Chemical Sensors Based on Cyclodextrin Derivatives
Abstract
:Introduction
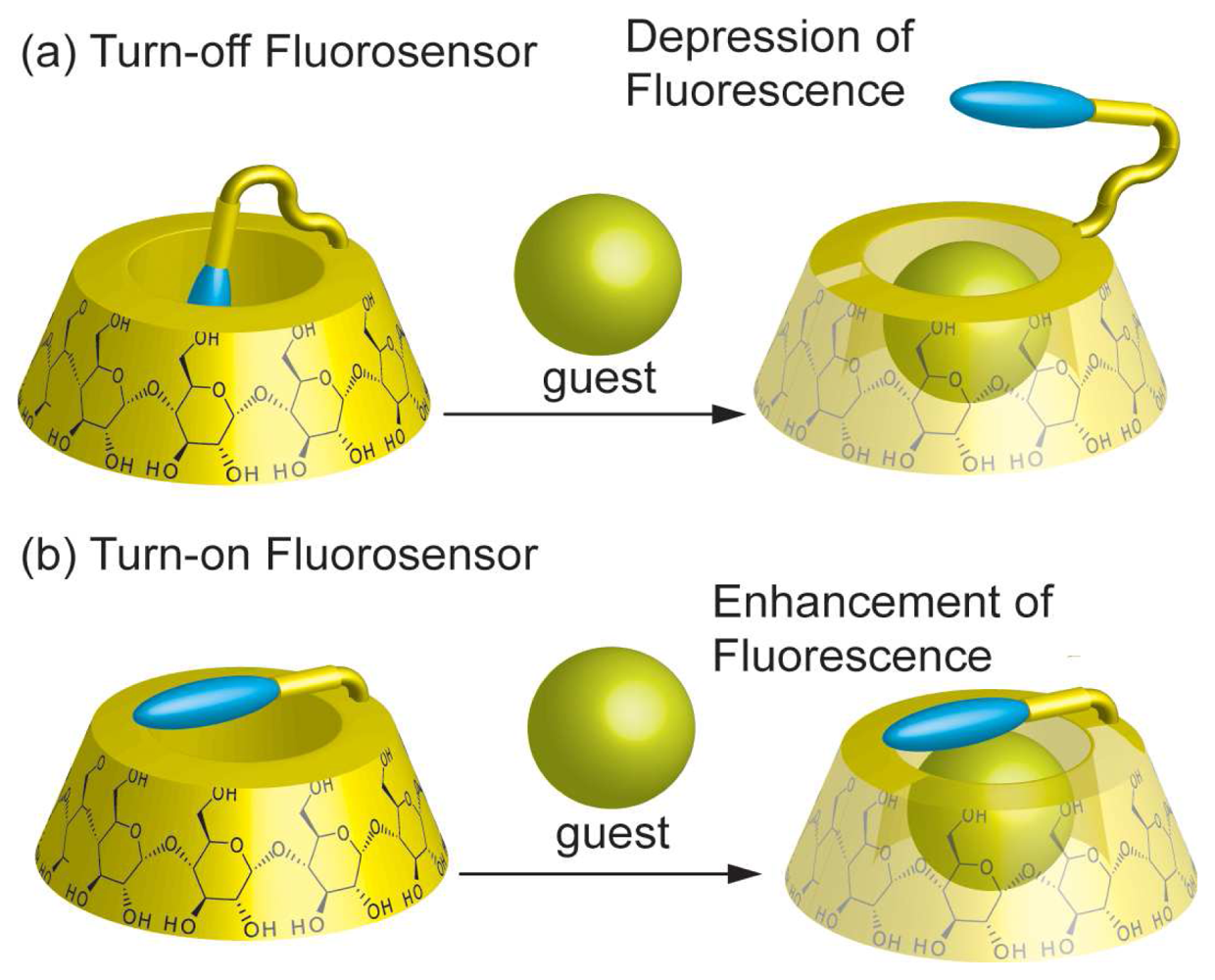
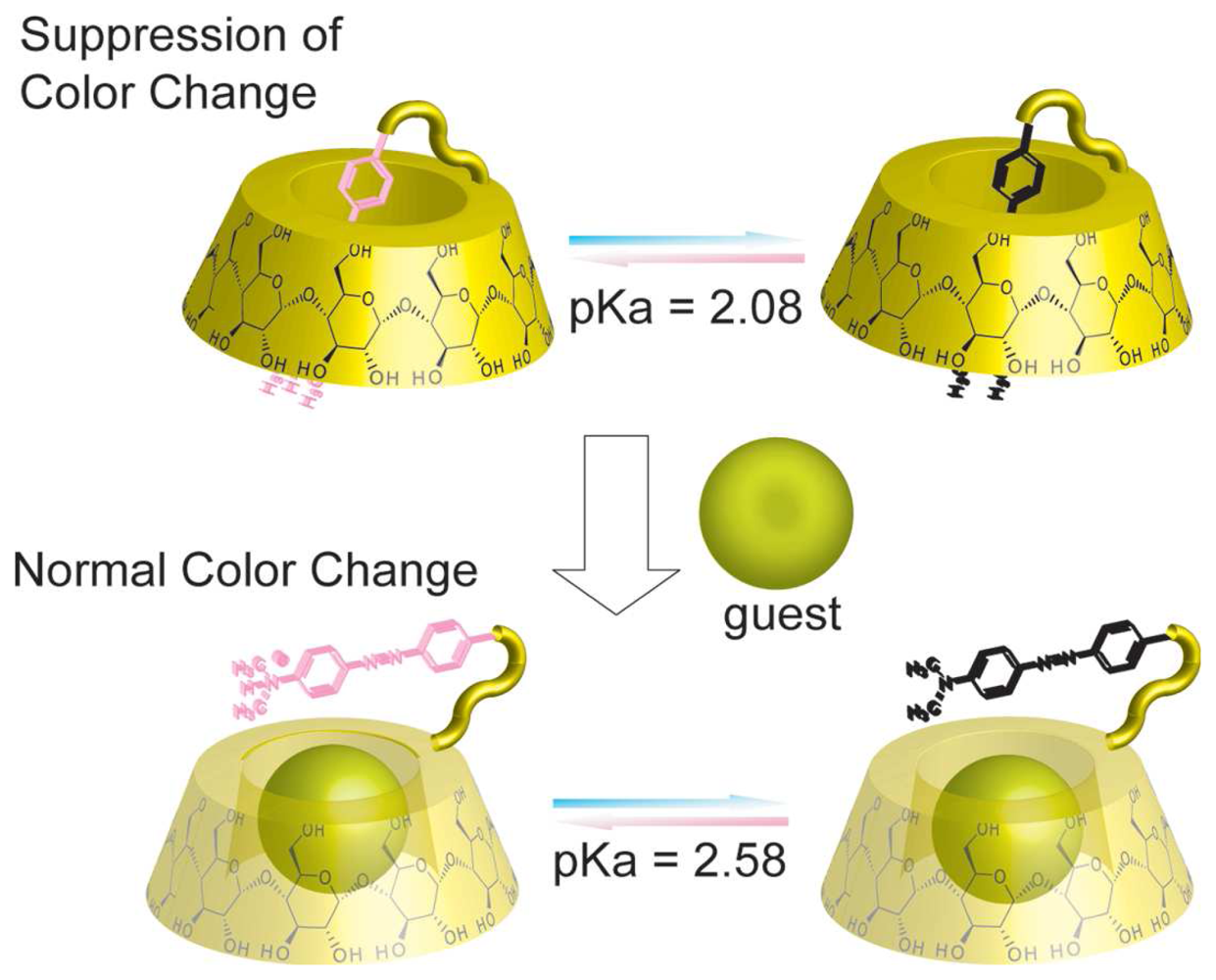
Chemical Sensors Using Chromophore Appended CDs
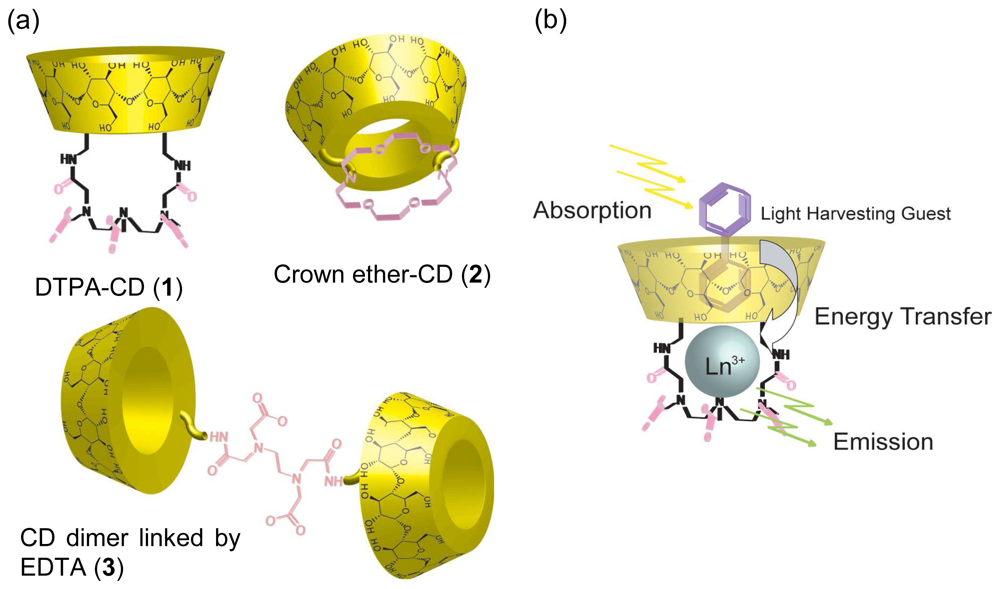
Chemical Sensors Using Metallocyclodextrins
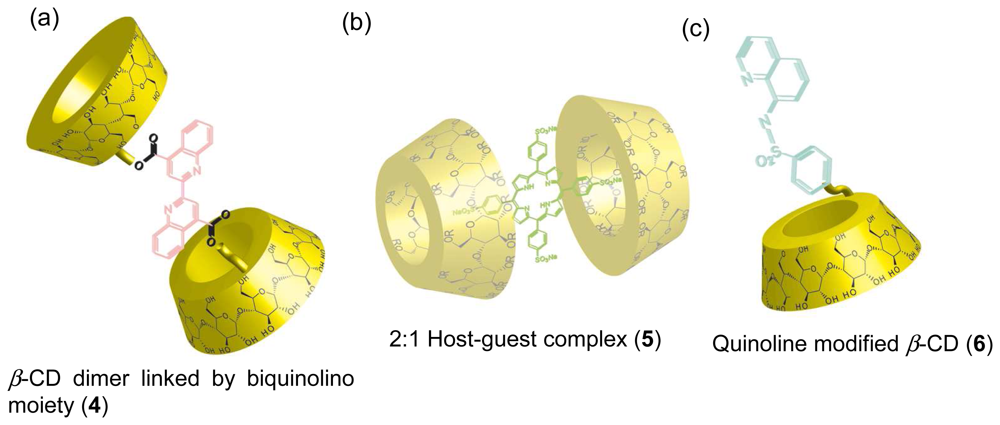
