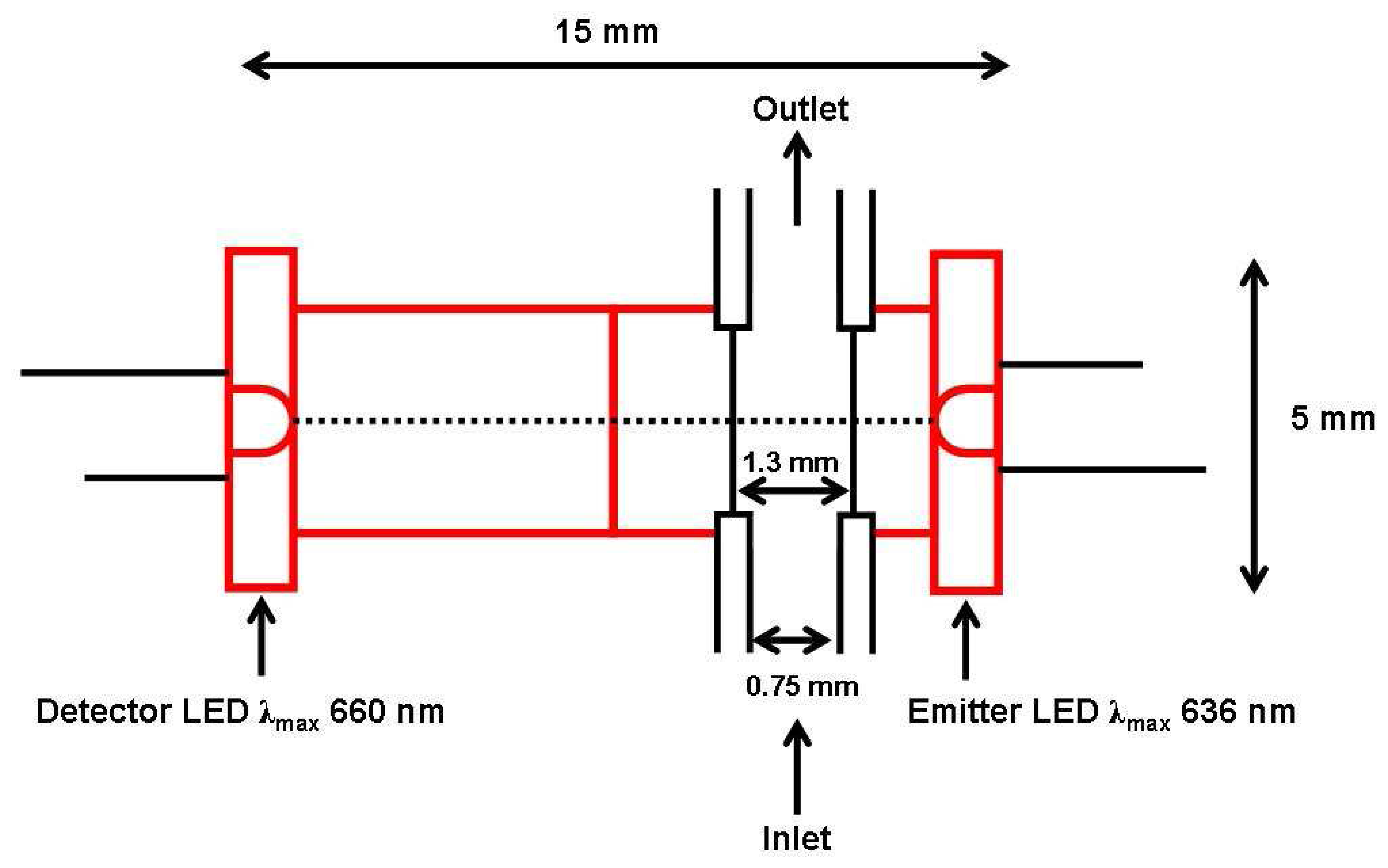
- O'Toole, M.; Lau, K.-T.; Diamond, D. Photometric detection in flow analysis systems using integrated PEDDs. Talanta 2005, 66, 1340–1344. [Google Scholar]
- Lau, K.-T.; Baldwin, S.; O'Toole, M.; Shepherd, R.; Yerazunis, W.J.; Izuo, S.; Ueyama, S.; Diamond, D. A low-cost optical sensing device based on paired emitter-detector light emitting diodes. Analytica Chimica Acta 2006, 557, 111–116. [Google Scholar]
- O'Toole, M.; Lau, K.-T.; Schazmann, B.; Shepherd, R.; Nesterenko, P.N.; Paull, B.; Diamond, D. Novel Integrated Paired Emitter Detector Diode as a Miniaturized Photometric Detector in HPLC. The Analyst 2006, 131, 938–943. [Google Scholar]
- Lau, K.-T.; Yerazunis, W.S.; Shepherd, R.L.; Diamond, D. Quantitative colorimetric analysis of dye mixtures using an optical photometer based on LED array. Sensors and Actuators B: Chemical 2006, 114, 819–825. [Google Scholar]
- Lau, K.T.; McHugh, E.; Baldwin, S.; Diamond, D. Paired emitter-detector light emitting diodes for the measurement of lead(II) and cadmium(II). Analytica Chimica Acta 2006, 569, 221–226. [Google Scholar]
- Barron, L.; Nesterenko, P.N.; Diamond, D.; O'Toole, M.; Lau, K.-T.; Paull, B. Low pressure ion chromatography with a low cost paired emitter-detector diode based detector for the determination of alkaline earth metals in water samples. Analytica Chimica Acta 2006, 577, 32–37. [Google Scholar]
- O'Toole, M.; Lau, K.-T.; Shepherd, R.; Slater, C.; Diamond, D. Determination of Phosphate using a Highly Sensitive Paired Emitter-Detector Diode Photometric Detector. Analytica Chimica Acta 2007, 597, 290–294. [Google Scholar]
- Mims, F.M., III. Sun photometer with light emitting diode as spectrally selective detectors. Applied Optics 1992, 31, 6965–6967. [Google Scholar]
- Acharya, Y.B.; Jayaraman, A.; Ramachandran, S.; Subbaraya, B.H. Compact light-emitting-diode sun photometer for atmospheric optical depth measurements. Applied Optics 1995, 34, 1209–1214. [Google Scholar]
- Berry, R.J.; Harris, J.E.; Williams, R.R. Light-Emitting Diodes as Sensors for Colorimetric Analyses. Applied Spectroscopy 1997, 51, 1521–1524. [Google Scholar]
- Eiichi, M.; Shin, I.; Tsutomu, A. Using a light-emitting diode as a high-speed, wavelength selective photodetector. Review of Scientific Instruments 1998, 69, 3751–3754. [Google Scholar]
