Utilizing of Adsorptive Transfer Stripping Technique Brdicka Reaction for Determination of Metallothioneins Level in Melanoma Cells, Blood Serum and Tissues
Abstract
:1. Introduction
2. Material and Methods
2.1 Chemicals and pH measurements
2.2 Melanoma Cell lines
2.3 Animals
2.4 Patients with malignant melanoma
2.5 Preparation of biological samples for electrochemical analysis
2.6 Apparatus
2.6.1 Electrochemical measurements
2.6.2 Adsorptive transfer stripping technique differential pulse voltammetry Brdicka reaction of MT
2.6.3 Dot Immunobinding Assay (DIA)
2.6.4 Cell counting
2.7 Statistical analyses
3. Results and Discussion
3.1 Influence of dilution and kind of real sample on MT signal
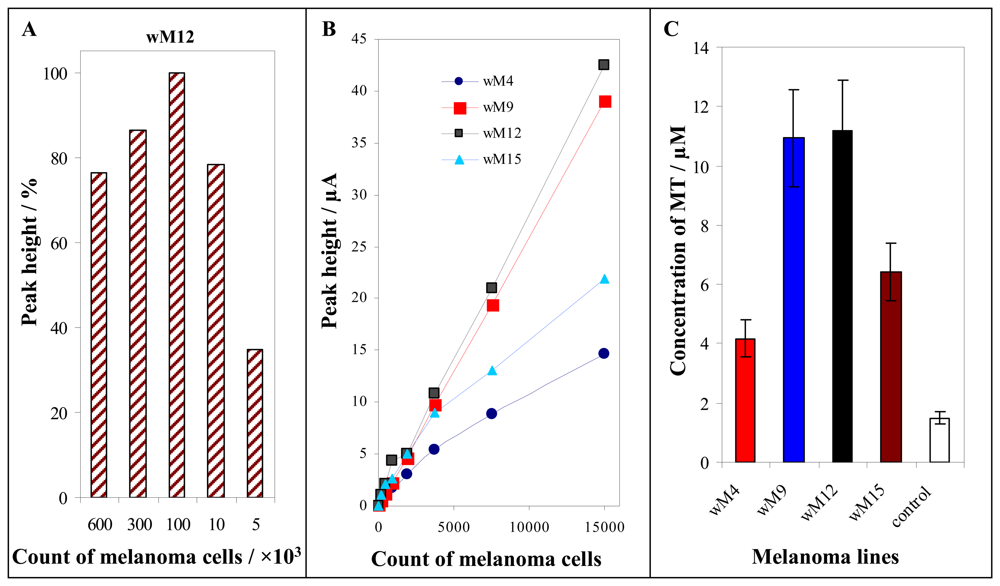
3.2 Detection of MT in tumour cell cultures
3.3 Metallothionein content in minipig melanoma tissues
3.4 Determination of metallothionein from the patients with melanoma
4. Conclusion
Acknowledgments
References
- Kagi, J.H.R.; Schaffer, A. Biochemistry of Metallothionein. Biochemistry 1988, 27, 8509–8515. [Google Scholar]
- Kizek, R.; Vacek, J.; Adam, V.; Vojtesek, B. Metallothionein - Cisplatin and anticancer therapy. Klin. Biochem. Metab. 2004, 12, 72–78. [Google Scholar]
- Studnickova, M.; Turanek, J.; Zabrsova, H.; Krejci, M.; Kysel, M. Rat liver metallothioneins are metal dithiolene clusters. J. Electroanal. Chem. 1997, 421, 25–32. [Google Scholar]
- Hamer, D.H. Metallothionein. Annu. Rev. Biochem. 1986, 55, 913–951. [Google Scholar]
- Cousins, R.J. Absorption, Transport, and Hepatic-Metabolism of Copper and Zinc - Special Reference to Metallothionein and Ceruloplasmin. Physiol. Rev. 1985, 65, 238–309. [Google Scholar]
- Kagi, J.H.R. Overview of Metallothionein. Method Enzymol. 1991, 205, 613–626. [Google Scholar]
- Roesijadi, G. Metallothioneins in Metal Regulation and Toxicity in Aquatic Animals. Aquat. Toxicol 1992, 22, 81–114. [Google Scholar]
- Theocharis, S.E.; Margeli, A.P.; Klijanienko, J.T.; Kouraklis, G.P. Metallothionein expression in human neoplasia. Histopathology 2004, 45, 103–118. [Google Scholar]
- Dutsch-Wicherek, M.; Popiela, T.J.; Klimek, M.; Rudnicka-Sosin, L.; Wicherek, L.; Oudinet, J.P.; Skladzien, J.; Tomaszewska, R. Metallothionein stroma reaction in tumor adjacent healthy tissue in head and neck squamous cell carcinoma and breast adenocarcinoma. Neuroendocrinol. Lett. 2005, 26, 567–574. [Google Scholar]
- Klimek, M.; Wicherek, L.; Galazka, K.; Tetlak, T.; Popiela, T.J.; Kulczycka, M.; Rudnicka-Sosin, L.; Dutsch-Wicherek, M. Cycle dependent expression of endometrial metallothionein. Neuroendocrinol. Lett. 2005, 26, 663–666. [Google Scholar]
- Klimek, R. Biology of cancer: Thermodynamic answers to some questions. Neuroendocrinol. Lett. 2001, 22, 413–416. [Google Scholar]