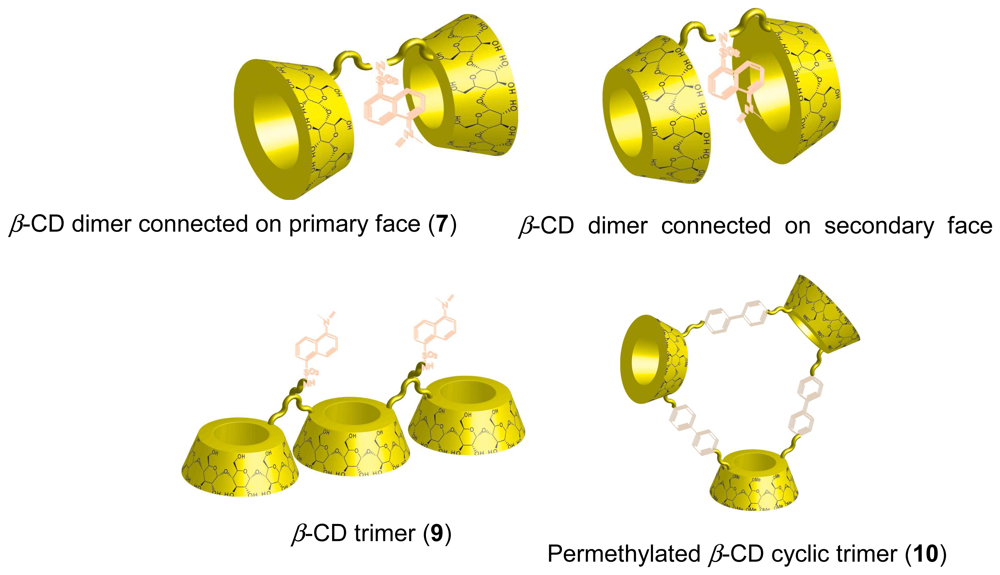
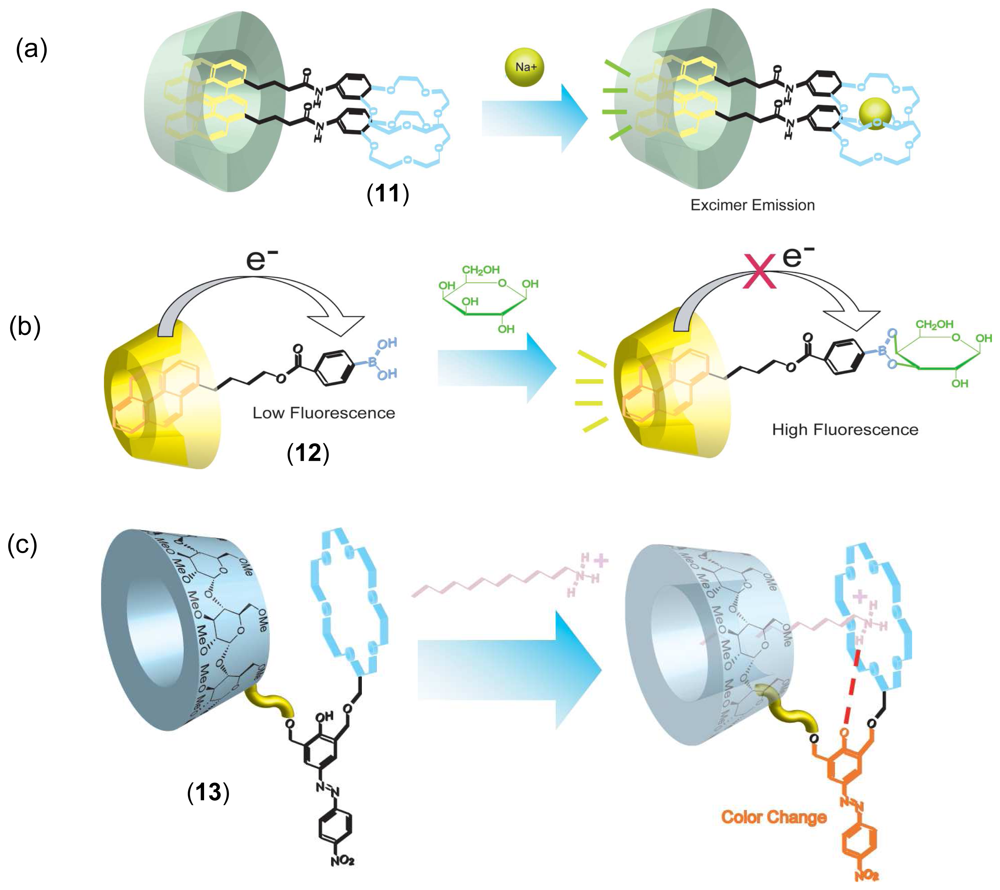
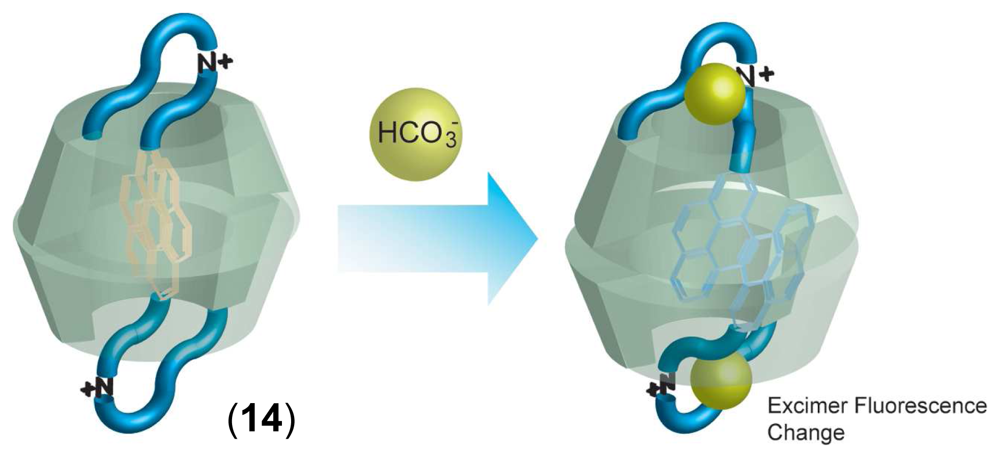
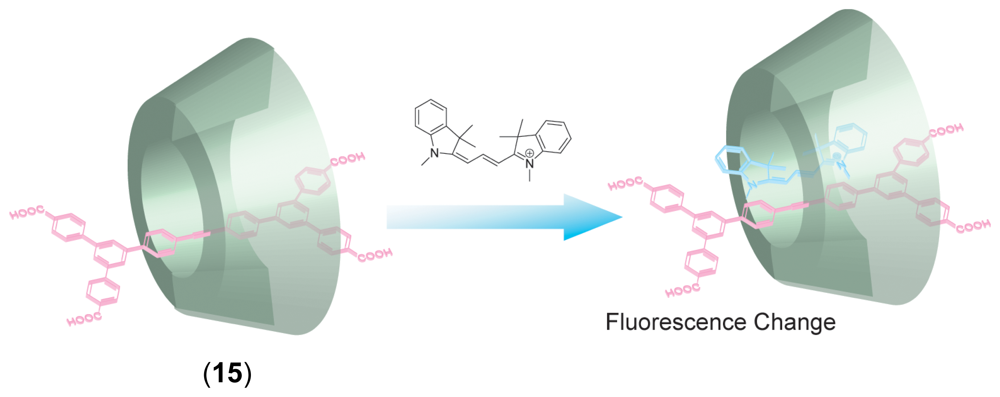
Chemical Sensors Using CD Supramolecular Systems
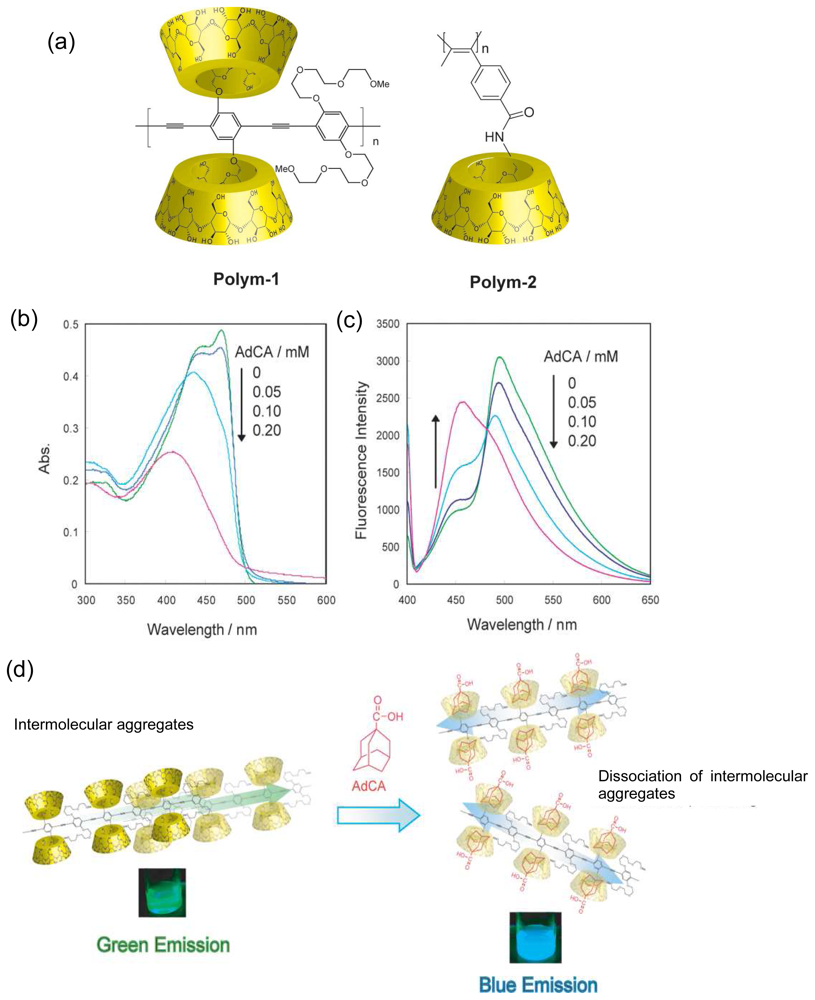
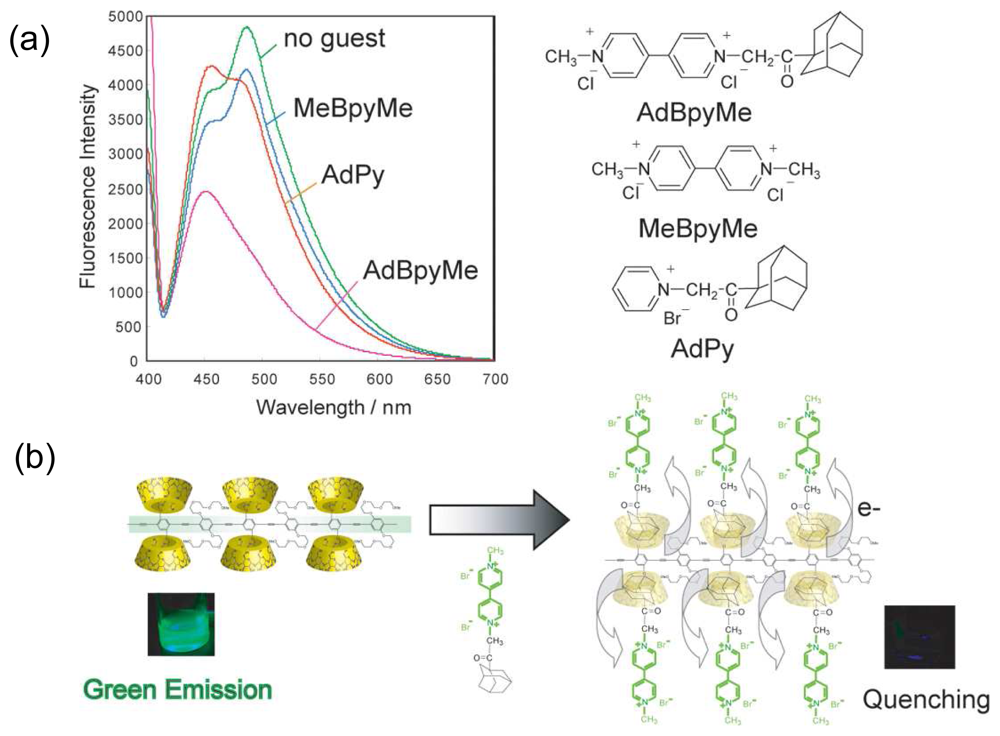