- Betteridge, D. Flow Injection Analysis. Analytical Chemistry 1978, 50, 832A–846A. [Google Scholar]
- Betteridge, D.; Sly, T.J.; Wade, A.P.; Porter, D.G. Versatile automatic development system for flow injection analysis. Analytical Chemistry 1986, 58, 2258–2265. [Google Scholar]
- Jambunathan, S.; Dasgupta, P.K.; Wolcott, D.K.; Marshall, G.D.; Olson, D.C. Optical fiber coupled light emitting diode based absorbance detector with a reflective flow cell. Talanta 1999, 50, 481–490. [Google Scholar]
- Daykin, R.N.C.; Haswell, S.J. Development of a micro flow injection manifold for the determination of orthophosphate. Analytica Chimica Acta 1995, 313, 155–159. [Google Scholar]
- Carroll, M.K.; Conboy, M.; Murfin, A.; Tyson, J.F. Solid-state microprocessor-controlled detector for doublet peak measurements in flow-injection analysis. Analytica Chimica Acta 1994, 295, 143–149. [Google Scholar]
- Johns, C.; Macka, M.; Haddad, P.R. Design and performance of a light-emitting diode detector compatible with a commercial capillary electrophoresis instrument. Electrophoresis 2004, 25, 3145–3152. [Google Scholar]
- Liu, S.; Dasgupta, P.K. Liquid Droplet. A Renewable Gas Sampling Interface. Analytical Chemistry 1995, 67, 2042–2049. [Google Scholar]
- Cardoso, A.A.; Dasgupta, P.K. Analytical Chemistry in a Liquid Film/Droplet. Analytical Chemistry 1995, 67, 2562–2566. [Google Scholar]
- Liu, H.; Dasgupta, P.K. A Renewable Liquid Droplet as a Sampler and a Windowless Optical Cell. Automated Sensor for Gaseous Chlorine. Analytical Chemistry 1995, 67, 4221–4228. [Google Scholar]
- King, M.; Paull, B.; Haddad, P.R.; Macka, M. Performance of a simple UV LED light source in the capillary electrophoresis of inorganic anions with indirect detection using a chromate background electrolyte. The Analyst 2002, 127, 1564–1567. [Google Scholar]
- Kuban, P.; Guchardi, R.; Hauser, P.C. Trace-metal analysis with separation methods. Trends in Analytical Chemistry 2005, 24, 192–198. [Google Scholar]
- Pacquit, A.; Lau, K.T.; McLaughlin, H.; Frisby, J.; Quilty, B.; Diamond, D. Development of a volatile amine sensor for the monitoring of fish spoliage. Talanta 2006, 69, 515–520. [Google Scholar]