- Kukacka, J.; Vajtr, D.; Huska, D.; Prusa, R.; Houstava, L.; Samal, F.; Diopan, V.; Kotaska, K.; Kizek, R. Blood Metallothionein, Neuron Specific Enolase, And Protein S100B In Patients With Trauma Brain Injury. Neuroendocrinol. Lett. 2006, 27, 116–120. [Google Scholar]
- Prusa, R.; Blastik, O.; Potesil, D.; Trnkova, L.; Zehnalek, J.; Adam, V.; Petrlova, J.; Jelen, F.; Kizek, R. Analytic method for determination of metallothioneins as tumor markers. Clin. Chem. 2005, 51, A56–A56. [Google Scholar]
- Prusa, R.; Kizek, R.; Vacek, J.; Trnkova, L.; Zehnalek, J. Study of relationship betwenn metallothionein and heavy metals by CPSA method. Clin. Chem. 2004, 50, A28–A29. [Google Scholar]
- Prusa, R.; Petrlova, J.; Kukacka, J.; Adam, V.; Sures, B.; Beklova, M.; Kizek, R. Study of interaction of glutathiones and metallothionein with cytostatics. Clin. Chem. 2006, 52, A175–A175. [Google Scholar]
- Prusa, R.; Svoboda, M.; Blastik, O.; Adam, V.; Zitka, O.; Beklova, M.; Eckschlager, T.; Kizek, R. Increase in content of metallothionein as marker of resistence to cisplatin treatment. Clin. Chem. 2006, 52, A174–A175. [Google Scholar]
- Dziegiel, P.; Suder, E.; Surowiak, P.; Kornafel, J.; Zabel, M. Expression of metallothionein in synovial sarcoma cells. Appl. Immunohistochem. 2002, 10, 357–362. [Google Scholar]
- Goldmann, T.; Moorkamp, A.; Wiedorn, K.H.; Suter, L.; Otto, F. The prognostic value of the expression of collagenase IV, cathepsin D and metallothionein in squamous cell carcinomas of the skin determined by immunohistochemistry. Arch. Dermatol. Res. 2001, 293, 115–120. [Google Scholar]
- Goldmann, T.; Ribbert, D.; Suter, L.; Brode, M.; Otto, F. Tumor characteristics involved in the metastatic behaviour as an improvement in primary cutaneous melanoma prognostics. J. Exp. Clin. Cancer Res. 1998, 17, 483–489. [Google Scholar]
- Jin, R.X.; Huang, J.X.; Tan, P.H.; Bay, B.H. Clinicopathological significance of metallothioneins in breast cancer. Pathol. Oncol. Res. 2004, 10, 74–79. [Google Scholar]
- Mitropoulos, D.; Kyroudi-Voulgari, A.; Theocharis, S.; Serafetinides, E.; Moraitis, E.; Zervas, A.; Kittas, C. Prognostic significance of metallothionein expression in renal cell carcinoma. World J. Gastroenterol. 2005, 3, 5. [Google Scholar]
- Ohshio, G.; Imamura, T.; Okada, N.; Wang, Z.H.; Yamaki, K.; Kyogoku, T.; Suwa, H.; Yamabe, H.; Imamura, M. Immunohistochemical study of metallothionein in pancreatic carcinomas. J. Cancer Res. Clin. Oncol. 1996, 122, 351–355. [Google Scholar]
- Sauerbrey, A.; Zintl, F.; Volm, M. Expression of Metallothionein in Initial and Relapsed Childhood Acute Lymphoblastic-Leukemia. Ann. Hematol. 1994, 69, 111–115. [Google Scholar]
- Shukla, V.K.; Aryya, N.C.; Pitale, A.; Pandey, M.; Dixit, V.K.; Reddy, C.D.; Gautam, A. Metallothionein expression in carcinoma of the gallbladder. Histopathology 1998, 33, 154–157. [Google Scholar]
- Sugita, K.; Yamamoto, O.; Asahi, M. Immunohistochemical analysis of metallothionein expression in malignant melanoma in Japanese patients. Am. J. Dermatopathol. 2001, 23, 29–35. [Google Scholar]