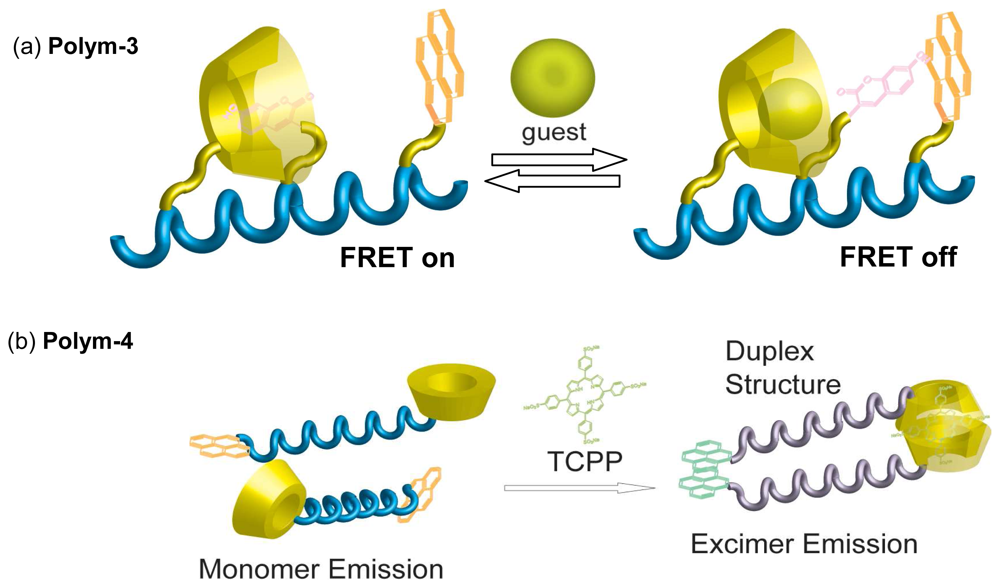
Chemical Sensors Using Polymers Carrying CDs
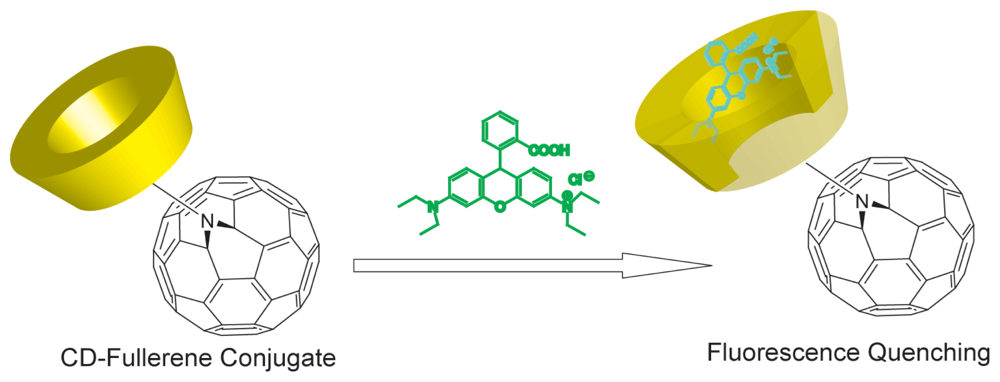
Chemical Sensors Using Cyclodextrin-Nanocarbon Hybrids
Chemical Sensors Using CD-Nanoparticle Hybrids
Conclusions and Outlook
Acknowledgments
References and Notes
- Bender, M.L.; Komiyama, M. Cyclodextrin chemistry; Springer-Verlag: Berlin, Germany, 1978. [Google Scholar]
- Szejtli, S. Cyclodextrin technology; Kluwer Academic Publishers: Dordrecht, Netherlands, 1998. [Google Scholar]
- Szejtli, J.; Osa, T. (Eds.) Comprehensive cyclodextrin chemistry; Pergmon: Oxford, U.K, 1996; Volume 3.