- Artur, D.; Wojciech, W.; Janusz, M.; Ryszard, S.R.; Zbigniew, B. Fiber optic probe for monitoring of drinking water. presented at Chemical, Biochemical and Environmental Fiber Sensors IX, Munich; 1997. [Google Scholar]
- Dybko, A.; Wroblewski, W.; Rozniecka, E.; Pozniakb, K.; Maciejewski, J.; Romaniuk, R.; Brzozka, Z. Assessment of water quality based on multiparameter fiber optic probe. Sensors and Actuators B: Chemical 1998, 51, 208–213. [Google Scholar]
- Smardzewski, R.R. Multi-element optical waveguide sensor: General concept and design. Talanta 1988, 35, 95–101. [Google Scholar]
- Li, Q.; Morris, K.J.; Dasgupta, P.K.; Raimundo, I.M.; Temkin, H. Portable flow-injection analyzer with liquid-core waveguide based fluorescence, luminescence, and long path length absorbance detector. Analytica Chimica Acta 2003, 479, 151–165. [Google Scholar]
- Ramırez-Garcıa, S.; Diamond, D. Biomimetic, low power pumps based on soft actuators. Sensors and Actuators A 2007, 135, 229–235. [Google Scholar]
- Ramirez-Garcia, S.; Diamond, D. Internet-scale Sensing: Are Biomimetic Approaches the Answer? Journal of Intelligent Material Systems and Structures 2007, 18, 159–164. [Google Scholar]
- Diamond, D.; Lau, K.T.; Brady, S.; Cleary, J. Integration of analytical measurements and wireless communications—Current issues and future strategies. Talanta 2007. In Press, Corrected Proof. [Google Scholar]
- Hooley, D.J.; Dessy, R.E. Continuous flow kinetic techniques in flow injection analysis. Analytical Chemistry 1983, 55, 313–320. [Google Scholar]
- Zagatto, E.A.G.; Arruda, M.A.Z.; Jacintho, A.O.; Mattos, I.L. Compensation of the schlieren effect in flow-injection analysis by using dual-wavelength spectrophotometry. Analytica Chimica Acta 1990, 234, 153–160. [Google Scholar]
- Eom, I.-Y.; Dasgupta, P.K. Frequency-selective absorbance detection: Refractive index and turbidity compensation with dual-wavelength measurement. Talanta 2006, 69, 906–913. [Google Scholar]
- Beach, J.M. A LED light calibration source for dual-wavelength microscopy. Cell Calcium 1997, 21, 63–68. [Google Scholar]
- Schnable, J.G.; Grochowski, P.J.; Wilhelm, L.; Harding, C.; Kiefer, M.; Orr, R.S. Portable LED-array VIS-NIR spectrophotometer/nephelometer. Field Analytical Chemistry and Technology 1998, 2, 21–28. [Google Scholar]