- Surowiak, P.; Materna, V.; Kaplenko, I.; Spaczynski, M.; Dietel, M.; Lage, H.; Zabel, M. Augmented expression of metallothionein and glutathione S-transferase pi as unfavourable prognostic factors in cisplatin-treated ovarian cancer patients. Virchows Arch. 2005, 447, 626–633. [Google Scholar]
- Zelger, B.; Hittmair, A.; Schir, M.; Ofner, C.; Ofner, D.; Fritsch, P.O.; Bocker, W.; Jasani, B.; Schmid, K.W. Immunohistochemically Demonstrated Metallothionein Expression in Malignant-Melanoma. Histopathology 1993, 23, 257–264. [Google Scholar]
- Weinlich, G.; Eisendle, K.; Hassler, E.; Baltaci, M.; Fritsch, P.O.; Zelger, B. Metallothionein - overexpression as a highly significant prognostic factor in melanoma: a prospective study on 1270 patients. Br. J. Cancer 2006, 94, 835–841. [Google Scholar]
- Meyskens, F.L.; Farmer, P.J.; Anton-Culver, H. Etiologic pathogenesis of melanoma: A unifying hypothesis for the missing attributable risk. Clin. Cancer Res. 2004, 10, 2581–2583. [Google Scholar]
- Weinlich, G.; Bitterlich, W.; Mayr, V.; Fritsch, P.O.; Zelger, B. Metallothionein-overexpression as a prognostic factor for progression and survival in melanoma. A prospective study on 520 patients. Br. J. Dermatol. 2003, 149, 535–541. [Google Scholar]
- Sarangarajan, R.; Apte, S.P. The polymerization of melanin: a poorly understood phenomenon with egregious biological implications. Melanoma Res. 2006, 16, 3–10. [Google Scholar]
- Happle, R.; Traupe, H.; Vakilzadeh, F.; Macher, E. Arguments in Favor of a Polygenic Inheritance of Precursor Nevi. J. Am. Acad. Dermatol. 1982, 6, 540–542. [Google Scholar]
- Kamb, A.; Shattuckeidens, D.; Eeles, R.; Liu, Q.; Gruis, N.A.; Ding, W.; Hussey, C.; Tran, T.; Miki, Y.; Weaverfeldhaus, J.; McClure, M.; Aitken, J.F.; Anderson, D.E.; Bergman, W.; Frants, R.; Goldgar, D.E.; Green, A.; Maclennan, R.; Martin, N.G.; Meyer, L.J.; Youl, P.; Zone, J.J.; Skolnick, M.H.; Cannonalbright, L.A. Analysis of the P16 Gene (Cdkn2) as a Candidate for the Chromosome 9p Melanoma Susceptibility Locus. Nature Genet. 1994, 8, 22–26. [Google Scholar]
- Gruis, N.A.; Vandervelden, P.A.; Sandkuijl, L.A.; Prins, D.E.; Weaverfeldhaus, J.; Kamb, A.; Bergman, W.; Frants, R.R. Homozygotes for Cdkn2 (P16) Germline Mutation in Dutch Familial Melanoma Kindreds. Nature Genet. 1995, 10, 351–353. [Google Scholar]
- Vasen, H.F.A.; Gruis, N.A.; Frants, R.R.; van der Velden, P.A.; Hille, E.T.M.; Bergman, W. Risk of developing pancreatic cancer in families with familial atypical multiple mole melanoma associated with a specific 19 deletion of p16 (p16-Leiden). Int. J. Cancer 2000, 87, 809–811. [Google Scholar]
- Kefford, R.; Bishop, J.N.; Tucker, M.; Bressac-de-Paillerets, B.; Bianchi-Scarra, G.; Bergman, W.; Goldstein, A.; Puig, S.; Mackie, R.; Elder, D.; Hansson, J.; Hayward, N.; Hogg, D.; Olsson, H. Genetic testing for melanoma. Lancet Oncol. 2002, 3, 653–654. [Google Scholar]