- Nepogodiev, S.A.; Stoddart, J.F. Cyclodextrin-based catenanes and rotaxanes. Chem. Rev. 1998, 98, 1959–1976. [Google Scholar]
- Gattuso, G.; Nepogodiev, S.A.; Stoddart, J.F. Synthetic cyclic oligosaccharides. Chem. Rev. 1998, 98, 1919–1958. [Google Scholar]
- Khan, A.R.; Forgo, P.; Stine, K.J.; D'Souza, V.T. Methods for selective modifications of cyclodextrins. Chem. Rev. 1998, 98, 1977–1996. [Google Scholar]
- Engeldinger, E.; Armspach, D.; Matt, D. Capped cyclodextrins. Chem. Rev. 2003, 103, 4147–4173. [Google Scholar]
- Rekharsky, M.V.; Inoue, Y. Complexation thermodynamics of cyclodextrins. Chem. Rev. 1998, 98, 1875–1918. [Google Scholar]
- Connors, K.A. The stability of cyclodextrin complexes in solution. Chem. Rev. 1997, 97, 1325–1358. [Google Scholar]
- Wenz, G.; Han, B.H.; Muller, A. Cyclodextrin rotaxanes and polyrotaxanes. Chem. Rev. 2006, 106, 782–817. [Google Scholar]
- Armspach, D.; Ashton, P.R.; Moore, C.P.; Spencer, N.; Stoddart, J.F.; Wear, T.J.; Williams, D.J. The self-assembly of catenated cyclodextrins. Angew. Chem. Int. Ed. 1993, 32, 854–858. [Google Scholar]
- Armspach, D.; Ashton, P.R.; Ballardini, R.; Balzani, V.; Godi, A.; Moore, C.P.; Prodi, L.; Spencer, N.; Stoddart, J.F.; Tolley, M.S.; Wear, T.J.; Williams, D.J. Catenated cyclodextrins. Chem. Eur. J. 1995, 1, 33–55. [Google Scholar]
- Ogino, H. Relatively high-yield syntheses of rotaxanes. Syntheses and properties of compounds consisting of cyclodextrins threaded by α,ω-diaminoalkanes coordinated to cobalt(III) complexes. J. Am. Chem. Soc. 1981, 103, 1303–1304. [Google Scholar]
- Ogino, H.; Ohta, K. Synthesis and properties of rotaxane complexes. [2]Rotaxanes consisting of α-or β-cyclodextrin threaded by (μ-α,ω-diaminoalkane)bis[chlorobis(ethylenediamine)cobalt(III)] complexes. Inorg. Chem. 1984, 23, 3312–3316. [Google Scholar]
- Isnin, R.; Kaifer, A.E. Self-assembling metal rotaxane complexes of α-cyclodextrin. J. Am. Chem. Soc. 1992, 114, 3136. [Google Scholar]
- Isnin, R.; Kaifer, A.E. Novel class of asymmetric zwitterionic rotaxanes based on α-cyclodextrin. J. Am. Chem. Soc 1991, 113, 8188. [Google Scholar]
- Harada, A.; Li, J.; Kamachi, M. Non-ionic [2]rotaxanes containing methylated α-cyclodextrins. Chem. Commun. 1997, 1413–1414. [Google Scholar]
- Harada, A.; Li, J.; Kamachi, M. The molecular necklace: a rotaxane containing many threaded α-cyclodextrins. Nature 1992, 356, 325–327. [Google Scholar]
- Harada, A.; Li, J.; Kamachi, M. Synthesis of a tubular polymer from threaded cyclodextrins. Nature 1993, 364, 516–518. [Google Scholar]
- Harada, A. Cyclodextrin-based molecular machines. Acc. Chem. Res. 2001, 34, 456–464. [Google Scholar]
- Kuad, P.; Miyawaki, A.; Takashima, Y.; Yamaguchi, H.; Harada, A. External stimulus-responsive supramolecular structures formed by a stilbene cyclodextrin dimer. J. Am. Chem. Soc. 2007, 129, 12630–12631. [Google Scholar]
- Harada, A. Supramolecular polymers based on cyclodextrins. J. Polym. Sci. A. Polym. Chem. 2006, 44, 5113–5119. [Google Scholar]
- Harada, A.; Hashidzume, A.; Takashima, Y. Cyclodextrin-based supramolecular polymers. Adv. Polym. Sci. 2006, 201, 1–43. [Google Scholar]
- Thomas, S.W.; Joly, G.D.; Swager, T.M. Chemical sensors based on amplifying fluorescent conjugated polymers. Chem. Rev. 2007, 107, 1339–1386. [Google Scholar]
- McQuade, D.T.; Pullen, A.E.; Swager, T.M. Conjugated polymer-based chemical sensors. Chem. Rev. 2000, 100, 2537–2574. [Google Scholar]
- Sherigara, B.S.; Kutner, W.; D'Souza, F. Electrocatalytic properties and sensor applications of fullerenes and carbon nanotubes. Electroanalysis 2003, 15, 753–772. [Google Scholar]
- Bonifazi, D.; Enger, O.; Diederich, F. Supramolecular [60]fullerene chemistry on surfaces. Chem. Soc. Rev. 2007, 36, 390–414. [Google Scholar]
- Anker, J.N.; Hall, W.P.; Lyandres, O.; Shah, N.C.; Zhao, J.; Van Duyne, R.P. Biosensing with plasmonic nanosensors. Nature Mater. 2008, 7, 442–453. [Google Scholar]
- Murphy, C.J.; Gole, A.M.; Hunyadi, S.E.; Stone, J.W.; Sisco, P.N.; Alkilany, A.; Kinard, B.E.; Hankins, P. Chemical sensing and imaging with metallic nanorods. Chem. Commun. 2008, 544–557. [Google Scholar]
- Ueno, A.; Minato, S.; Suzuki, I.; Fukushima, M.; Ohkubo, M.; Osa, T.; Hamada, F.; Murai, K. Host–guest sensory system of dansyl-modified β-cyclodextrin for detecting steroidal compounds by dansyl fluorescence. Chem. Lett. 1990, 605–608. [Google Scholar]
- Hamada, F.; Kondo, Y.; Ito, R.; Suzuki, I.; Osa, T.; Ueno, A. Dansyl-modified γ-cyclodextrin as a fluorescent sensor for molecular recognition. J. Incl. Phenom. 1993, 15, 273–279. [Google Scholar]