- Suzuki, Y.; Hori, H.; Iwatsuki, M.; Yamane, T. A four-wavelength channel absorbance detector with a light emitting diode-fiber optics assembly for simplifying the flow-injection analysis system. Analytical Sciences 2003, 19, 1025–1028. [Google Scholar]
- Cantrell, K.M.; Ingle, J.D. The SLIM Spectrometer. Analytical Chemistry 2003, 75, 27–35. [Google Scholar]
- Zude, M.; Birlouez-Aragon, I.; Paschold, P.-J.; Rutledge, D.N. Non-invasive spectrophotometric sensing of carrot quality from harvest to consumption. Postharvest Biology and Technology 2007. In Press, Corrected Proof. [Google Scholar]
- Fonseca, A.; Raimundo, I.M., Jr. A simple method for water discrimination based on an light emitting diode (LED) photometer. Analytica Chimica Acta 2007, 596, 66–72. [Google Scholar]
- Gros, N. A novel type of tri-colour light-emitting-diode-based spectrometric detector for low-budget flow-injection analysis. Sensors 2007, 7, 166–184. [Google Scholar]
- Yang, P.K.; Chen, J.C.; Chuang, Y.H. Improvement on reflective color measurement using a tricolor LED by multi-point calibration. Optics Communications 2007, 272, 320–324. [Google Scholar]
- Rocha, F.R.P.; Reis, B.F. A flow system exploiting multicommutation for speciation of inorganic nitrogen in waters. Analytica Chimica Acta 2000, 409, 227–235. [Google Scholar]
- Mims, F.M., III. How to monitor ultraviolet radiation from the sun. Scientific American 1990, 263, 106–109. [Google Scholar]
- Riley, M.R.; Jordan, K.A.; Cox, M.L. Development of a cell-based sensing device to evaluate toxicity of inhaled materials. Biochemical Engineering journal 2004, 19, 95–99. [Google Scholar]
- Kawamura, K.; Vestergaard, M.d.; Ishiyama, M.; Nagatani, N.; Hashiba, T.; Tamiya, E. Development of a novel hand-held toluene gas sensor: Possible use in the prevention and control of sick building syndrome. Measurement 2006, 39, 490–496. [Google Scholar]
- Smiddy, M.; Papkovskaia, N.; Papkovsky, D.B.; Kerry, J.P. Use of oxygen sensors for the nondestructive measurement of the oxygen content in modified atmosphere and vacuum packs of cooked chicken patties; impact of oxygen content on lipid oxidation. Food Research International 2002, 35, 577–584. [Google Scholar]
- Firstenberg-Eden, R.; Shelef, L.A. A new rapid automated method for the detection of Listeria from environmental swabs and sponges. International Journal of Food Microbiology 2000, 56, 231–237. [Google Scholar]