- Goldstein, A.M.; Chan, M.; Harland, M.; Hayward, N.K.; Demenais, F.; Bishop, D.T.; Azizi, E.; Bergman, W.; Bianchi-Scarra, G.; Bruno, W.; Calista, D.; Albright, L.A.C.; Chaudru, V.; Chompret, A.; Cuellar, F.; Elder, D.E.; Ghiorzo, P.; Gillanders, E.M.; Gruis, N.A.; Hansson, J.; Hogg, D.; Holland, E.A.; Kanetsky, P.A.; Kefford, R.F.; Landi, M.T.; Lang, J.; Leachman, S.A.; MacKie, R.M.; Magnusson, V.; Mann, G.J.; Bishop, J.N.; Palmer, J.M.; Puig, S.; Puig-Butille, J.A.; Stark, M.; Tsao, H.; Tucker, M.A.; Whitaker, L.; Yakobson, E. Features associated with germline CDKN2A mutations: a GenoMEL study of melanoma-prone families from three continents. J. Med. Genet. 2007, 44, 99–106. [Google Scholar]
- Alvarado, N.E.; Cancio, I.; Hylland, K.; Marigomez, I.; Soto, M. Immunolocalization of metallothioneins in different tissues of turbot (Scophthalmus maximus) exposed to Cd. Histol. Histopath. 2007, 22, 719–728. [Google Scholar]
- Alves, S.; Cardoso, S.V.; Bernardes, V.D.F.; Machado, V.C.; Mesquita, R.A.; do Carmo, M.A.V.; Aguiar, M.C.F. Metallothionein immunostaining in adenoid cystic carcinomas of the salivary glands. Oral Oncol. 2007, 43, 252–256. [Google Scholar]
- Lobinski, R.; Chassaigne, H.; Szpunar, J. Analysis for metallothioneins using coupled techniques. Talanta 1998, 46, 271–289. [Google Scholar]
- Szpunar, J. Bio-inorganic speciation analysis by hyphenated techniques. Analyst 2000, 125, 963–988. [Google Scholar]
- Szpunar, J.; Lobinski, R.; Prange, A. Hyphenated techniques for elemental speciation in biological systems. Appl. Spectrosc. 2003, 57, 102A–112A. [Google Scholar]
- Chassaigne, H.; Lobinski, R. Characterization of metallothionein isoforms by reversed-phase high-performance liquid chromatography with on-line post-column acidification and electrospray mass spectrometric detection. J. Chromatogr. A 1998, 829, 127–136. [Google Scholar]
- Chassaigne, H.; Lobinski, R. Characterization of horse kidney metallothionein isoforms by electrospray MS and reversed-phase HPLC-electrospray MS. Analyst 1998, 123, 2125–2130. [Google Scholar]
- Chassaigne, H.; Lobinski, R. Polymorphism and identification of metallothionein isoforms by reversed-phase HPLC with on-line ion spray mass spectrometric detection. Anal. Chem. 1998, 70, 2536–2543. [Google Scholar]
- Kizek, R.; Vacek, J.; Trnkova, L.; Klejdus, B.; Havel, L. Application of catalytic reactions on a mercury electrode for metallothionein electrochemical detection. Chem. Listy. 2004, 98, 166–173. [Google Scholar]
- Thompson, J.A.J.; Cosson, R.P. An improved electrochemical method for the quantification of metallothioneins in marine organisms. Marine Environ. Res. 1984, 11, 137–152. [Google Scholar]