- Wang, Y.; Ikeda, T.; Ueno, A.; Toda, F. Syntheses and molecular recognition abilities of 6-O-, 2-O-, and 3-O-dansyl-γ-cyclodextrins. Chem. Lett. 1992, 863–866. [Google Scholar]
- Wang, Y.; Ikeda, T.; Ikeda, H.; Ueno, A.; Toda, F. Dansyl-β-cyclodextrins as fluorescent sensors responsive to organic compounds. Bull. Chem. Soc. Jpn. 1994, 67, 1598–1607. [Google Scholar]
- Corradini, R.; Dossena, A.; Marchelli, R.; Panagia, A.; Sartor, G.; Saviano, M.; Lombardi, A.; Pavone, V. A modified cyclodextrin with a fully encapsulated dansyl group: self-inclusion in the solid state and in solution. Chem. Eur. J. 1996, 2, 373–381. [Google Scholar]
- Pagliari, S.; Corradini, R.; Galaverna, G.; Sforza, S.; Dossena, A.; Montalti, M.; Prodi, L.; Zaccheroni, N.; Marchelli, R. Enaitioselective fluorescence sensing of amino acids by modified cyclodextrins: role of the cavity and sensing mechanism. Chem. Eur. J. 2004, 10, 2749–2758. [Google Scholar]
- Hamasaki, K.; Ikeda, H.; Nakamura, A.; Ueno, A.; Toda, F.; Suzuki, I.; Osa, T. Fluorescent sensors of molecular recognition. Modified cyclodextrins capable of exhibiting guest-responsive twisted intramolecular charge transfer fluorescence. J. Am. Chem. Soc. 1993, 115, 5035–5040. [Google Scholar]
- Hamasaki, K.; Ueno, A.; Toda, F.; Suzuki, I.; Osa, T. Molecular recognition indicators of modified cyclodextrins using twisted intramolecular charge transfer fluorescence. Bull. Chem. Soc. Jpn. 1994, 67, 516–523. [Google Scholar]
- Hamasaki, K.; Ueno, A.; Toda, F. A fluorescent α-cyclodextrin as a sensor for detecting aliphatic alcohols by dual fluorescence arising from normal planar and twisted intramolecular charge transfer excited states. J. Chem. Soc, Chem. Commun. 1993, 331–333. [Google Scholar]
- Ueno, A.; Moriwaki, F.; Osa, T.; Hamada, F.; Murai, K. Fluorescence and circular dichroism studies on host-guest complexation of γ-cyclodextrin bearing two 2-naphthyl moieties. Bull. Chem. Soc. Jpn. 1986, 59, 465–470. [Google Scholar]
- Ueno, A.; Minato, S.; Osa, T. Host-guest sensors of 6A,6B-, 6A,6C-, 6A,6D-, and 6A,6E-bis(2-naphthylsulfenyl)-γ-cyclodextrins for detecting organic compounds by fluorescence enhancements. Anal. Chem. 1992, 64, 1154–1157. [Google Scholar]
- Moriwaki, F.; Kaneko, H.; Ueno, A.; Osa, T.; Hamada, F.; Murai, K. Excimer formation and induced-fit type of complexation of β-cyclodextrin capped by two naphthyl moieties. Bull. Chem. Soc. Jpn. 1987, 60, 3619–3623. [Google Scholar]
- Suzuki, I.; Ohkubo, M.; Ueno, A.; Osa, T. Detection of organic compounds by dual fluorescence of bis(1-pyrenecarbonyl)-γ-cyclodextrins. Chem. Lett. 1992, 269–272. [Google Scholar]
- Ueno, A.; Moriwaki, F.; Osa, T.; Hamada, F.; Murai, K. Association, photodimerization, and induced-fit types of host-guest complexation of anthracene-appended γ-cyclodextrin derivatives. J. Am. Chem. Soc. 1988, 110, 4323–4328. [Google Scholar]
- Ueno, A.; Moriwaki, F.; Iwata, Y.; Suzuki, I.; Osa, T.; Ohta, T.; Nozoe, S. γ-Cyclodextrin template method for controlling stereochemistry of bimolecular interactions and reactions. J. Am. Chem. Soc. 1991, 113, 7034–7036. [Google Scholar]
- Ikeda, H.; Murayama, T.; Ueno, A. Skeleton-selective fluorescent chemosensor based on cyclodextrin bearing a 4-amino-7-nitrobenz-2-oxa-1,3-diazole moiety. Org. Biomol. Chem. 2005, 3, 4262–4267. [Google Scholar]
- Liu, Y.; Shi, J.; Guo, D.S. Novel permethylated β-cyclodextrin derivatives appended with chromophores as efficient fluorescent sensors for the molecular recognition of bile salts. J. Org. Chem. 2007, 72, 8227–8234. [Google Scholar]
- Kuwabara, T.; Nakamura, A.; Ueno, A.; Toda, F. Inclusion complexes and guest-induced color changes of pH-indicator-modified β-cyclodextrins. J. Phys. Chem. 1994, 98, 6297–6303. [Google Scholar]
- Ueno, A.; Kuwabara, T.; Nakamura, A.; Toda, F. A modified cyclodextrin as a guest responsive color-change indicator. Nature 1992, 356, 136–137. [Google Scholar]
- Kuwabara, T.; Nakamura, A.; Ueno, A.; Toda, F. Supramolecular thermochromism of a dye-appended □-cyclodextrin. J. Chem. Soc, Chem. Commun. 1994, 689–690. [Google Scholar]
- Aoyagi, T.; Nakamura, A.; Ikeda, H.; Ikeda, T.; Mihara, H.; Ueno, A. Alizarin yellow-modified β-cyclodextrin as a guest-responsive absorption change sensor. Anal. Chem. 1997, 69, 659–663. [Google Scholar]
- Barcza, L.; Buravi, A. Complex formation of cyclomalto-octaose with tetrabromophenolphthalein and some related compounds. Carbohyd. Res. 1989, 192, 103–109. [Google Scholar]
- Haider, J.M.; Pikramenou, Z. Photoactive metallocyclodextrins: sophisticated supramolecular arrays for the construction of light activated miniature devices. Chem. Soc. Rev. 2005, 34, 120–132. [Google Scholar]