- Pacquit, A.; Frisby, J.; Diamond, D.; Lau, K.T.; Farrell, A.; Quilty, B.; Diamond, D. Development of a smart packaging for the monitoring of fish spoilage. Food Chemistry 2007, 102, 466–470. [Google Scholar]
- Ichikawa, T.; Horiuchi, M.; Ichiba, H.; Matsumoto, N. Trial production and application of an optical system for measuring the movements of organs composed of soft tissue. The Journal of Prosthetic Dentistry 1990, 64, 227–231. [Google Scholar]
- Mitrani, A.A.; Gonzalez, M.L.; O'Connell, M.T.; Guerra, J.; Harwood, R.B.; Gardner, L.B. Detection of clinically suspected deep vein thrombosis using light reflection rheography. The American Journal of Surgery 1991, 161, 646–650. [Google Scholar]
- Teshima, N.; Li, J.; Toda, K.; Dasgupta, P.K. Determination of acetone in breath. Analytica Chimica Acta 2005, 535, 189–199. [Google Scholar]
- Singh, S. Sensors-An effective approach for the detection of explosives. Journal of Hazardous Materials 2007. In Press. [Google Scholar]
- Lee, R.S.; Aldis, D.F.; Garrett, D.W.; Lai, F.S. Improved diagnostics for determination of minimum explosive concentration, ignition energy and ignition temperature of dusts. Powder Technology 1982, 31, 51–62. [Google Scholar]
- Pamula, V.K.; Svinivasan, V.; Chakrapani, H.; Fair, R.B.; Toone, E.J. A droplet-based lab-on-a-chip for coloflumetric detection of nitroaromatic explosives. presented at Micro Electro Mechanical Systems, 2005; 2005. [Google Scholar]
- Koch, S.; Wolf, H.; Danapel, C.; Feller, K.A. Optical flow-cell multichannel immunosensor for the detection of biological warfare agents. Biosensors and Bioelectronics 2000, 14, 779–784. [Google Scholar]
- Higgins, J.A.; Nasarabadi, S.; Karns, J.S.; Shelton, D.R.; Cooper, M.; Gbakima, A.; Koopman, R.P. A handheld real time thermal cycler for bacterial pathogen detection. Biosensors and Bioelectronics 2003, 18, 115–1123. [Google Scholar]
- Lyddy-Meaney, A.J.; Ellis, P.S.; Worsfold, P.J.; Butler, E.C.V.; McKelvie, I.D. A compact flow injection analysis system for surface mapping of phosphate in marine waters. Talanta 2002, 58, 1043–1053. [Google Scholar]
- Ellis, P.S.; Lyddy-Meaney, A.J.; Worsfold, P.J.; McKelvie, I.D. Multi-reflection photometric flow cell for use in flow injection analysis of estuarine waters. Analytica Chimica Acta 2003, 499, 81–89. [Google Scholar]