- Dabrio, M.; Rodriguez, A.R.; Bordin, G.; Bebianno, M.J.; De Ley, M.; Šestakova, I.; Vasak, M.; Nordberg, M. Recent developments in quantification methods for metallothionein. J. Inorg. Biochem. 2002, 88, 123–134. [Google Scholar]
- Sestakova, I.; Navratil, T. Voltammetric methods in metallothionein research. Bioinorg. Chem. Appl. 2005, 3, 43–53. [Google Scholar]
- Adam, V.; Blastik, O.; Krizkova, S.; Lubal, P.; Kukacka, J.; Prusa, R.; Kizek, R. Application of the Brdicka reaction in determination of metallothionein in patients with tumours. Chem. Listy 2008, 102, 51–58. [Google Scholar]
- Adam, V.; Beklova, M.; Pikula, J.; Hubalek, J.; Trnkova, L.; Kizek, R. Shapes of differential pulse voltammograms and level of metallothionein at different animal species. Sensors 2007, 7, 2419–2429. [Google Scholar]
- Kukacka, J.; Vajtr, D.; Huska, D.; Prusa, R.; Houstava, L.; Samal, F.; Diopan, V.; Kotaska, K.; Kizek, R. Blood metallothionein, neuron specific enolase, and protein S100B in patients with traumatic brain injury. Neuroendocrinol. Lett. 2006, 27, 116–120. [Google Scholar]
- Adam, V.; Krizkova, S.; Zitka, O.; Trnkova, L.; Petrlova, J.; Beklova, M.; Kizek, R. Determination of apo-metallothionein using adsorptive transfer stripping technique in connection with differential pulse voltammetry. Electroanalysis 2007, 19, 339–347. [Google Scholar]
- Petrlova, J.; Potesil, D.; Mikelova, R.; Blastik, O.; Adam, V.; Trnkova, L.; Jelen, F.; Prusa, R.; Kukacka, J.; Kizek, R. Attomole voltammetric determination of metallothionein. Electrochim. Acta 2006, 51, 5112–5119. [Google Scholar]
- Yang, M.L.; Zhang, Z.J.; Hu, Z.B.; Li, J.H. Differential pulse anodic stripping voltammetry detection of metallothionein at bismuth film electrodes. Talanta 2006, 69, 1162–1165. [Google Scholar]
- Adam, V.; Petrlova, J.; Potesil, D.; Zehnalek, J.; Sures, B.; Trnkova, L.; Jelen, F.; Kizek, R. Study of metallothionein modified electrode surface behavior in the presence of heavy metal ions-biosensor. Electroanalysis 2005, 17, 1649–1657. [Google Scholar]
- Lopez, M.J.; Arino, C.; Diaz-Cruz, S.; Diaz-Cruz, J.M.; Tauler, R.; Esteban, M. Voltammetry assisted by multivariate analysis as a tool for speciation of metallothioneins: Competitive complexation of alpha- and beta-metallothionein domains with cadmium and zinc. Environ. Sci. Technol. 2003, 37, 5609–5616. [Google Scholar]
- Diaz-Cruz, M.S.; Lopez, M.J.; Diaz-Cruz, J.M.; Esteban, M. Comparison of the zinc-cadmium exchange properties of the metallothionein related peptide {Lys-Cys-Thr-Cys-Cys-Ala} and a zinc-containing metallothionein: study by voltammetry and multivariate curve resolution. J. Electroanal. Chem. 2002, 523, 114–125. [Google Scholar]
- Sestakova, I.; Mader, P. Voltammetry on mercury and carbon electrodes as a tool for studies of metallothionein interactions with metal ions. Cell. Mol. Biol. 2000, 46, 257–267. [Google Scholar]