- Pikramenou, Z.; Yu, J.A.; Lessard, R.B.; Ponce, A.; Wong, P.A.; Nocera, D.G. Luminescence from supramolecules triggered by the molecular recognition of substrate. Coord. Chem. Rev. 1994, 132, 181–194. [Google Scholar]
- Mortellaro, M.A.; Nocera, D.G. A supramolecular chemosensor for aromatic hydrocarbons. J. Am. Chem. Soc. 1996, 118, 7414–7415. [Google Scholar]
- Michels, J.J.; Huskens, J.; Reinhoudt, D.N. Nonconvalent binding of sensitizers for lanthanide(III) luminescence in an EDTA-bis(β-cyclodextrin) ligand. J. Am. Chem. Soc. 2002, 124, 2056–2064. [Google Scholar]
- Haider, J.M.; Pikramenou, Z. Metal assembly of cyclodextrin recognition sites. Eur. J. Inorg. Chem. 2001, 189–194. [Google Scholar]
- Heck, R.; Dumarcay, F.; Marsura, A. New scaffolds for supramolecular chemistry: upper-rim fully tethered 5-methyleneureido-5′-methyl-2,2′-bipyridyl cyclodextrins. Chem. Eur. J. 2002, 8, 2438–2445. [Google Scholar]
- Liu, Y.; Song, Y.; Chen, Y.; Li, X.Q.; Ding, F.; Zhong, R.Q. Biquinolino-modified β-cyclodextrin dimers and their metal complexes as efficient fluorescent sensors for the molecular recognition of steroids. Chem. Eur. J. 2004, 10, 3685–3696. [Google Scholar]
- Yang, R.; Li, K.; Wang, K.; Zhao, F.; Li, N.; Liu, F. Porphyrin assembly on β-cyclodextrin for selective sensing and detection of a zinc ion based on the dual emission fluorescence ratio. Anal. Chem. 2003, 75, 612–621. [Google Scholar]
- Liu, Y.; Zhang, N.; Chen, Y.; Wang, L.H. Fluorescence sensing and binding behavior of aminobenzenesulfonamidoquinolino-β-cyclodextrin to Zn2+. Org. Lett. 2007, 9, 315–318. [Google Scholar]
- Nakamura, M.; Ikeda, T.; Nakamura, A.; Ikeda, H.; Ueno, A.; Toda, F. Remarkable molecular recognition of dansyl-modified cyclodextrin dimer. Chem. Lett. 1995, 343–344. [Google Scholar]
- de Jong, M.R.; Engbersen, J.F.J.; Huskens, J.; Reinhoudt, D.N. Cyclodextrin dimers as receptor molecules for steroid sensors. Chem. Eur. J. 2000, 6, 4034–4040. [Google Scholar]
- Kikuchi, T.; Narita, M.; Hamada, F. Synthesis of bis dansyl-modified β-cyclodextrin liner trimer having multi-recognition sites and high hydrophobic environment. Tetrahedron 2001, 57, 9317–9324. [Google Scholar]
- Sasaki, K.; Nagasawa, M.; Kuroda, Y. New cyclodextrin dimer and trimer: formation of biphenyl excimer and their molecular recognition. Chem. Commun. 2001, 2630–2631. [Google Scholar]
- Yamauchi, A.; Hayashita, T.; Kato, A.; Nishizawa, S.; Watanabe, M.; Teramae, N. Selective potassium ion recognition by benzo-15-crown-5 fluoroionophore/γ-cyclodextrin complex sensors in water. Anal. Chem. 2000, 72, 5841–5846. [Google Scholar]
- Hayashita, T.; Qing, D.; Minagawa, M.; Lee, J.C.; Ku, C.H.; Teramae, N. Highly selective recognition of lead ion in water by a podand fluoroionophore/γ-cyclodextrin complex sensor. Chem. Commun. 2003, 2160–2161. [Google Scholar]
- Tong, A.J.; Yamauchi, A.; Hayashita, T.; Zhang, Z.Y.; Smith, B.D.; Teramae, N. Boronic acid fluorophore/β-cyclodextrin complex sensors for selective sugar recognition in water. Anal. Chem. 2001, 73, 1530–1536. [Google Scholar]
- Jung, J.H.; Lee, S. J.; Kim, J.S.; Lee, W.S.; Sakata, Y.; Kaneda, T. α-CD/crown-appended diazophenol for selective sensing of amines. Org. Lett. 2006, 8, 3009–3012. [Google Scholar]
- Jung, J.H.; Lee, H.Y.; Jung, S.H.; Lee, S.J.; Sakata, Y.; Kaneda, T. A color version of the hinsberg test: permethylated cyclodextrin and crown-appended azophenol for highly selective sensing of amines. Tetrahedron 2008, 64, 6705–6710. [Google Scholar]
- Bügler, J.; Engbersen, J.F.J.; Reinhoudt, D.N. Novel water-soluble β-cyclodextrin-calix[4]arene couples as fluorescent sensor molecules for the detection of neutral analytes. J. Org. Chem. 1998, 63, 5339–5344. [Google Scholar]
- Liu, Y; Chen, Y.; Li, L.; Huang, G.; You, C.C.; Zhang, H.Y.; Wada, T.; Inoue, Y. Cooperative multiple recognition by novel calix[4]arene-tethered β-cyclodextrin and calix[4]arene-bridged bis(β-cyclodextrin). J. Org. Chem. 2001, 66, 7209–7215. [Google Scholar]
- Suzuki, I.; Ui, M.; Yamauchi, A. Supramolecular probe for bicarbonate exhibiting anomalous pyrene fluorescence in aqueous media. J. Am. Chem. Soc. 2006, 128, 4498–4499. [Google Scholar]
- Klotz, E.J.F.; Claridge, T.D.W.; Anderson, H.L. Homo- and hetero-[3]rotaxanes with two π-systems clasped in a single macrocycle. J. Am. Chem. Soc. 2006, 128, 15374–15375. [Google Scholar]
- Deng, W.; Yamaguchi, H.; Takashima, Y.; Harada, A. A chemical-responsive supramolecular hydrogel from modified cyclodextrins. Angew. Chem. Int. Ed. 2007, 46, 5144–5147. [Google Scholar]