- Blundell, N.J.; Worsfold, P.J.; Casey, H.; Smith, S. The design and performance of a portable, automated flow injection monitor for the in-situ analysis of nutrients in natural waters. Environmental International 1995, 21, 205–209. [Google Scholar]
- Gardolinski, P.C.F.C.; David, A.R.J.; Worsfold, P.J. Miniature flow injection analyser for laboratory, shipboard and in situ monitoring of nitrate in estuarine and coastal waters. Talanta 2002, 58, 1015–1027. [Google Scholar]
- David, A.R.J.; McCormack, T.; Morris, A.W.; Worsfold, P.J. A submersible flow injection-based sensor for the determination of total oxidised nitrogen in coastal waters. Analytica Chimica Acta 1998, 361, 63–72. [Google Scholar]
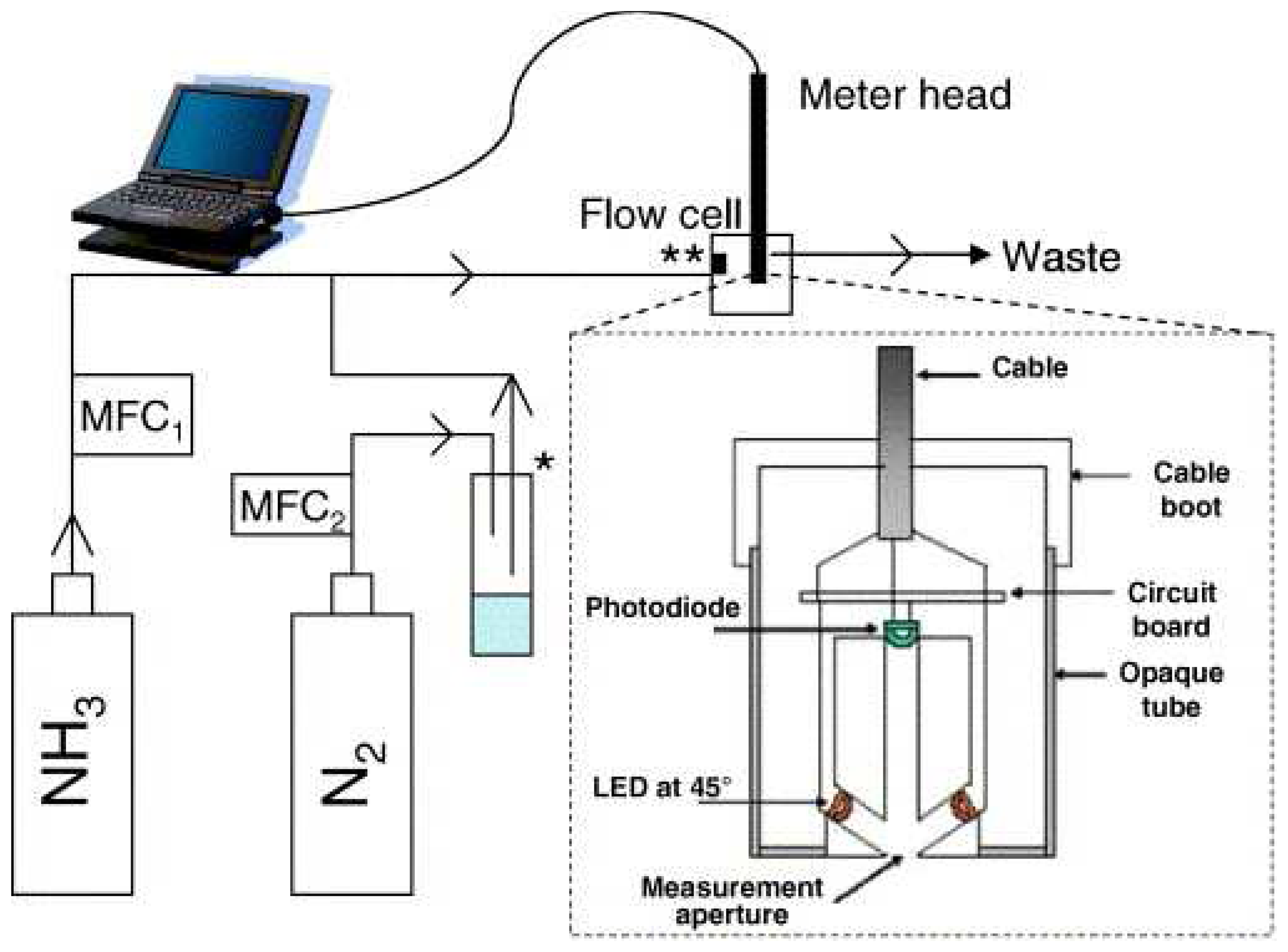
- Andrew, K.N.; Worsfold, P.J.; Comber, M. On-line flow injection monitoring of ammonia in industrial liquid effluents. Analytica Chimica Acta 1995, 314, 33–43. [Google Scholar]
- Benson, R.L.; McKelvie, I.D.; Hart, B.T.; Truong, Y.B.; Hamilton, I.C. Determination of total phosphorus in waters and wastewaters by on-line UV/thermal induced digestion and flow injection analysis. Analytica Chimica Acta 1996, 326, 29–39. [Google Scholar]
- Dasgupta, P.K.; Huang, H.; Zhang, G.; Cobb, G.P. Photometric measurement of trace As(III) and As(V) in drinking water. Talanta 2002, 58, 153–164. [Google Scholar]
- Vachirapatama, N.; Macka, M.; Haddad, P.R. Separation and determination of vanadium in fertiliser by capillary electrophoresis with light-emitting diode detector. Analytical Bioanalytical Chemistry 2002, 374, 1082–1085. [Google Scholar]
- Hauser, P.C.; Rupasinghe, T.W.T.; Lucas, C.C.; McClure, A. Process monitor for an ammoniacal nickel solution employing an infrared light-emitting diode and a log-ratio amplifier. The Analyst 1995, 120, 2635–2638. [Google Scholar]
- Chapin, T.P.; Jannasch, H.W.; Johnson, K.S. In situ osmotic analyzer for the year-long continuous determination of Fe in hydrothermal systems. Analytica Chimica Acta 2002, 463, 265–274. [Google Scholar]



| Analyte | Chemical Basis for Detection | Detection Sensor | LOD | Reference |
|---|---|---|---|---|
| Phosphorus | ||||
| Total phosphorus | Ascorbic acid reduction of phosphomolybdate | LED (λmax 880 nm)-PD | ≤10 μ g L-1 | [65] |
| Total phosphorus | Phosphomolybdenum blue | LED (λmax 635 nm)-PD | 0.15 mg P L-1 | [138] |