- Erk, M.; Raspor, B. Advantages and disadvantages of voltammetric method in studying cadmium-metallothionein interactions. Cell. Mol. Biol. 2000, 46, 269–281. [Google Scholar]
- Erk, M.; Ivankovic, D.; Raspor, B.; Pavicic, J. Evaluation of different purification procedures for the electrochemical quantification of mussel metallothioneins. Talanta 2002, 57, 1211–1218. [Google Scholar]
- Raspor, B. Elucidation of the mechanism of the Brdicka reaction. J. Electroanal. Chem. 2001, 503, 159–162. [Google Scholar]
- Brdicka, R. Polarographic investigation in serological cancer diagnosis. Nature 1937, 139, 1020–1021. [Google Scholar]
- Brdicka, R. Application of the polarographic effect of proteins in cancer diagnosis. Nature 1937, 139, 330–330. [Google Scholar]
- Huska, D.; Krizkova, S.; Beklova, M.; Havel, L.; Zehnalek, J.; Diopan, V.; Adam, V.; Zeman, L.; Babula, P.; Kizek, R. Influence of cadmium(II) ions and brewery sludge on metallothionein level in earthworms (Eisenia fetida) - Biotransforming of toxic wastes. Sensors 2008, 8, 1039–1047. [Google Scholar]
- Kizek, R.; Trnkova, L.; Palecek, E. Determination of metallothionein at the femtomole level by constant current stripping chronopotentiometry. Anal. Chem. 2001, 73, 4801–4807. [Google Scholar]
- Kizek, R.; Vacek, J.; Trnkova, L.; Klejdus, B.; Havel, L. Application of catalytic reactions on a mercury electrode for electrochemical detection of metallothioneins. Chem. Listy 2004, 98, 166–173. [Google Scholar]
- Krizkova, S.; Zitka, O.; Adam, V.; Beklova, M.; Horna, A.; Svobodova, Z.; Sures, B.; Trnkova, L.; Zeman, L.; Kizek, R. Possibilities of electrochemical techniques in metallothionein and lead detection in fish tissues. Czech J. Anim. Sci. 2007, 52, 143–148. [Google Scholar]
- Ostatna, V.; Palecek, E. Native, denatured and reduced BSA - Enhancement of chronopotentiometric peak H by guanidinium chloride. Electrochim. Acta 2008, 53, 4014–4021. [Google Scholar]
- Petrlova, J.; Krizkova, S.; Zitka, O.; Hubalek, J.; Prusa, R.; Adam, V.; Wang, J.; Beklova, M.; Sures, B.; Kizek, R. Utilizing a chronopotentiometric sensor technique for metallothionein determination in fish tissues and their host parasites. Sens. Actuator B-Chem. 2007, 127, 112–119. [Google Scholar]
- Serrano, N.; Sestakova, I.; Diaz-Cruz, J.M. Constant current stripping chronopotentiometry for the study of adsorbing inert and electrochemically nonreversible metal complexes at low concentrations: Application to Cd and Zn metallothioneins. Electroanalysis 2006, 18, 169–176. [Google Scholar]
- Serrano, N.; Sestakova, I.; Diaz-Cruz, J.M.; Arino, C. Adsorptive accumulation in constant current stripping chronopotentiometry as an alternative for the electrochemical study of metal complexation by thiol-containing peptides. J. Electroanal. Chem. 2006, 591, 105–117. [Google Scholar]