- Deng, W.; Yamaguchi, H.; Takashima, Y.; Harada, A. Construction of chemical-responsive supramolecular hydrogels from guest-modified cyclodextrins. Chem. Asian J. 2008, 3, 687–695. [Google Scholar]
- Ogoshi, T.; Takashima, Y.; Yamaguchi, H.; Harada, A. Cyclodextrin-grafted poly(phenylene ethynylene) with chemical-responsive properties. Chem. Commun. 2006, 3702–3704. [Google Scholar]
- Yashima, E.; Maeda, K.; Sato, O. Switching of macromolecular helicity for visual distinction of molecular recognition events. J. Am. Chem. Soc. 2001, 123, 8159–8160. [Google Scholar]
- Onouchi, H.; Hasegawa, T.; Kashiwagi, D.; Ishiguro, H.; Maeda, K.; Yashima, E. Chirality sensing of various biomolecules with helical poly(phenylacetylene)s bearing acidic functional groups in water. J. Polym. Sci. Part A: Polym. Chem. 2006, 44, 5039–5048. [Google Scholar]
- Maeda, K.; Mochizuki, H.; Watanabe, M.; Yashima, E. Switching of macromolecular helicity of optically active poly(phenylacetylene)s bearing cyclodextrin pendants induced by various external stimuli. J. Am. Chem. Soc. 2006, 128, 7639–3650. [Google Scholar]
- Hossain, M. A.; Mihara, H.; Ueno, A. Novel peptides bearing pyrene and coumarin units with or without β-cyclodextrin in their side chains exhibit intramolecular fluorescence resonance energy transfer. J. Am. Chem. Soc. 2003, 125, 11178–11179. [Google Scholar]
- Hossain, M.A.; Mihara, H.; Ueno, A. Fluorescence resonance energy transfer in a novel cyclodextrin–peptide conjugate for detecting steroid molecules. Bioorg. Med. Chem. Lett. 2000, 10, 1857–1861. [Google Scholar]
- Yana, D.; Shimizu, T.; Hamasaki, K.; Mihara, H.; Ueno, A. Double naphthalene-tagged cyclodextrin-peptide capable of exhibiting guest-induced naphthalene excimer fluorescence. Macromol. Rapid. Commun. 2002, 23, 11–15. [Google Scholar]
- Furukawa, S.; Mihara, H.; Ueno, A. Sensing behavior of fluorescent cyclodextrin/peptide hybrids bearing a macrocyclic metal complex. Macromol. Rapid. Commun. 2003, 24, 202–206. [Google Scholar]
- Hossain, M.A.; Hamasaki, K.; Takahashi, K.; Mihara, H.; Ueno, A. Guest-induced diminishment in fluorescence quenching and molecule sensing ability of a novel cyclodextrin-peptide conjugate. J. Am. Chem. Soc. 2001, 123, 7435–7436. [Google Scholar]
- Fujumoto, K.; Muto, Y.; Inouye, M. A DNA duplex-based, tailor-made fluorescent sensor for porphyrin derivatives. Bioconjugate Chem. 2008, 19, 1132–1134. [Google Scholar]
- Yuan, D.Q.; Koga, K.; Kourogi, Y.; Fujita, K. Synthesis of fullerene-cyclodextrin conjugates. Tetrahedron Lett. 2001, 42, 6727–6729. [Google Scholar]
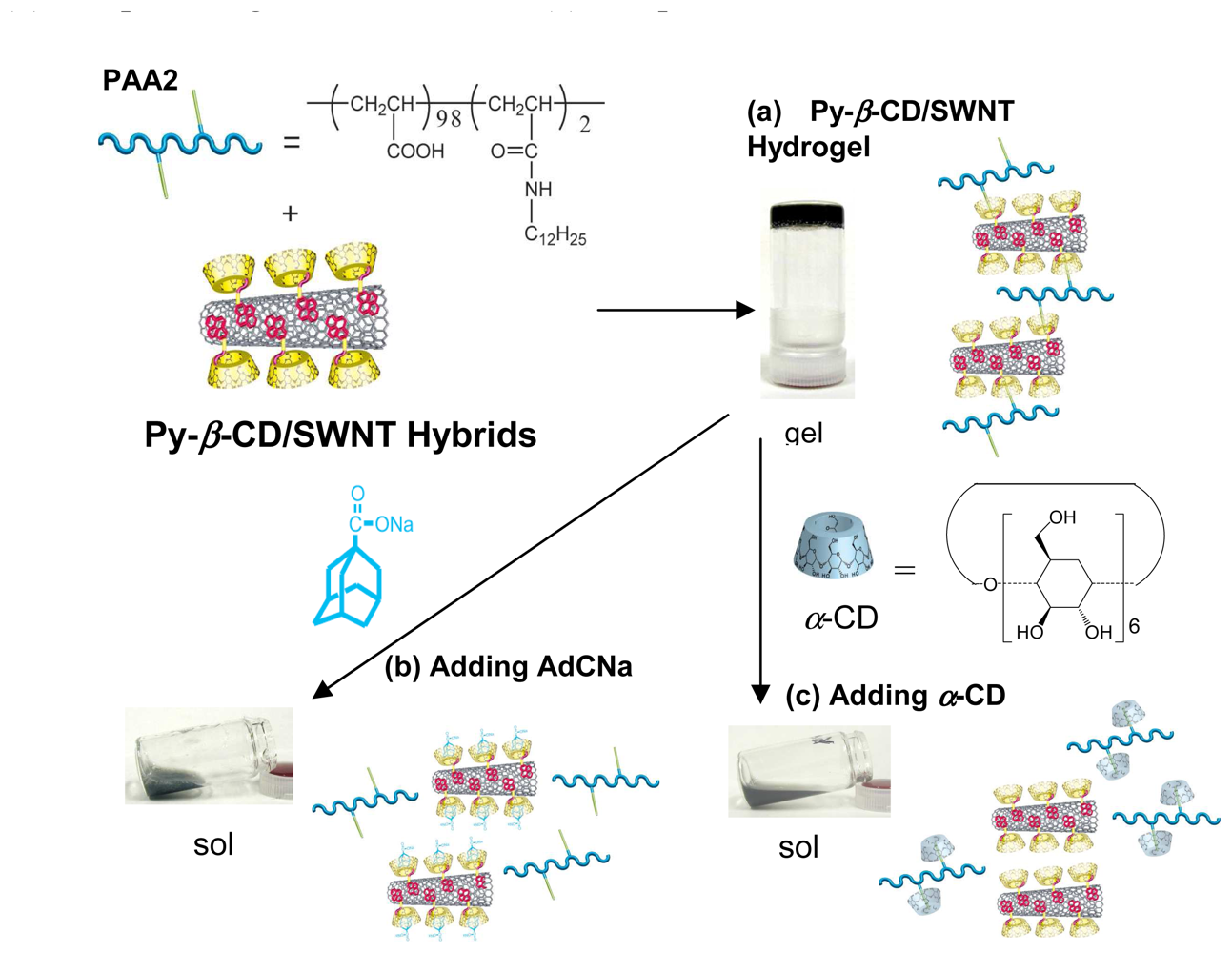
- Ogoshi, T.; Takashima, Y.; Yamaguchi, H.; Harada, A. Chemically-responsive sol-gel transition of supramolecular single-walled carbon nanotubes (SWNTs) hydrogel made by hybrids of SWNTs and cyclodextrins. J. Am. Chem. Soc. 2007, 129, 4878–4879. [Google Scholar]
- Zhao, Y.L.; Hu, L.; Stoddart, J.F.; Grüner, G. Pyrenecyclodextrin-decorated single-walled carbon nanotube field-effect transistors as chemical sensors. Adv. Mater. 2008, 20, 1910–1915. [Google Scholar]
- Liang, P.; Zhang, H.Y.; Yu, Z.L.; Liu, Y. Solvent-controlled photoinduced electron transfer between porphyrin and carbon nanotubes. J. Org. Chem. 2008, 73, 2163–2168. [Google Scholar]
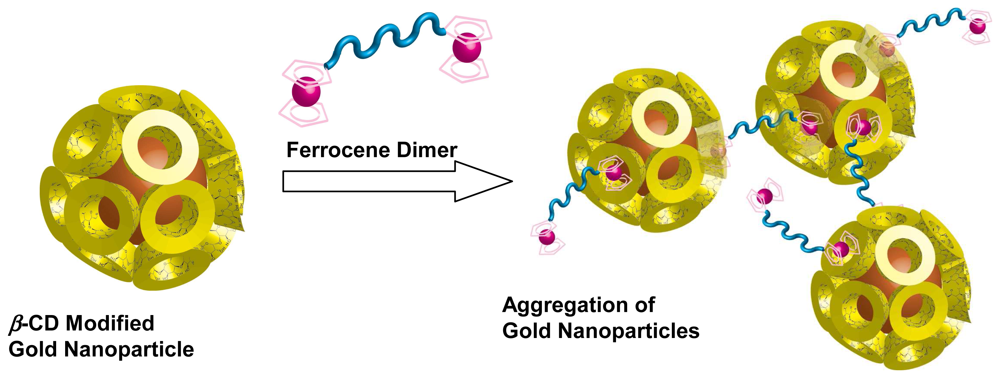
- Liu, J.; Mendoza, S.; Román, E.; Lynn, M.J.; Xu, R.; Kaifer, A.E. Cyclodextrin-modified gold nanoparticles. Host-guest interactions at work to control colloidal properties. J. Am. Chem. Soc. 1999, 121, 4304–4305. [Google Scholar]
- Liu, J.; Ong, W.; Román, E.; Lynn, M.J.; Kaifer, A.E. Cyclodextrin-modified gold nanospheres. Langmuir 2000, 16, 3000–3002. [Google Scholar]
- Liu, J.; Alvarez, J.; Ong, W.; Kaifer, A.E. Network aggregates formed by C60 and gold nanoparticles capped with cyclodextrin hosts. Nano Lett. 2001, 1, 57–60. [Google Scholar]
- Tang, B.; Cao, L.; Xu, K.; Zhuo, L.; Ge, J.; Li, Q.; Yu, L. A new nanobiosensor for glucose with high sensitivity and selectivity in serum based on fluorescence resonance energy transfer (FRET) between CdTe quantum dots and Au nanoparticles. Chem. Eur. J. 2008, 14, 3637–3644. [Google Scholar]

© 2008 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ogoshi, T.; Harada, A. Chemical Sensors Based on Cyclodextrin Derivatives. Sensors 2008, 8, 4961-4982. https://doi.org/10.3390/s8084961
Ogoshi T, Harada A. Chemical Sensors Based on Cyclodextrin Derivatives. Sensors. 2008; 8(8):4961-4982. https://doi.org/10.3390/s8084961
Chicago/Turabian StyleOgoshi, Tomoki, and Akira Harada. 2008. "Chemical Sensors Based on Cyclodextrin Derivatives" Sensors 8, no. 8: 4961-4982. https://doi.org/10.3390/s8084961