| Dissolved reactive phosphorus | Molybdophosphate blue | LED (Red)-PD | 0.1 μg P L-1 | [60] |
| Filterable reactive phosphate | Phosphomolybdenum blue | LED (λmax 650 nm)-PD | 0.15 μM | [132] |
| Reactive phosphate | Ascorbic acid reduction of phosphomolybdate | LED (λmax 660 nm)-PD | 12 μg L-1 P | [66] |
| Reactive phosphate | Phosphomolybdenum blue | LED (λmax 652 nm)-PD | 3 μg L-1 P | [133] |
| Orthophosphate | Phosphomolybdenum blue | LED (λmax 700 nm)-PD | 0.7 ppb (PO43-) | [89] |
| Orthophosphate | Yellow vanamolybdophosphoric acid | LED (λmax 390 nm)-PDA | 5 ppm (PO43-) | [3] |
| Phosphate | Molybdenum blue | LED (λmax 820 nm)-PT | 0.5 mg L-1 (P) | [56] |
| Orthophosphate | Malachite Green Reaction | LED (λmax 621 nm)- LED (λmax 660 nm) | 2 nM (PO43-) | [81] |
| Nitrate | ||||
| NO3- | Griess reaction | LED (λmax 560 nm)-PD | 15 μg L-1 | [116] |
| NO3- | Griess reaction | LED (λmax 526 nm)-PDA | 0.51μM (NO3-) | [31] |
| NO3- | Griess reaction | LED (λmax 565 nm)-PD | 24 μg L-1 (NO3-N) | [55] |
| NO3- | Griess reaction | LED (λmax 540 nm)-PT | 30 μg L-1 (NO3-) | [50] |
| NO3- | Griess reaction | LED (λmax 540 nm)-PD | 2.8 μg L-1 (N) | [135] |
| Nitrite | ||||
| NO2- | Griess reaction | LED (λmax 560 nm)-PD | 5 μg L-1 | [116] |
| NO2- | Griess reaction | LED (λmax 526 nm)-PDA | 0.2 μM | [29] |
| NO2- | Griess reaction | LED (λmax 525 nm)-PD | 4 μM | [58] |
| NO2- | Griess reaction | LED (λmax 540 nm)-PT | 18 μg L-1 (NO2-) | [50] |
| NOx | Griess reaction | LED (λmax 540 nm)-PD | 1.4 μg L-1 (N) | [136] |
| Ammonia | ||||
| NH4+ | Indophenol blue reaction | LED (λmax 660 nm)-PD | 25 μg L-1 | [116] |
| NH4+ | Reaction with (NaOH, cresol red and thymol blue) | LED (λmax 605 nm)-PT | 0.5 mg L-1 (N) | [56] |
| NH4+ | Bromocresol green | LED (λmax 590 nm)-PD | [97] | |
| Metals/Cations | ||||
| Cd (II) and Pb(II) | Malachite green-iodide method | LED (λmax 621 nm)-LED (λmax 621 nm) | 5 ng mL-1 (Cd2+) 20 ng mL-1 (Pb2+) | [79] |
| Uranium | AIII metal complexes | LED (λmax 660 nm)-PDA | 383 ppb (UO22+) | [30] |
| Co (II) and Mn (II) | PAR complexes | LED (λmax 540 nm)-PMT | 450 ppb (Co2+) 1.3 ppm (Mn2+) | [30] |
| Al and Zn | Xylenol orange | LED (λmax 563 nm)-PD | 0.2 μg L-1 (Al) 0.2 μg L-1 (Zn) | [59] |
| Fe (II) | Phenanthroline in ammonium acetate | LED (λmax 525 nm)-PD | 33 μM | [58] |