- Sestakova, I.; Kopanica, M.; Havran, L.; Palecek, E. Constant current chronopotentiometric stripping analysis of Cd-metallothionein on carbon and mercury electrodes. Comparison with voltammetry. Electroanalysis 2000, 12, 100–104. [Google Scholar]
- Strouhal, M.; Kizek, R.; Vecek, J.; Trnkova, L.; Nemec, M. Electrochemical study of heavy metals and metallothionein in yeast Yarrowia lipolytica. Bioelectrochemistry 2003, 60, 29–36. [Google Scholar]
- Tomschik, M.; Havran, L.; Fojta, M.; Palecek, E. Constant current chronopotentiometric stripping analysis of bioactive peptides at mercury and carbon electrodes. Electroanalysis 1998, 10, 403–409. [Google Scholar]
- Tomschik, M.; Havran, L.; Palecek, E.; Heyrovsky, M. The “presodium” catalysis of electroreduction of hydrogen ions on mercury electrodes by metallothionein. An investigation by constant current derivative stripping chronopotentiometry. Electroanalysis 2000, 12, 274–279. [Google Scholar]
- Trnkova, L.; Kizek, R.; Vacek, J. Catalytic signal of rabbit liver metallothionein on a mercury electrode: a combination of derivative chronopotentiometry with adsorptive transfer stripping. Bioelectrochemistry 2002, 56, 57–61. [Google Scholar]
- Lauerova, L.; Dusek, L.; Simickova, M.; Kocak, I.; Vagundova, M.; Zaloudik, J.; Kovarik, J. Malignant melanoma associates with Th1/Th2 imbalance that coincides with disease progression and immunotherapy response. Neoplasma 2002, 49, 159–166. [Google Scholar]
- Supalkova, V.; Petrek, J.; Baloun, J.; Adam, V.; Bartusek, K.; Trnkova, L.; Beklova, M.; Diopan, V.; Havel, L.; Kizek, R. Multi-instrumental investigation of affecting of early somatic embryos of Spruce by cadmium(II) and lead(II) ions. Sensors 2007, 7, 743–759. [Google Scholar]
- Erk, M.; Ivanković, D.; Raspor, B.; Pavičić, J. Evaluation of different purification procedures for the electrochemical quantification of mussel metallothioneins. Talanta 2002, 57, 1211–1218. [Google Scholar]
- Petrlova, J.; Potesil, D.; Zehnalek, J.; Sures, B.; Adam, V.; Trnkova, L.; Kizek, R. Cisplatin electrochemical biosensor. Electrochim. Acta 2006, 51, 5169–5173. [Google Scholar]
- Fortyn, K.; Hruban, V.; Horak, V.; Tichy, J. Exceptional occurrence and extent of malignant melanoma in pig. Vet. Med. 1998, 43, 87–91. [Google Scholar]
- Horak, V.; Fortyn, K.; Hruban, V.; Klaudy, J. Hereditary melanoblastoma in miniature pigs and its successful therapy by devitalization technique. Cell. Mol. Biol. 1999, 45, 1119–1129. [Google Scholar]
- Borovansky, J.; Horak, V.; Elleder, M.; Fortyn, K.; Smit, N.P.M.; Kolb, A.M. Biochemical characterization of a new melanoma model the minipig MeLiM strain. Melanoma Res. 2003, 13, 543–548. [Google Scholar]
- Geffrotin, C.; Crechet, F.; Le Roy, P.; Le Chalony, C.; Leplat, J.J.; Iannuccelli, N.; Barbosa, A.; Renard, C.; Gruand, J.; Milan, D.; Horak, V.; Tricaud, Y.; Bouet, S.; Franck, M.; Frelat, G.; Vincent-Naulleau, S. Identification of five chromosomal regions involved in predisposition to melanoma by genome-wide scan in the MeLiM swine model. Int. J. Cancer 2004, 110, 39–50. [Google Scholar]
- Vincent-Naulleau, S.; Le Chalony, C.; Leplat, J.J.; Bouet, S.; Bailly, C.; Spatz, A.; Vielh, P.; Avril, M.F.; Tricaud, Y.; Gruand, J.; Horak, V.; Frelat, G.; Geffrotin, C. Clinical and histopathological characterization of cutaneous melanomas in the melanoblastoma-bearing Libechov minipig model. Pigm. Cell. Res. 2004, 17, 24–35. [Google Scholar]
- Svoboda, M.; Eichlerova, K.; Horak, V.; Hradecky, J. Development of haematological indices in melanoma-bearing Libechov Minipigs. Acta Vet. BRNO 2005, 74, 603–611. [Google Scholar]
- Le Chalony, C.; Renard, C.; Vincent-Naulleau, S.; Crechet, F.; Leplat, J.J.; Tricaud, Y.; Horak, V.; Gruand, J.; Le Roy, P.; Frelat, G.; Geffrotin, C. CDKN2A region polymorphism and genetic susceptibility to melanoma in the melim swine model of familial melanoma. Int. J. Cancer 2003, 103, 631–635. [Google Scholar]
- Geffrotin, C.; Horak, V.; Crechet, F.; Tricaud, Y.; Lethias, C.; Vincent-Naulleau, S.; Vielh, P. Opposite regulation of tenascin-C and tenascin-X in MeLiM swine heritable cutaneous malignant melanoma. Biochim. Biophys. Acta-Gen. Subj. 2000, 1524, 196–202. [Google Scholar]
- Potesil, D.; Mikelova, R.; Adam, V.; Kizek, R.; Prusa, R. Change of the protein p53 electrochemical signal according to its structural form - Quick and sensitive distinguishing of native, denatured, and aggregated form of the “guardian of the genome”. Protein J. 2006, 25, 23–32. [Google Scholar]
- Breazeale, R.I.; Fishburn, J.; Buchanan, T.; Stone, J. Overexpression of metallothionein and survival in uveal melanoma. Invest. Ophthalmol. Vis. Sci. 1996, 37, 2877–2877. [Google Scholar]



© 2008 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Krizkova, S.; Fabrik, I.; Adam, V.; Kukacka, J.; Prusa, R.; Chavis, G.J.; Trnkova, L.; Strnadel, J.; Horak, V.; Kizek, R. Utilizing of Adsorptive Transfer Stripping Technique Brdicka Reaction for Determination of Metallothioneins Level in Melanoma Cells, Blood Serum and Tissues. Sensors 2008, 8, 3106-3122. https://doi.org/10.3390/s8053106
Krizkova S, Fabrik I, Adam V, Kukacka J, Prusa R, Chavis GJ, Trnkova L, Strnadel J, Horak V, Kizek R. Utilizing of Adsorptive Transfer Stripping Technique Brdicka Reaction for Determination of Metallothioneins Level in Melanoma Cells, Blood Serum and Tissues. Sensors. 2008; 8(5):3106-3122. https://doi.org/10.3390/s8053106
Chicago/Turabian StyleKrizkova, Sona, Ivo Fabrik, Vojtech Adam, Jiri Kukacka, Richard Prusa, Grace J. Chavis, Libuse Trnkova, Jan Strnadel, Vratislav Horak, and Rene Kizek. 2008. "Utilizing of Adsorptive Transfer Stripping Technique Brdicka Reaction for Determination of Metallothioneins Level in Melanoma Cells, Blood Serum and Tissues" Sensors 8, no. 5: 3106-3122. https://doi.org/10.3390/s8053106