| Cr | Oxidation to dichromate with periodate | LED (λmax 460 nm)-PD | 6 ppm | [57] |
| Mn | Formaldoxime method | LED (λmax 460 nm)-PD | 0.2 ppm | [57] |
| Zn | PAR complex | LED (λmax 460 nm)-PD | 0.02 ppm | [57] |
| Fe | Phenanthroline method | LED (λmax 460 nm)-PD | 0.4 ppm | [57] |
| Cu, Pb, Zn, Ni, Co, Cd, Fe and Mn | PAR complexes | LED (λmax 550 nm)-PD | 320, 47, 79, 230, 5.4, 10, 24 and 33 ng mL-1 | [54] |
| Cd, Pb, Co and Ni | 5-Br-PAPS | LED (λmax 570 nm)-PMT | 6, 1.8, 0.15 and 0.48 μg L-1 | [41] |
| Co, V, Ni, Cu, Fe, Mn and Cd | PAR complexes | LED (λmax 540 nm)-PMT | 0.47, 0.97, 0.40, 0.41, 1, 1.15, 0.54 ppm | [35] |
| Co and Cd | PAR complexes | LED (λmax 565 nm)-PT | 0.6 ppb (Co) | [86] |
| Ba, Ca, Mg, Ni and Cu | EDTA metal complexes | LED (λmax 460 nm)-PMT | 11.9, 5.5, 8.3, 3.7 and 6.6 μM | [36] |
| As (III) and As (V) | Arsenomolybdate method | LED (λmax 565 nm)-PD | 4 μg L-1 As(t) | [139] |
| V | PAR | LED (λmax 568 nm)-Waters Quanta 4000 capillary electrophoresis system | 19 ppb | [140] |
| Ni | Hexamine complex | LED (λmax 950 nm)-PD | [141] | |
| Fe (II) | Ferrozine method | LED (λmax 565 nm)-PD | 0.1 μM | [142] |
| Mn and Co | PAR | LED (λmax 500 nm)- LED (λmax 621 nm) | 90 nM Mn and Co | [77] |
| Anions | ||||
| Cl-, NO3-, SO42-, F-, | Chromate-diethanolamine background | LED (λmax 379.5 nm)-Waters | 5, 9, 14, 3 and 5 μg | [95] |
| PO43- | electrolyte | CIA | L-1 | |
| Cl- | Thiocynate method | LED (λmax 525 nm)-PD | 158 μM | [58] |
| Cl- | Thiocynate method | LED (λmax 460 nm)-PD | 0.2 ppm | [57] |
| Lactate, butyrate, salicylate, propionate, acetate, phosphate, formate and citrate | EDTA anion complexes | LED (λmax 460 nm)-PMT | 13.7, 12.1, 14.5, 4.7, 4.7, 12.8, 14.6 and 7.6 μM | [36] |
© 2008 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
O’Toole, M.; Diamond, D. Absorbance Based Light Emitting Diode Optical Sensors and Sensing Devices. Sensors 2008, 8, 2453-2479. https://doi.org/10.3390/s8042453
O’Toole M, Diamond D. Absorbance Based Light Emitting Diode Optical Sensors and Sensing Devices. Sensors. 2008; 8(4):2453-2479. https://doi.org/10.3390/s8042453
Chicago/Turabian StyleO’Toole, Martina, and Dermot Diamond. 2008. "Absorbance Based Light Emitting Diode Optical Sensors and Sensing Devices" Sensors 8, no. 4: 2453-2479. https://doi.org/10.3390/s8042453





